9,10-Phenanthrenedione as Visible-Light Photoredox Catalyst: A Green Methodology for the Functionalization of 3,4-Dihydro-1,4-Benzoxazin-2-Ones through a Friedel-Crafts Reaction
Abstract
1. Introduction
2. Results
3. Materials and Methods
3.1. General Information
3.2. General Procedure: Friedel-Crafts Reaction between 4-Benzyl-3,4-Dihydro-1,4-Benzoxazin-2-Ones and Indoles, Pyrroles and 1,3,5-Trimethoxybenzene
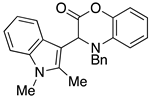
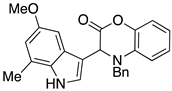
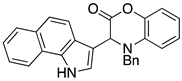
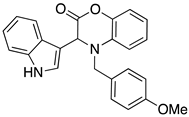
3.3. Characterization Data for Compounds 3, 6 and 7
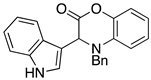
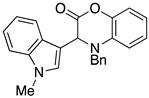
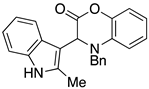

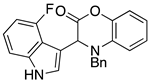
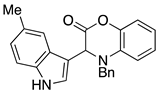

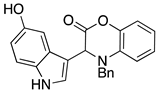


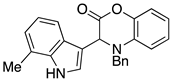

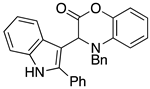
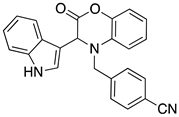


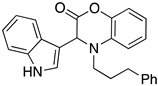
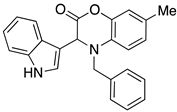
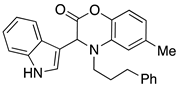
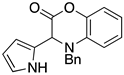
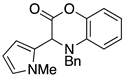

3.4. Synthesis and Characterization of Cephalandole A (8)
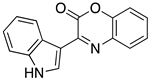
3.5. Synthesis and Characterization of Compound 9

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ciamician, G. The Photochemistry of the Future. Science 1912, 36, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Albani, A.; Fagnoni, M. Green chemistry and photochemistry were born at the same time. Green Chem. 2004, 6, 1–6. [Google Scholar] [CrossRef]
- Ravelli, D.; Protti, S.; Fagnoni, M. Carbon–Carbon Bond Forming Reactions via Photogenerated Intermediates. Chem. Rev. 2016, 116, 9850–9913. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.; Zhao, J.; Chen, X. Cooperative photoredox catalysis. Chem. Soc. Rev. 2016, 45, 3026–3038. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.P. Photochemical Stereocontrol Using Tandem Photoredox–Chiral Lewis Acid Catalysis. Acc. Chem. Res. 2016, 49, 2307–2315. [Google Scholar] [CrossRef]
- Narayanam, J.M.R.; Stephenson, C.R.J. Visible light photoredox catalysis: Applications in organic synthesis. Chem. Soc. Rev. 2011, 40, 102–113. [Google Scholar] [CrossRef]
- Chen, J.-R.; Hu, X.-Q.; Lu, L.-Q.; Xiao, W.-J. Visible light photoredox-controlled reactions of N-radicals and radical ions. Chem. Soc. Rev. 2016, 45, 2044–2056. [Google Scholar] [CrossRef]
- Shaw, M.H.; Twilton, J.; MacMillan, D.W.C. Photoredox Catalysis in Organic Chemistry. J. Org. Chem. 2016, 81, 6898–6926. [Google Scholar] [CrossRef]
- Fabry, D.C.; Rueping, M. Merging Visible Light Photoredox Catalysis with Metal Catalyzed C–H Activations: On the Role of Oxygen and Superoxide Ions as Oxidants. Acc. Chem. Res. 2016, 49, 1969–1979. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef]
- Arias-Rotondo, D.M.; McCusker, J.K. The photophysics of photoredox catalysis: A roadmap for catalyst design. Chem. Soc. Rev. 2016, 45, 5803–5820. [Google Scholar] [CrossRef] [PubMed]
- Tucker, J.W.; Stephenson, C.R.J. Shining Light on Photoredox Catalysis: Theory and Synthetic Applications. J. Org. Chem. 2012, 77, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Tellis, J.C.; Kelly, C.B.; Primer, D.N.; Jouffroy, M.; Patel, N.R.; Molander, G.A. Single-Electron Transmetalation via Photoredox/Nickel Dual Catalysis: Unlocking a New Paradigm for sp3–sp2 Cross-Coupling. Acc. Chem. Res. 2016, 49, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Alfonzo, E.; Alfonso, F.S.; Beeler, A.B. Redesign of a Pyrylium Photoredox Catalyst and Its Application to the Generation of Carbonyl Ylides. Org. Lett. 2017, 19, 2989–2992. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Meng, L.-G.; Wang, L. Visible-Light-Promoted [2 + 2 + 2] Cyclization of Alkynes with Nitriles to Pyridines Using Pyrylium Salts as Photoredox Catalysts. Org. Lett. 2017, 19, 1958–1961. [Google Scholar] [CrossRef]
- Kottisch, V.; Michaudel, Q.; Fors, B.P. Cationic Polymerization of Vinyl Ethers Controlled by Visible Light. J. Am. Chem. Soc. 2016, 138, 15535–15538. [Google Scholar] [CrossRef]
- Perkowski, A.J.; You, W.; Nicewicz, D.A. Visible Light Photoinitiated Metal-Free Living Cationic Polymerization of 4-Methoxystyrene. J. Am. Chem. Soc. 2015, 137, 7580–7583. [Google Scholar] [CrossRef]
- Perkowski, A.J.; Cruz, C.L.; Nicewicz, D.A. Ambient-Temperature Newman–Kwart Rearrangement Mediated by Organic Photoredox Catalysis. J. Am. Chem. Soc. 2015, 137, 15684–15687. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Ohkubo, K. Organic synthetic transformations using organic dyes as photoredox catalysts. Org. Biomol. Chem. 2014, 12, 6059–6071. [Google Scholar] [CrossRef]
- Margrey, K.A.; Nicewicz, D.A. A General Approach to Catalytic Alkene Anti-Markovnikov Hydrofunctionalization Reactions via Acridinium Photoredox Catalysis. Acc. Chem. Res. 2016, 49, 1997–2006. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Kotani, H.; Ohkubo, K.; Ogo, S.; Tkachenko, N.V.; Lemmetyinen, H. Electron-Transfer State of 9-Mesityl-10-methylacridinium Ion with a Much Longer Lifetime and Higher Energy Than That of the Natural Photosynthetic Reaction Center. J. Am. Chem. Soc. 2004, 126, 1600–1601. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.; Xia, X.-D.; Zeng, T.-T.; Feng, Z.-J.; Chen, J.-R.; Lu, L.-Q.; Xiao, W.-J. Visible-Light-Induced Formal [3+2] Cycloaddition for Pyrrole Synthesis under Metal-Free Conditions. Angew. Chem. Int. Ed. 2014, 53, 5653–5656. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.S.; Nicewicz, D.A. Direct Catalytic Anti-Markovnikov Hydroetherification of Alkenols. J. Am. Chem. Soc. 2012, 134, 18577–18580. [Google Scholar] [CrossRef] [PubMed]
- Romero, N.A.; Margrey, K.A.; Tay, N.E.; Nicewicz, D.A. Site-selective arene C-H amination via photoredox catalysis. Science, 2015, 349, 1326–1330. [Google Scholar] [CrossRef]
- Romero, N.A.; Nicewicz, D.A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, D.; Fagnoni, M. Dyes as Visible Light Photoredox Organocatalysts. ChemCatChem 2012, 4, 169–171. [Google Scholar] [CrossRef]
- Haria, D.P.; König, B. Synthetic applications of eosin Y in photoredox catalysis. Chem. Commun. 2014, 50, 6688–6699. [Google Scholar] [CrossRef]
- Srivastava, V.; Singh, P.P. Eosin Y catalysed photoredox synthesis: A review. RSC Adv. 2017, 7, 31377–31392. [Google Scholar] [CrossRef]
- Pan, Y.; Kee, C.W.; Chen, L.; Tan, C.-H. Dehydrogenative coupling reactions catalysed by Rose Bengal using visible light irradiation. Green Chem. 2011, 13, 2682–2685. [Google Scholar] [CrossRef]
- Neumann, M.; Füldner, S.; König, B.; Zeitler, K. Metal-Free, Cooperative Asymmetric Organophotoredox Catalysis with Visible Light. Angew. Chem. Int. Ed. 2011, 50, 951–954. [Google Scholar] [CrossRef]
- Hari, D.P.; Hering, T.; König, B. The Photoredox-Catalyzed Meerwein Addition Reaction: Intermolecular Amino-Arylation of Alkenes. Angew. Chem. Int. Ed. 2014, 53, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.C.; Yang, D.-D. H. Photochemical Reactions of Ketones in Solution. J. Am. Chem. Soc. 1958, 80, 2913–2914. [Google Scholar] [CrossRef]
- Coyle, J.D.; Carless, H.A. Selected aspects of photochemistry. I Photochemistry of carbonyl compounds. Chem. Soc. Rev. 1972, 1, 465–480. [Google Scholar] [CrossRef]
- Hoffmann, N. Photochemical Reactions as Key Steps in Organic Synthesis. Chem. Rev. 2008, 108, 1052–1103. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Verma, A.; Kadu, R.; Kumar, S. Visible-light-induced oxidant and metal-free dehydrogenative cascade trifluoromethylation and oxidation of 1,6-enynes with water. Chem. Sci. 2017, 8, 6633–6644. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mu, X.; Liu, W.; Wang, Y.; Mi, Z.; Li, C.-J. Simple and Clean Photoinduced Aromatic Trifluoromethylation Reaction. J. Am. Chem. Soc. 2016, 138, 5809–5812. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Caiuby, C.A.D.; Paixão, M.W.; Li, C.-J. Synthesis of 6-Trifluoromethylphenanthridines through Radical Trifluoromethylation of Isocyanides with Sodium Triflinate under Visible Light. Eur. J. Org. Chem. 2018, 2018, 2498–2512. [Google Scholar] [CrossRef]
- Beatty, J.W.; Stephenson, C.R.J. Amine Functionalization via Oxidative Photoredox Catalysis: Methodology Development and Complex Molecule Synthesis. Acc. Chem. Res. 2015, 48, 1474–1484. [Google Scholar] [CrossRef]
- Shi, L.; Xia, W. Photoredox functionalization of C–H bonds adjacent to a nitrogen atom. Chem. Soc. Rev. 2012, 41, 7687–7697. [Google Scholar] [CrossRef]
- Nakajima, K.; Miyake, Y.; Nishibayashi, Y. Synthetic Utilization of α-Aminoalkyl Radicals and Related Species in Visible Light Photoredox Catalysis. Acc. Chem. Res. 2016, 49, 1946–1956. [Google Scholar] [CrossRef]
- Hu, J.; Wang, J.; Nguyen, T.H.; Zheng, N. The chemistry of amine radical cations produced by visible light photoredox catalysis. Beilstein J. Org. Chem. 2013, 9, 1977–2001. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; He, X.; Wang, F.; Chen, J.; Xu, J.; Wang, X.; Han, X. Engineering an N-doped Cu2O@N–C interface with long-lived photo-generated carriers for efficient photoredox catalysts. J. Mater. Chem. A, 2017, 5, 10220–10226. [Google Scholar] [CrossRef]
- Chen, C.-C.; Hong, B.-C.; Li, W.-S.; Chang, T.-T.; Lee, G.-H. Synthesis of Biologically Active Bis(Indolyl)Methane Derivatives by Bisindole Alkylation of Tetrahydroisoquinolines with Visible-Light Induced Ring-Opening Fragmentation. Asian J. Org. Chem. 2017, 6, 426–431. [Google Scholar] [CrossRef]
- Li, Q.-Y.; Ma, Z.; Zhang, W.-Q.; Xu, J.-L.; Wei, W.; Lu, H.; Zhao, X.; Wang, X.-J. AIE-active tetraphenylethene functionalized metal–organic framework for selective detection of nitroaromatic explosives and organic photocatalysis. Chem. Commun. 2016, 52, 11284–11287. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Shelar, D.P.; Han, X.; Li, T.; Guan, X.; Lu, W.; Liu, K.; Chen, Y.; Fu, W.; Che, C. Long-Lived Excited States of Zwitterionic Copper(I) Complexes for Photoinduced Cross-Dehydrogenative Coupling Reactions. Chem. Eur. J. 2015, 21, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Wu, C.; Meng, Q.; Gao, X.; Lei, T.; Tung, C.; Wu, L. A Cascade Cross-Coupling and in Situ Hydrogenation Reaction by Visible Light Catalysis. Adv. Synth. Catal. 2014, 356, 2846–2852. [Google Scholar] [CrossRef]
- Wu, C.-J.; Zhong, J.-J.; Meng, Q.-Y.; Lei, T.; Gao, X.-W.; Tung, C.-H.; Wu, L.-Z. Cobalt-Catalyzed Cross-Dehydrogenative Coupling Reaction in Water by Visible Light. Org. Lett. 2015, 17, 884–887. [Google Scholar] [CrossRef]
- Zhong, J.-J.; Meng, Q.-Y.; Liu, B.; Li, X.-B.; Gao, X.-W.; Lei, T.; Wu, C.-J.; Li, Z.-J.; Tung, C.-H.; Wu, L.-Z. Cross-Coupling Hydrogen Evolution Reaction in Homogeneous Solution without Noble Metals. Org. Lett. 2014, 16, 1988–1991. [Google Scholar] [CrossRef]
- Meng, Q.-Y.; Zhong, J.-J.; Liu, Q.; Gao, X.-W.; Zhang, H.-H.; Lei, T.; Li, Z.-J.; Feng, K.; Chen, B.; Tung, C.-H.; et al. A Cascade Cross-Coupling Hydrogen Evolution Reaction by Visible Light Catalysis. J. Am. Chem. Soc. 2013, 135, 19052–19055. [Google Scholar] [CrossRef]
- Zhong, J.-J.; Meng, Q.-Y.; Wang, G.-X.; Liu, Q.; Chen, B.; Feng, K.; Tung, C.-H.; Wu, L.-Z. A Highly Efficient and Selective Aerobic Cross-Dehydrogenative-Coupling Reaction Photocatalyzed by a Platinum(II) Terpyridyl Complex. Chem. Eur. J. 2013, 19, 6443–6450. [Google Scholar] [CrossRef]
- Freeman, D.B.; Furst, L.; Condie, A.G.; Stephenson, C.R.J. Functionally Diverse Nucleophilic Trapping of Iminium Intermediates Generated Utilizing Visible Light. Org. Lett. 2012, 14, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; He, X.; Sun, L.; Han, X.; Zhan, W.; Xu, J.; Wang, X.; Chen, J. Increasing Effectiveness of Photogenerated Carriers by in Situ Anchoring of Cu2O Nanoparticles on a Nitrogen-Doped Porous Carbon Yolk–Shell Cuboctahedral Framework. ACS Catal. 2018, 8, 3348–3356. [Google Scholar] [CrossRef]
- Hepburn, H.B.; Melchiorre, P. Brønsted acid-catalysed conjugate addition of photochemically generated α-amino radicals to alkenylpyridines. Chem. Commun. 2016, 52, 3520–3523. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.J.; Bastida, D.; Paria, S.; Fagnonia, M.; Melchiorre, P. Asymmetric catalytic formation of quaternary carbons by iminium ion trapping of radicals. Nature 2016, 532, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Li, D.; Li, W.; Yu, W.; Bian, F. The Reaction of Tertiary Anilines with Maleimides under Visible Light Redox Catalysis. Adv. Synth. Catal. 2012, 354, 3561–3567. [Google Scholar] [CrossRef]
- Uraguchi, D.; Kinoshita, N.; Kizu, T.; Ooi, T. Synergistic Catalysis of Ionic Brønsted Acid and Photosensitizer for a Redox Neutral Asymmetric α-Coupling of N-Arylaminomethanes with Aldimines. J. Am. Chem. Soc. 2015, 137, 13768–13771. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Das, A.; Bui, L.; Zhou, H.; Curran, D.P.; Rueping, M. Oxygen Switch in Visible-Light Photoredox Catalysis: Radical Additions and Cyclizations and Unexpected C–C-Bond Cleavage Reactions. J. Am. Chem. Soc. 2013, 135, 1823–1829. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Rueping, M. Merging visible-light photoredox and Lewis acid catalysis for the functionalization and arylation of glycine derivatives and peptides. Chem. Commun. 2012, 48, 11960–11962. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Q.; Hu, M.; Huang, X.-C.; Gong, L.-B.; Xie, Y.-X.; Li, J.-H. Direct α-Arylation of α-Amino Carbonyl Compounds with Indoles Using Visible Light Photoredox Catalysis. J. Org. Chem. 2012, 77, 8705–8711. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-H.; Xiang, Y.; Yang, D.-C.; Guan, Z. Combining enzyme and photoredox catalysis for aminoalkylation of indoles via a relay catalysis strategy in one pot. Green Chem. 2016, 18, 5325–5330. [Google Scholar] [CrossRef]
- Gao, X.-W.; Meng, Q.-Y.; Li, J.-X.; Zhong, J.-J.; Lei, T.; Li, X.-B.; Tung, C.-H.; Wu, L.-Z. Visible Light Catalysis Assisted Site-Specific Functionalization of Amino Acid Derivatives by C–H Bond Activation without Oxidant: Cross-Coupling Hydrogen Evolution Reaction. ACS Catal. 2015, 5, 2391–2396. [Google Scholar] [CrossRef]
- Zhang, G.-Y.; Xiang, Y.; Guan, Z.; He, Y.-H. Enzyme and photoredox sequential catalysis for the synthesis of 1,3-oxazine derivatives in one pot. Catal. Sci. Technol. 2017, 7, 1937–1942. [Google Scholar] [CrossRef]
- Ilaš, J.; Anderluh, P.Š.; Dolenc, M.S.; Kikelj, D. Recent advances in the synthesis of 2H-1,4-benzoxazin-3-(4H)-ones and 3,4-dihydro-2H-1,4-benzoxazines. Tetrahedron 2005, 61, 7325–7348. [Google Scholar] [CrossRef]
- Achari, B.; Mandal, S.B.; Duttaand, P.K.; Chowdhury, C. Perspectives on 1,4-Benzodioxins, 1,4-Benzoxazines and their 2,3-Dihydro Derivatives. Synlett 2004, 2449–2467. [Google Scholar] [CrossRef]
- Patel, M.; McHush, R.J.; Cordova, B.C.; Klabe, R.M.; Erickson-Viitanen, S.; Trainor, G.L.; Rodgers, J.D. Synthesis and evaluation of quinoxalinones as HIV-1 reverse transcriptase inhibitors. Bioorg. Med. Chem. Lett. 2000, 10, 1729–1731. [Google Scholar] [CrossRef]
- Pamerla, M.; Reddy, D.R.S.; Battula, S.; Bodipati, N.; Murthy, Y.L.N. Antimicrobial evaluation of 1,4-benzoxazine derivatives. Med. Chem. Res. 2015, 24, 611–615. [Google Scholar] [CrossRef]
- Bouyssou, T.; Casarosa, P.; Naline, E.; Pestel, S.; Konetzki, I.; Devillier, P.; Schnapp, A. Pharmacological characterization of olodaterol, a novel inhaled beta2-adrenoceptor agonist exerting a 24-h-long duration of action in preclinical models. J. Pharmacol. Exp. Ther. 2010, 334, 53–62. [Google Scholar] [CrossRef]
- Liu, C.; Tan, J.L.; Xiao, S.Y.; Liao, J.F.; Zou, G.R.; Ai, X.X.; Chen, J.B.; Xiang, Y.; Yang, Q.; Zuo, H. 1,4-Benzoxazine-3(4H)-ones as Potent Inhibitors of Platelet Aggregation: Design, Synthesis and Structure–Activity Relations. Chem. Pharm. Bull. 2014, 62, 915–920. [Google Scholar] [CrossRef]
- Zidar, N.; Kikelj, D. A convenient synthesis of 3,4-dihydro-1,4-benzoxazin-2-ones. Tetrahedron 2008, 64, 5756–5761. [Google Scholar] [CrossRef]
- Huo, C.; Dong, J.; Su, Y.; Tang, J.; Chen, F. Iron-catalyzed oxidative sp3 carbon–hydrogen bond functionalization of 3,4-dihydro-1,4-benzoxazin-2-ones. Chem. Commun. 2016, 52, 13341–13344. [Google Scholar] [CrossRef]
- Dong, J.; Min, W.; Li, H.; Quan, Z.; Yang, C.; Huo, C. Iron-Catalyzed C(sp3)−C(sp3) Bond Formation in 3,4-Dihydro-1,4-benzoxazin-2-ones. Adv. Synth. Catal. 2017, 359, 3940–3944. [Google Scholar] [CrossRef]
- De Munck, L.; Vila, C.; Pons, C.; Pedro, J.R. Synthesis of Multisubstituted 1,4-Dihydrobenzoxazin-2-ones through a One-Pot Nucleophilic N-Alkylation/C-Alkylation of Cyclic α-Imino Esters. Synthesis 2017, 49, 2683–2690. [Google Scholar] [CrossRef]
- Blay, G.; Fernández, I.; Muñoz, M.C.; Pedro, J.R.; Vila, C. Synthesis of Functionalized Indoles with a Trifluoromethyl-Substituted Stereogenic Tertiary Carbon Atom Through an Enantioselective Friedel–Crafts Alkylation with β-Trifluoromethyl-α,β-enones. Chem. Eur. J. 2010, 16, 9117–9122. [Google Scholar] [CrossRef] [PubMed]
- Blay, G.; Fernández, I.; Monleón, A.; Muñoz, M.C.; Pedro, J.R.; Vila, C. Synthesis of Functionalized Indoles with an α-Stereogenic Ketone Moiety Through an Enantioselective Friedel–Crafts Alkylation with (E)-1,4-Diaryl-2-butene-1,4-diones. Adv. Synth. Catal. 2009, 351, 2433–2440. [Google Scholar] [CrossRef]
- Blay, G.; Fernández, I.; Pedro, J.R.; Vila, C. Highly Enantioselective Friedel−Crafts Alkylations of Indoles with Simple Enones Catalyzed by Zirconium(IV)−BINOL Complexes. Org. Lett. 2007, 9, 2601–2604. [Google Scholar] [CrossRef]
- Zhang, G.-Y.; Yu, K.-K.; Zhang, C.; Guan, Z.; He, Y.-H. Oxidative Cross-Dehydrogenative-Coupling Reaction of 3,4-Dihydro-1,4-Benzoxazin-2-ones through Visible-Light Photoredox Catalysis. Eur. J. Org. Chem. 2018, 525–531. [Google Scholar] [CrossRef]
- Akula, P.S.; Hong, B.-C.; Lee, G.-H. Visible-light-induced C(sp3)–H activation for a C–C bond forming reaction of 3,4-dihydroquinoxalin-2(1H)-one with nucleophiles using oxygen with a photoredox catalyst or under catalyst-free conditions. RSC Adv. 2018, 8, 19580–19584. [Google Scholar] [CrossRef]
- Mason, J.J.; Bergman, J.; Janosik, T. Synthetic Studies of Cephalandole Alkaloids and the Revised Structure of Cephalandole A. J. Nat. Prod. 2008, 71, 1447–1450. [Google Scholar] [CrossRef]
- Cornford, E.M.; Crane, P.D.; Braun, L.D.; Bocash, W.D.; Nyerges, A.M.; Oldendorf, W.H. Reduction in Brain Glucose Utilization Rate after Tryptophol (3-Indole Ethanol) Treatment. J. Neurochem. 1981, 36, 1758–1765. [Google Scholar] [CrossRef]
- Khedekar, V.; Tillack, A.; Michalik, M.; Beller, M. Convenient synthesis of tryptophols and tryptophol homologues by hydroamination of alkynes. Tetrahedron 2005, 61, 7622–7631. [Google Scholar] [CrossRef]
- Fernando, I.N.; Francis, P.L.; Smith, I. Acyltryptophols reversibly inhibit muscle contractions caused by the actions of acetylcholine and raised potassium ion concentrations. J. Neural. Transm. 1983, 56, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Blay, G.; Fernandez, I.; Muñoz, M.C.; Pedro, J.R.; Vila, C. Enantioselective Friedel–Crafts Alkylation of Indoles with (E)-1-Aryl-4-benzyloxybut-2-en-1-ones Catalyzed by an (R)-3,3′-Br2BINOLate–Hafnium(IV) Complex. Eur. J. Org. Chem. 2013, 1902–1907. [Google Scholar] [CrossRef]







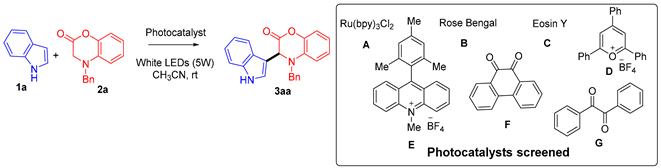
| Entry | Photocatalyst (mol%) | 1a (mmol) | 2a (mmol) | t (h) | Yield of 3aa (%) b |
|---|---|---|---|---|---|
| 1 | A (1%) | 0.15 | 0.1 | 24 | 28 |
| 2 | B (5%) | 0.15 | 0.1 | 27 | 38 |
| 3 | C (5%) | 0.15 | 0.1 | 46 | 27 |
| 4 | D (5%) | 0.15 | 0.1 | 48 | 13 |
| 5 | E (5%) | 0.15 | 0.1 | 48 | 35 |
| 6 | F (10%) | 0.15 | 0.1 | 25 | 33 |
| 7 | A (1%) | 0.1 | 0.15 | 24 | 48 |
| 8 | B (5%) | 0.1 | 0.15 | 24 | 53 |
| 9 | E (5%) | 0.1 | 0.15 | 48 | 27 |
| 10 | F (10%) | 0.1 | 0.15 | 24 | 53 |
| 11 | G (10%) | 0.1 | 0.15 | 24 | 15 |
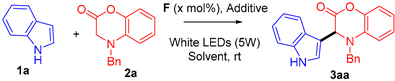
| Entry | Photocat. (mol%) | Additive (mol%) | Solvent | t (h) | Yield of 3aa (%) b |
|---|---|---|---|---|---|
| 1 | F (10%) | - | CH3CN | 24 | 53 |
| 2 | F (10%) | PhCO2H (10 mol%) | CH3CN | 24 | 36 |
| 3 | F (10%) | AcOH (10 mol%) | CH3CN | 24 | 26 |
| 4 | F (10%) | Zn(OAc)2 (10 mol%) | CH3CN | 24 | 37 |
| 5 | F (10%) | Zn(OTf)2 (10 mol%) | CH3CN | 9 | 76 |
| 6 | F (10%) | Fe(OTf)2 (10 mol%) | CH3CN | 20 | 22 |
| 7 | F (10%) | Cu(OTf)2 (10 mol%) | CH3CN | 17 | 19 |
| 8 | F (10%) | Sc(OTf)3 (10 mol%) | CH3CN | 19 | 16 |
| 9 | F (10%) | Zn(OTf)2 (5 mol%) | CH3CN | 9 | 74 |
| 10 | F (10%) | Zn(OTf)2 (5 mol%) | Toluene | 8 | 40 |
| 11 | F (10%) | Zn(OTf)2 (5 mol%) | CH2Cl2 | 20 | 30 |
| 12 | F (10%) | Zn(OTf)2 (5 mol%) | DMF | 72 | 12 |
| 13 | F (10%) | Zn(OTf)2 (5 mol%) | THF | 9 | 34 |
| 14 | F (10%) | Zn(OTf)2 (5 mol%) | MeOH | 17 | 22 |
| 15 | F (5%) | Zn(OTf)2 (5 mol%) | CH3CN | 10 | 74 |
| 16 | F (5%) | Zn(OTf)2 (2.5 mol%) | CH3CN | 10 | 75 |
| 17 | B (5%) | Zn(OTf)2 (2.5 mol%) | CH3CN | 17 | 38 |
| 18 c | F (5%) | Zn(OTf)2 (2.5 mol%) | CH3CN | 15 | 45 |
| 19 d | F (5%) | Zn(OTf)2 (2.5 mol%) | CH3CN | 72 | n.d. e |
| 20 | - | Zn(OTf)2 (2.5 mol%) | CH3CN | 72 | <5 e |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rostoll-Berenguer, J.; Blay, G.; Pedro, J.R.; Vila, C. 9,10-Phenanthrenedione as Visible-Light Photoredox Catalyst: A Green Methodology for the Functionalization of 3,4-Dihydro-1,4-Benzoxazin-2-Ones through a Friedel-Crafts Reaction. Catalysts 2018, 8, 653. https://doi.org/10.3390/catal8120653
Rostoll-Berenguer J, Blay G, Pedro JR, Vila C. 9,10-Phenanthrenedione as Visible-Light Photoredox Catalyst: A Green Methodology for the Functionalization of 3,4-Dihydro-1,4-Benzoxazin-2-Ones through a Friedel-Crafts Reaction. Catalysts. 2018; 8(12):653. https://doi.org/10.3390/catal8120653
Chicago/Turabian StyleRostoll-Berenguer, Jaume, Gonzalo Blay, José R. Pedro, and Carlos Vila. 2018. "9,10-Phenanthrenedione as Visible-Light Photoredox Catalyst: A Green Methodology for the Functionalization of 3,4-Dihydro-1,4-Benzoxazin-2-Ones through a Friedel-Crafts Reaction" Catalysts 8, no. 12: 653. https://doi.org/10.3390/catal8120653
APA StyleRostoll-Berenguer, J., Blay, G., Pedro, J. R., & Vila, C. (2018). 9,10-Phenanthrenedione as Visible-Light Photoredox Catalyst: A Green Methodology for the Functionalization of 3,4-Dihydro-1,4-Benzoxazin-2-Ones through a Friedel-Crafts Reaction. Catalysts, 8(12), 653. https://doi.org/10.3390/catal8120653







