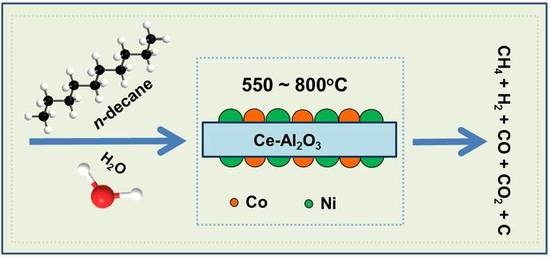
Catalytic Performance and Characterization of Ni-Co Bi-Metallic Catalysts in n-Decane Steam Reforming: Effects of Co Addition
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalytic Performance
2.1.1. n-Decane Conversion and H2 Selectivity
2.1.2. Thermal Stability and Regeneration of C12-NCA
2.2. Fresh Catalyst Characterization
2.2.1. N2 Adsorption-Desorption Measurements
2.2.2. X-ray Diffraction (XRD) Analysis
2.2.3. H2-Temperature-Programmed Reduction (H2-TPR) and NH3-Temperature-Programmed Desorption (NH3-TPD) Analysis
2.2.4. Transmission Electron Microscope (TEM) Analysis
2.3. Carbon Deposition
2.4. Used Catalyst Characterization
3. Experimental Section
3.1. Catalysts Preparation
3.2. Catalysts Characterization
3.3. Catalytic Performance Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cheekatamarla, P.K.; Finnerty, C.M. Reforming catalysts for hydrogen generation in fuel cell applications. J. Power Sources 2006, 160, 490–499. [Google Scholar] [CrossRef]
- Kaewpanha, M.; Guan, G.; Hao, X.; Wang, Z.; Kasai, Y.; Kakuta, S.; Kusakabe, K.; Abudula, A. Steam reforming of tar derived from the steam pyrolysis of biomass over metal catalyst supported on zeolite. J. Taiwan Inst. Chem. Eng. 2013, 44, 1022–1026. [Google Scholar] [CrossRef]
- Liu, X.; Yang, X.; Liu, C.; Chen, P.; Yue, X.; Zhang, S. Low-temperature catalytic steam reforming of toluene over activated carbon supported nickel catalysts. J. Taiwan Inst. Chem. Eng. 2016, 65, 233–241. [Google Scholar] [CrossRef]
- Yang, R.X.; Chuang, K.H.; Wey, M.Y. Hydrogen production through methanol steam reforming: Effect of synthesis parameters on Ni-Cu/CaO-SiO2 catalysts activity. Int. J. Hydrog. Energy 2014, 39, 19494–19501. [Google Scholar] [CrossRef]
- Oararteta, L.; Remiro, A.; Aguayo, A.T.; Bilbao, J.; Gayubo, A.G. Effect of operating conditions on dimethyl ether steam reforming over a CuFe2O4/γ-Al2O3 bifunctional catalyst. Ind. Eng. Chem. Res. 2015, 54, 9722–9732. [Google Scholar] [CrossRef]
- Dou, B.; Song, Y.; Wang, C.; Chen, H.; Xu, Y. Hydrogen production from catalytic steam reforming of biodiesel byproduct glycerol: Issues and challenges. Renew. Sust. Energy Rev. 2014, 30, 950–960. [Google Scholar] [CrossRef]
- Chen, H.; Ding, Y.; Cong, N.T.; Dou, B.; Dupont, V.; Ghadiri, M.; Williams, P.T. Progress in low temperature hydrogen production with simultaneous CO2 abatement. Chem. Eng. Res. Des. 2011, 89, 1774–1782. [Google Scholar] [CrossRef]
- Jiao, Y.; Sun, D.; Zhang, J.; Du, Y.; Kang, J.; Li, C.; Lu, J.; Wang, J.; Chen, Y. Steam reforming of n-decane toward H2 production over Ni/Ce-Al2O3 composite catalysts: Effects of M (M = Fe, Co, Cu, Zn) promoters. J. Anal. Appl. Pyrol. 2016, 120, 238–246. [Google Scholar] [CrossRef]
- Dan, M.; Mihet, M.; Tasnadi-Asztalos, Z.; Imre-Lucaci, A.; Katona, G.; Lazar, M.D. Hydrogen Production by Ethanol Steam Reforming on Nickel Catalysts: Effect of Support Modification by CeO2 and La2O3. Fuel 2015, 147, 260–268. [Google Scholar] [CrossRef]
- Bambal, A.S.; Vecchio, K.S.; Cattolica, R.J. Catalytic effect of Ni and Fe addition to gasifier bed material in the steam reforming of producer gas. Ind. Eng. Chem. Res. 2014, 53, 13656–13666. [Google Scholar] [CrossRef]
- Sangjun, Y.; Youngchan, C.; Jaegoo, L. Hydrogen production from biomass tar by catalytic steam reforming. Energy Convers. Manag. 2010, 51, 42–47. [Google Scholar]
- Jiao, Y.; Du, Y.; Zhang, J.; Li, C.; Xue, Y.; Lu, J.; Wang, J.; Chen, Y. Steam reforming of n-decane for H2 production over Ni modified La-Al2O3 catalysts: Effects of the active component Ni content. J. Anal. Appl. Pyrol. 2015, 116, 58–67. [Google Scholar] [CrossRef]
- Dieuzeide, M.L.; Laborde, M.; Amadeo, N.; Cannilla, C.; Bonura, G.; Frusteri, F. Hydrogen production by glycerol steam reforming: How Mg doping affects the catalytic behaviour of Ni/Al2O3 catalysts. Int. J. Hydrog. Energy 2016, 41, 157–166. [Google Scholar] [CrossRef]
- Sun, L.Z.; Tan, Y.S.; Zhang, Q.D.; Xie, H.J.; Song, F.E.; Sun, Y.Z. Effects of Y2O3-modification to Ni/γ-Al2O3 catalysts on auto thermal reforming of methane with CO2 to syngas. Int. J. Hydrog. Energy 2013, 38, 1892–1900. [Google Scholar] [CrossRef]
- Quitete, C.P.B.; Bittencourt, R.C.P.; Souza, M.M.V.M. Coking resistance evaluation of tar removal catalysts. Catal. Commun. 2015, 71, 79–83. [Google Scholar] [CrossRef]
- Rossetti, I.; Biffi, C.; Bianchi, C.L.; Nichele, V.; Signoretto, M.; Menegazzo, F.; Finocchio, E.; Ramis, G.; Di Michele, A. Ni/SiO2 and Ni/ZrO2 catalysts for the steam reforming of ethanol. Appl. Catal. B Environ. 2012, 117, 384–396. [Google Scholar] [CrossRef]
- Mondal, T.; Pant, K.K.; Dalai, A.K. Catalytic oxidative steam reforming of bio-ethanol for hydrogen production over Rh promoted Ni/CeO2-ZrO2 catalyst. Int. J. Hydrog. Energy 2015, 40, 2529–2544. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, J.; Du, Y.; Li, F.; Li, C.; Lu, J.; Wang, J.; Chen, Y. Hydrogen production by catalytic steam reforming of hydrocarbon fuels over Ni/Ce-Al2O3, bifunctional catalysts: Effects of SrO addition. Int. J. Hydrog. Energy 2016, 41, 13436–13447. [Google Scholar] [CrossRef]
- Bizkarra, K.; Barrio, V.L.; Yartu, A.; Requies, J.; Arias, P.L.; Cambra, J.F. Hydrogen production from n-butanol over alumina and modified alumina nickel catalysts. Int. J. Hydrog. Energy 2015, 40, 5272–5280. [Google Scholar] [CrossRef]
- Souza, G.D.; Marcilio, N.R.; Perezlopez, O.W. Dry reforming of methane at moderate temperatures over modified Co-Al Co-precipitated catalysts. Mater. Res. 2014, 17, 1047–1055. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Chong, C.; Chen, L.; Wang, Z.; Zhong, Z.; Campos-Cuerva, C.; Lin, J. Monometallic carbonyl-derived CeO2-supported Rh and Co bicomponent catalysts for CO-Free, high-yield H2 generation from low-temperature ethanol steam reforming. ChemCatChem 2013, 5, 220–234. [Google Scholar] [CrossRef]
- Liguras, D.K.; Kondarides, D.I.; Verykios, X.E. Production of hydrogen for fuel cells by steam reforming of ethanol over supported noble metal catalysts. Appl. Catal. B Environ. 2003, 43, 345–354. [Google Scholar] [CrossRef]
- Cavallaro, S. Ethanol steam reforming on Rh/Al2O3 catalysts. Energy Fuels 2000, 14, 1195–1199. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Chen, S.; Liu, Y. Co-Ni bimetal catalyst supported on perovskite-type oxide for steam reforming of ethanol to produce hydrogen. Int. J. Hydrog. Energy 2014, 39, 5644–5652. [Google Scholar] [CrossRef]
- De Lima, S.M.; da Cruz, I.O.; Jacobs, G.; Davis, B.H.; Mattos, L.V.; Noronha, F.B. Study of catalyst deactivation and reaction mechanism of steam reforming, partial oxidation, and oxidative steam reforming of ethanol over Co/CeO2 catalyst. J. Catal. 2009, 268, 268–281. [Google Scholar] [CrossRef]
- Frusteri, F.; Freni, S.; Spadaro, L.; Chiodo, V.; Bonura, G.; Donato, S.; Cavallaro, S. H2 production for MC fuel cell by steam reforming of ethanol over MgO supported Pd, Rh, Ni and Co catalysts. Catal. Commun. 2004, 5, 611–615. [Google Scholar] [CrossRef]
- Freni, S.; Cavallaro, S.; Mondello, N.; Spadaro, L.; Frusteri, F. Production of hydrogen for MC fuel cell by steam reforming of ethanol over MgO supported Ni and Co catalysts. Catal. Commun. 2003, 4, 259–268. [Google Scholar] [CrossRef]
- Ashok, J.; Kawi, S. Steam reforming of toluene as a biomass tar model compound over CeO2 promoted Ni/CaO-Al2O3 catalytic system. Int. J. Hydrog. Energy 2013, 38, 13938–13949. [Google Scholar] [CrossRef]
- Tao, J.; Zhao, L.Q.; Dong, C.Q.; Qiang, L.; Du, X.Z.; Dahlquist, E. Catalytic steam reforming of toluene as a model biomass gasification tar compound using Ni-CeO2/SBA-15 catalysts. Energies 2013, 6, 3284–3296. [Google Scholar] [CrossRef]
- Guan, G.; Chen, G.; Kasai, Y.; Lim, E.W.C.; Hao, X.; Kaewpanha, M.; Abuliti, A.; Fushimi, C.; Tsutsumi, A. Catalytic steam reforming of biomass tar over iron- or nickel-based catalyst supported on calcined scallop shell. Appl. Catal. B Environ. 2012, 115–116, 159–168. [Google Scholar] [CrossRef]
- Blanco, P.H.; Wu, C.; Onwudili, J.A.; Williams, P.T. Characterization and evaluation of Ni/SiO2 catalysts for hydrogen production and tar reduction from catalytic steam pyrolysis-reforming of refuse-derived fuel. Appl. Catal. B Environ. 2013, 134–135, 238–250. [Google Scholar] [CrossRef]
- Garbarino, G.; Finocchio, E.; Lagazzo, A.; Valsamakis, I.; Riani, P.; Escribano, V.S.; Busca, G. Steam reforming of ethanol-phenol mixture on Ni/Al2O3: Effect of magnesium and boron on catalytic activity in the presence and absence of sulphur. Appl. Catal. B Environ. 2014, 147, 813–826. [Google Scholar] [CrossRef]
- Koike, M.; Ishikawa, C.; Li, D.; Wang, L.; Nakagawa, Y.; Tomishige, K. Catalytic performance of manganese-promoted nickel catalysts for the steam reforming of tar from biomass pyrolysis to synthesis gas. Fuel 2013, 103, 122–129. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Niu, G.; Yang, Z.; Bian, M.; He, A. High temperature thermal stabilization of alumina modified by lanthanum species. Appl. Catal. A Gen. 2001, 205, 159–172. [Google Scholar] [CrossRef]
- Oudet, F.; Courtine, P.; Vejux, A. Thermal stabilization of transition alumina by structural coherence with LnAlO3 (Ln = La, Pr, Nd). J. Catal. 1988, 114, 112–120. [Google Scholar] [CrossRef]
- Borowiecki, T.; Denis, A.; Rawski, M.; Gołębiowski, A.; Stołecki, K.; Dmytrzyk, J.; Kotarba, A. Studies of potassium-promoted nickel catalysts for methane steam reforming: Effect of surface potassium location. Appl. Surf. Sci. 2014, 300, 191–200. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhang, J.C. Hydrogen production on Ni-Pd-Ce/γ-Al2O3 catalyst by partial oxidation and steam reforming of hydrocarbons for potential application in fuel cells. Fuel 2005, 84, 1926–1932. [Google Scholar] [CrossRef]
- Fauteux-Lefebvre, C.; Abatzoglou, N.; Braidy, N.; Achouri, I.E. Diesel steam reforming with a nickel-alumina spinel catalyst for solid oxide fuel cell application. J. Power Sources 2011, 196, 7673–7680. [Google Scholar] [CrossRef]
- Al-Musa, A.A.; Ioakeimidis, Z.S.; Al-Saleh, M.S.; Al-Zahrany, A.; Marnellos, G.E.; Konsolakis, M. Steam reforming of iso-octane toward hydrogen production over mono- and bi-metallic Cu-Co/CeO2 Catalysts: Structure-activity correlations. Int. J. Hydrog. Energy 2014, 39, 19541–19554. [Google Scholar] [CrossRef]
- Ramasamy, K.K.; Gray, M.; Job, H.; Wang, Y. Direct syngas hydrogenation over a Co-Ni bimetallic catalyst: Process parameter optimization. Chem. Eng. Sci. 2015, 135, 266–273. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Sutthisripok, W.; Charojrochkul, S.; Assabumrungrat, S. Development of Ni-Fe bimetallic based catalysts for biomass tar cracking/reforming: Effects of catalyst support and Co-fed reactants on tar conversion characteristics. Fuel Process. Technol. 2014, 127, 26–32. [Google Scholar] [CrossRef]
- Dave, C.D.; Pant, K.K. Renewable hydrogen generation by steam reforming of glycerol over zirconia promoted ceria supported catalyst. Renew Energy 2011, 36, 3195–3202. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; Carrero, A.; Calles, J.A. Hydrogen production by ethanol steam reforming over Cu-Ni supported catalysts. Int. J. Hydrog. Energy 2007, 32, 1450–1461. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, J.; Du, Y.; Sun, D.; Wang, J.; Chen, Y.; Lu, J. Steam reforming of hydrocarbon fuels over M (Fe, Co, Ni, Cu, Zn)-Ce bimetal catalysts supported on Al2O3. Int. J. Hydrog. Energy 2016, 41, 10473–10482. [Google Scholar] [CrossRef]
- Goula, M.A.; Charisiou, N.D.; Papageridis, K.N.; Delimitis, A.; Pachatouridou, E.; Iliopoulou, E.F. Nickel on alumina catalysts for the production of hydrogen rich mixtures via the biogas dry reforming reaction: Influence of the synthesis method. Int. J. Hydrog. Energy 2015, 40, 9183–9200. [Google Scholar] [CrossRef]
- Bereketidou, O.A.; Goula, M.A. Biogas reforming for syngas production over nickel supported on ceria-alumina catalysts. Catal. Today 2012, 195, 93–100. [Google Scholar] [CrossRef]
- Papageridis, K.N.; Siakavelas, G.; Charisiou, N.D.; Avraam, D.G.; Tzounis, L.; Kousi, K.; Goula, M.A. Comparative study of Ni, Co, Cu supported on γ-alumina catalysts for hydrogen production via the glycerol steam reforming reaction. Fuel Process. Technol. 2016, 152, 156–175. [Google Scholar] [CrossRef]
- Jiao, Y.; He, Z.; Wang, J.; Chen, Y. n-decane steam reforming for hydrogen production over mono- and bi-metallic Co-Ni/Ce-Al2O3 catalysts: Structure-activity correlations. Energy Convers. Manag. 2017, 148, 954–962. [Google Scholar] [CrossRef]
- Cassinelli, W.H.; Feio, L.S.F.; Araújo, J.C.S.; Hori, C.E.; Noronha, F.B.; Marques, C.M.P.; Bueno, J.M.C. Effect of CeO2 and La2O3 on the activity of CeO2-La2O3/ Al2O3-supported Pd catalysts for steam reforming of methane. Catal. Lett. 2008, 120, 86–94. [Google Scholar] [CrossRef]
- Peña, J.A.; Herguido, J.; Guimon, C.; Monzón, A.; Santamarí, J. Hydrogenation of acetylene over Ni/NiAl2O4 catalyst: Characterization, coking, and reaction studies. J. Catal. 1996, 159, 313–322. [Google Scholar] [CrossRef]
- Boukha, Z.; Jiménez-González, C.; Rivas, B.D.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Synthesis, characterisation and performance evaluation of spinel-derived Ni/Al2O3 catalysts for various methane reforming reactions. Appl. Catal. B Environ. 2014, 158–159, 190–201. [Google Scholar] [CrossRef]
- Nandini, A.; Pant, K.K.; Dhingra, S.C. CeO2 and Mn-Promoted Ni/Al2O3 Catalysts for Stable CO2 Reforming of Methane. Appl. Catal. A Gen. 2005, 290, 166–174. [Google Scholar] [CrossRef]
- Xu, G.; Shi, K.; Gao, Y.; Xu, H.; Wei, Y. Studies of Reforming Natural Gas with Carbon Dioxide to Produce Synthesis Gas: The Role of CeO2 and MgO Promoters. J. Mol. Catal. A Chem. 1999, 147, 47–54. [Google Scholar] [CrossRef]
- Siew, K.W.; Lee, H.C.; Gimbun, J.; Cheng, C.K. Production of CO-Rich Hydrogen Gas from Glycerol Gry Reforming over La-promoted Ni/Al2O3 Catalyst. Int. J. Hydrog. Energy 2014, 39, 6927–6936. [Google Scholar] [CrossRef]
- Zeng, S.H.; Zhang, L.; Zhang, X.H.; Wang, Y.; Pan, H.; Su, H.Q. Modification Effect of Natural Mixed Rare Earths on Co/g-Al2O3 catalysts for CH4/CO2 Reforming to Synthesis Gas. Int. J. Hydrog. Energy 2012, 37, 9994–10001. [Google Scholar] [CrossRef]
- Djaidja, A.; Libs, S.; Kiennemann, A.; Barama, A. Characterization and Activity in Dry Reforming of Methane on NiMg/Al and Ni/MgO Catalysts. Catal. Today 2006, 113, 194–200. [Google Scholar] [CrossRef]
- Resini, C.; Delgado, M.C.H.; Presto, S.; Alemany, L.J.; Riani, P.; Marazza, R.; Ramis, G.; Busca, G. Yttria-stabilized zirconia (YSZ) supported Ni-Co alloys (precursor of SOFC anodes) as catalysts for the steam reforming of ethanol. Int. J. Hydrog. Energy 2008, 33, 3728–3735. [Google Scholar] [CrossRef]
- Fenelonov, V.B.; Derevyankin, A.Y.; Okkel, L.G.; Avdeeva, L.B.; Zaikovskii, V.I.; Moroz, E.M.; Salanov, A.N.; Rudina, N.A.; Likholobov, V.A.; Shaikhutdinov, S.K. Structure and texture of filamentous carbons produced by methane decomposition on Ni and Ni-Cu catalysts. Carbon 1997, 35, 1129–1140. [Google Scholar] [CrossRef]









| Catalysts | Textural Properties | ||
|---|---|---|---|
| Surface Area *(m2/g) | Pore Volume (mL/g) | Mean Pore Diameter (nm) | |
| CA | 155.9 (69.3) * | 0.49 | 5.42 |
| NCA | 150.2 (83.6) | 0.47 | 5.35 |
| C3-NCA | 149.3 (89.1) | 0.47 | 5.32 |
| C6-NCA | 147.6 (88.6) | 0.47 | 5.34 |
| C9-NCA | 143.9 (92.3) | 0.45 | 5.28 |
| C12-NCA | 140.5 (96.7) | 0.44 | 5.26 |
| C15-NCA | 136.9 (92.4) | 0.44 | 5.26 |
| Catalysts | Carbon Deposition | |
|---|---|---|
| [mg/(gCat·h)] | [mg/gCFeed] | |
| CA | 258.9 | 11.6 |
| NCA | 194.3 | 8.55 |
| C3-NCA | 178.8 | 7.87 |
| C6-NCA | 143.2 | 6.30 |
| C9-NCA | 114.1 | 5.02 |
| C12-NCA | 88.6 | 3.90 |
| C15-NCA | 75.0 | 3.30 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Q.; Jiao, Y.; Wang, W.; Du, Y.; Li, C.; Yang, J.; Lu, J. Catalytic Performance and Characterization of Ni-Co Bi-Metallic Catalysts in n-Decane Steam Reforming: Effects of Co Addition. Catalysts 2018, 8, 518. https://doi.org/10.3390/catal8110518
Yu Q, Jiao Y, Wang W, Du Y, Li C, Yang J, Lu J. Catalytic Performance and Characterization of Ni-Co Bi-Metallic Catalysts in n-Decane Steam Reforming: Effects of Co Addition. Catalysts. 2018; 8(11):518. https://doi.org/10.3390/catal8110518
Chicago/Turabian StyleYu, Qinwei, Yi Jiao, Weiqiang Wang, Yongmei Du, Chunying Li, Jianming Yang, and Jian Lu. 2018. "Catalytic Performance and Characterization of Ni-Co Bi-Metallic Catalysts in n-Decane Steam Reforming: Effects of Co Addition" Catalysts 8, no. 11: 518. https://doi.org/10.3390/catal8110518