Low Pt Alloyed Nanostructures for Fuel Cells Catalysts
Abstract
:1. Introduction
2. Design and Synthesis for Pt Alloyed Nanostructures
2.1. Design of Low Pt Alloyed Nanocatalyst with High Performance
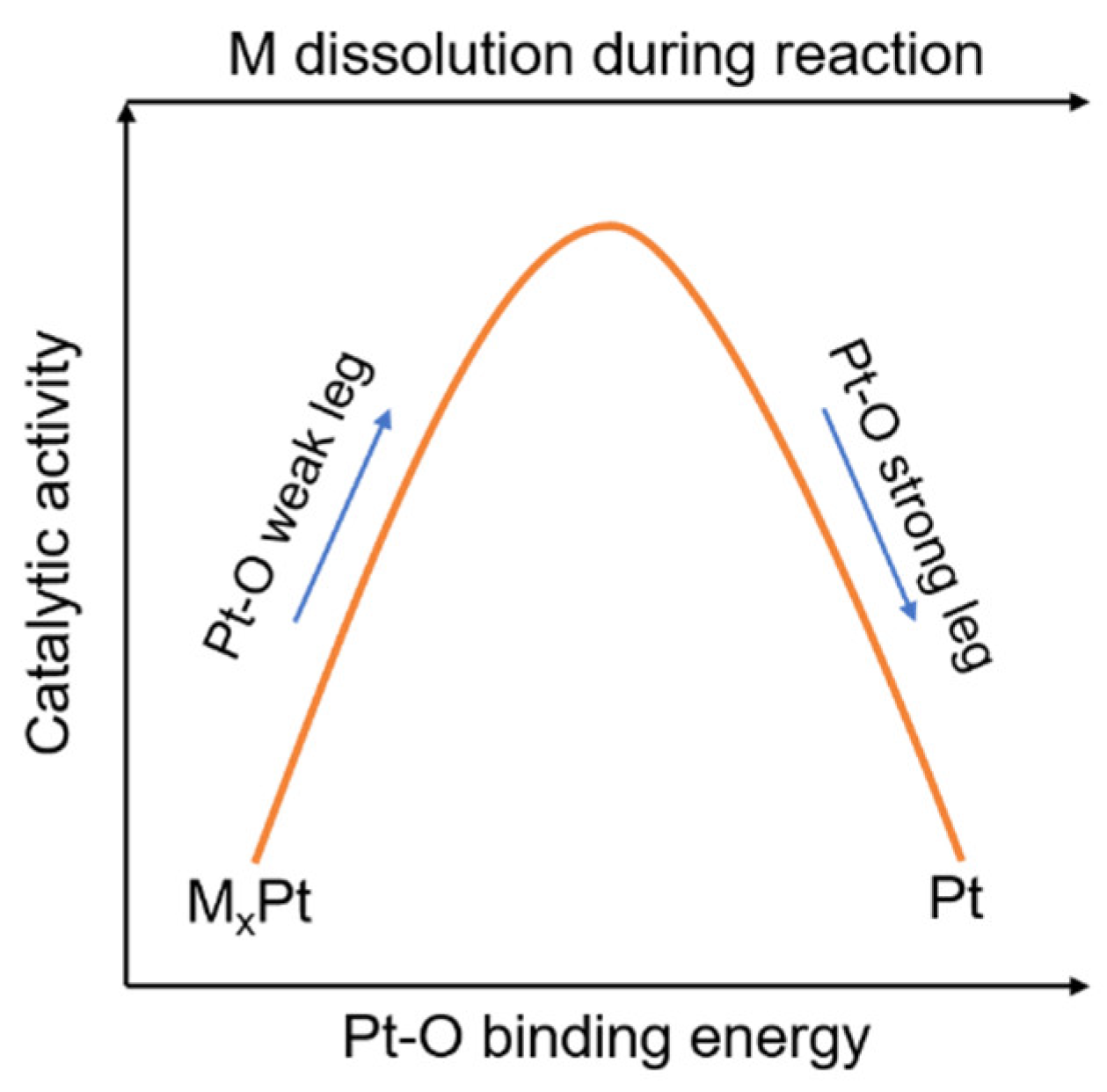
- the kernel of design is to balance the competition between activity and durability;
- moderate transition metal M content is crucial such that the resulting Pt–O binding strength falls over the region in volcano plot with both high activity and durability; and,
- structural design that brings about composition segregation as well as selective facets exposure with high fractions of electrochemically favored and/or high-index facets provides additional activity boost into pre-balanced activity and durability.
2.2. Structure Design
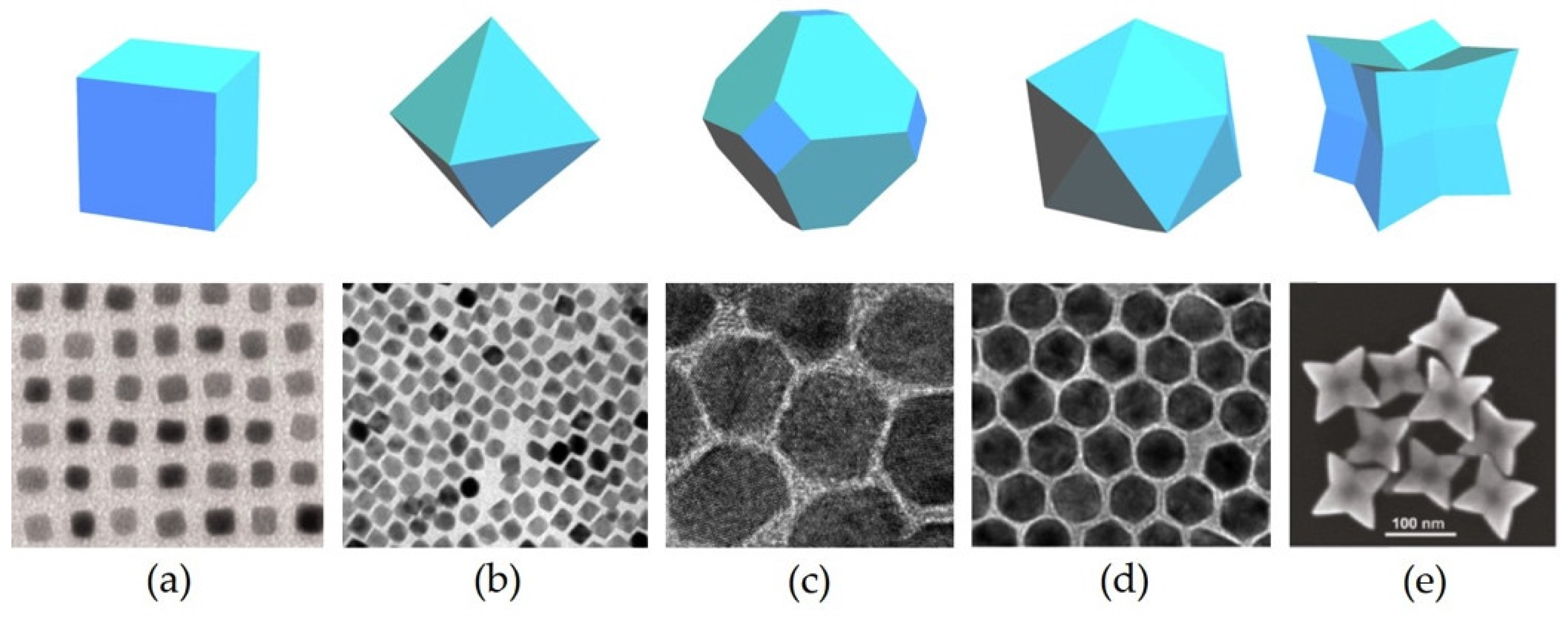
2.2.1. Alloy Polyhedra
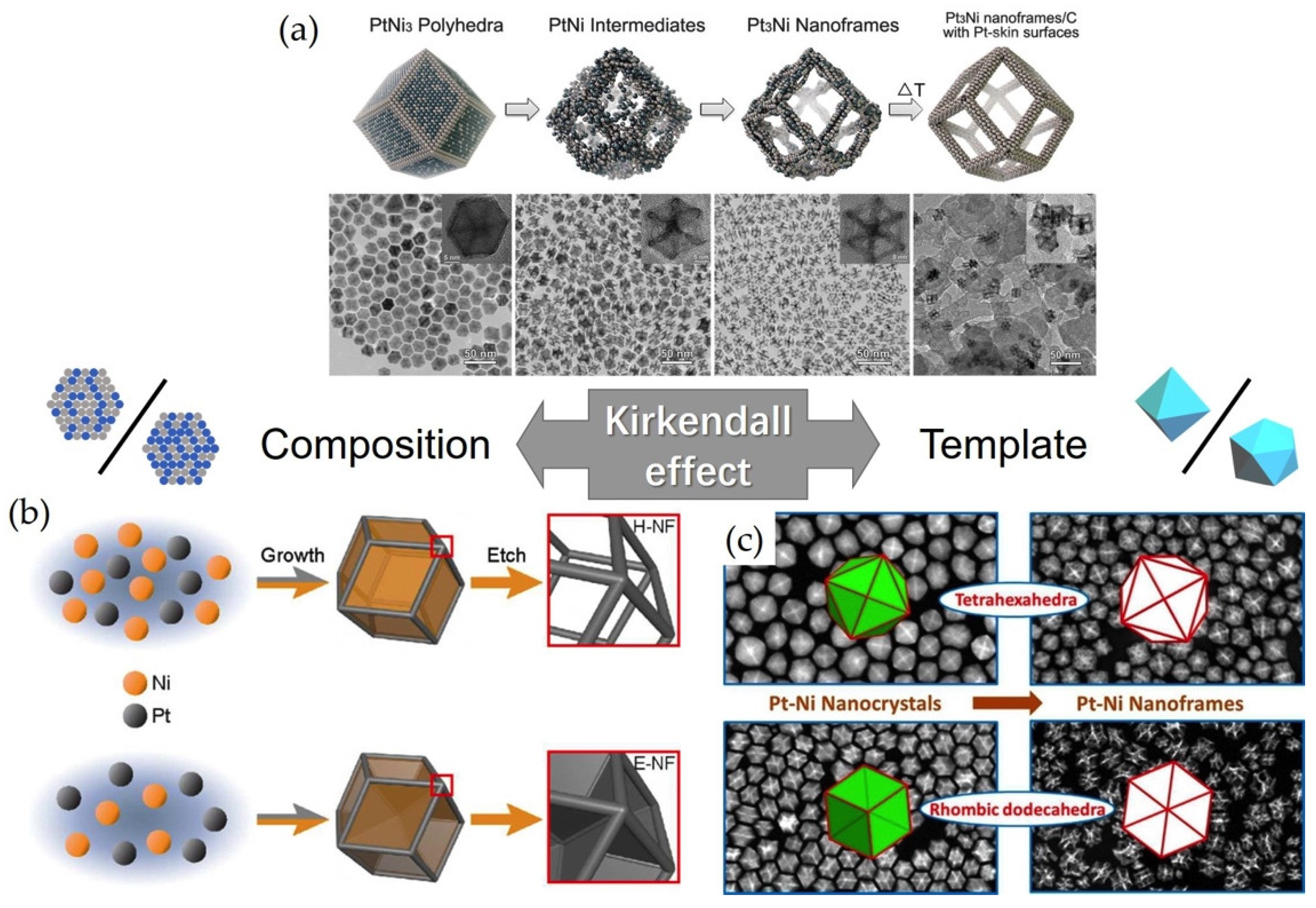
2.2.2. Hollow and Frame Structures
2.2.3. Pt-Skin
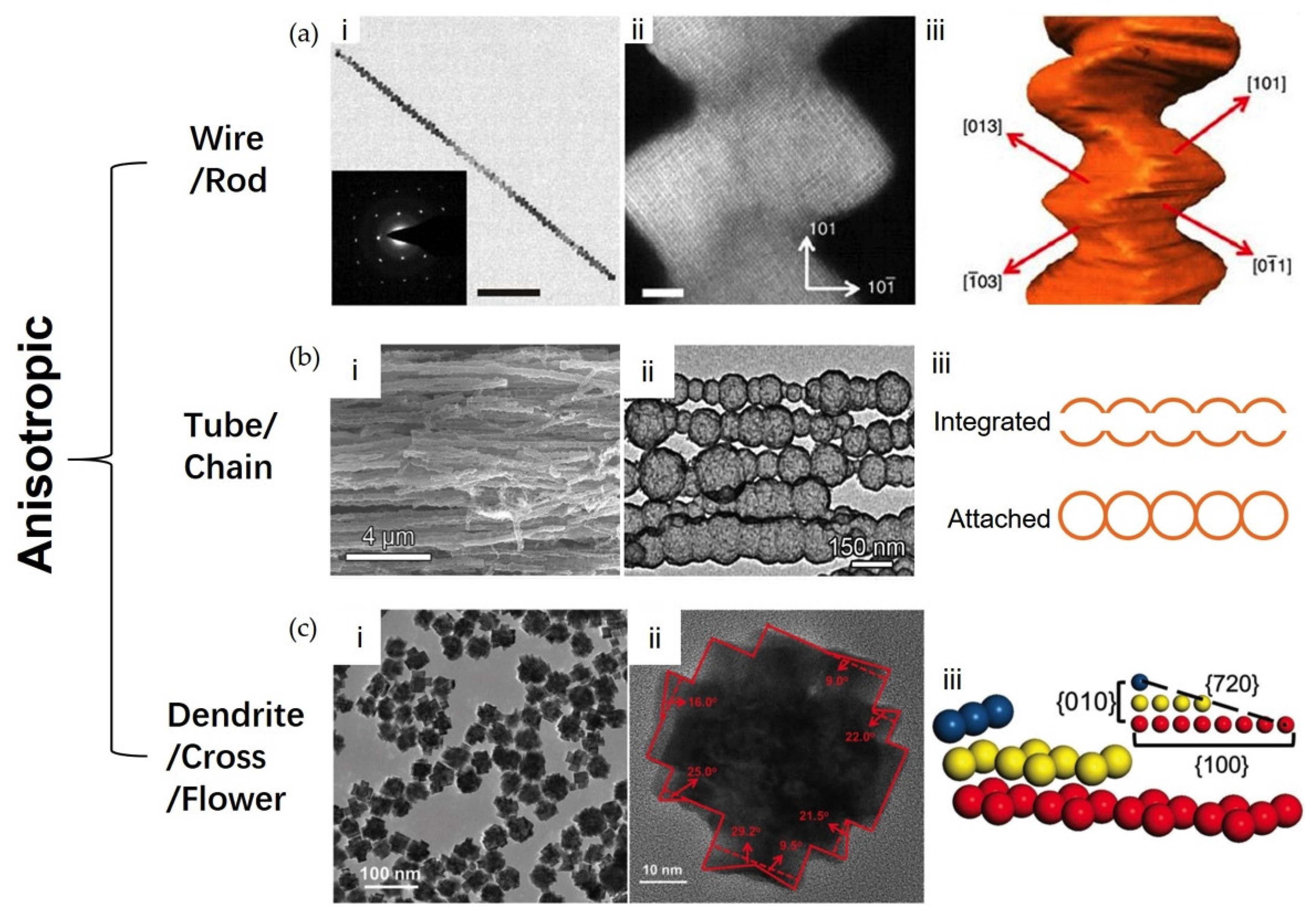
2.2.4. Anisotropies
2.3. Composition Control
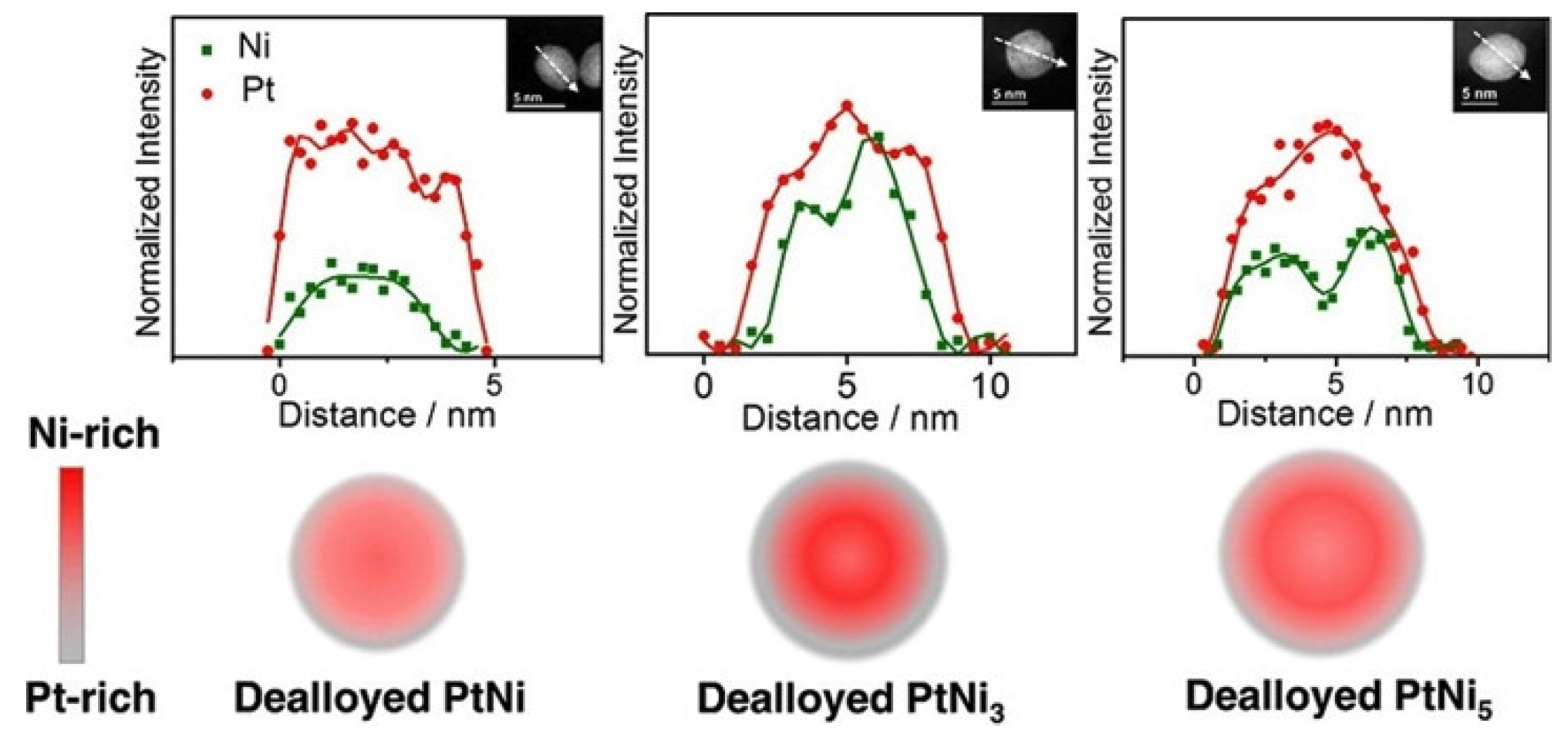
3. Microstructure Characterization
3.1. Electron Probing
3.2. X-Ray And Infrared Probing
4. Progress in Electrocatalytic Performances
4.1. ORR
4.2. MOR
4.3. Bifunctional Catalysts
5. Summaries and Perspectives
Funding
Conflicts of Interest
References
- Sulaiman, N.; Hannan, M.A.; Mohamed, A.; Majlan, E.H.; Daud, W.R.W. A review on energy management system for fuel cell hybrid electric vehicle: Issues and challenges. Renew. Sustain. Energy Rev. 2015, 52, 802–814. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.C.; Koh, S.; Yu, C.F.; Strasser, P. Synthesis, dealloying, and orr electrocatalysis of pdda-stabilized cu-rich pt alloy nanoparticles. J. Electrochem. Soc. 2007, 154, B1192–B1199. [Google Scholar] [CrossRef]
- Wang, C.; Chi, M.; Li, D.; van der Vliet, D.; Wang, G.; Lin, Q.; Mitchell, J.F.; More, K.L.; Markovic, N.M.; Stamenkovic, V.R. Synthesis of homogeneous pt-bimetallic nanoparticles as highly efficient electrocatalysts. ACS Catal. 2011, 1, 1355–1359. [Google Scholar] [CrossRef]
- Greeley, J.; Stephens, I.E.L.; Bondarenko, A.S.; Johansson, T.P.; Hansen, H.A.; Jaramillo, T.F.; Rossmeisl, J.; Chorkendorff, I.; Nørskov, J.K. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 2009, 1, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Alonso-Vante, N.; Lamy, C.; Akins, D.L. High methanol tolerance of carbon-supported pt-cr alloy nanoparticle electrocatalysts for oxygen reduction. J. Electrochem. Soc. 2005, 152, A704–A709. [Google Scholar] [CrossRef]
- Kang, Y.; Murray, C.B. Synthesis and electrocatalytic properties of cubic mn−pt nanocrystals (nanocubes). J. Am. Chem. Soc. 2010, 132, 7568–7569. [Google Scholar] [CrossRef] [PubMed]
- Miura, A.; Wang, H.; Leonard, B.M.; Abruña, H.D.; DiSalvo, F.J. Synthesis of intermetallic ptzn nanoparticles by reaction of pt nanoparticles with zn vapor and their application as fuel cell catalysts. Chem. Mater. 2009, 21, 2661–2667. [Google Scholar] [CrossRef]
- Strasser, P.; Koh, S.; Anniyev, T.; Greeley, J.; More, K.; Yu, C.; Liu, Z.; Kaya, S.; Nordlund, D.; Ogasawara, H.; et al. Lattice-strain control of the activity in dealloyed core–shell fuel cell catalysts. Nat. Chem. 2010, 2, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, S.; Jia, Q.; Bates, M.K.; Li, J.; Xu, C.; Gath, K.; Yang, J.; Waldecker, J.; Che, H.; Liang, W.; et al. Tuning nb–pt interactions to facilitate fuel cell electrocatalysis. ACS Catal. 2017, 7, 4936–4946. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.Z.; Fang, J.Y.; Zou, S.Z. Synthesis and oxygen reduction activity of shape-controlled pt3ni nanopolyhedra. Nano Lett. 2010, 10, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.J.; Tian, N.; Zhou, Z.Y.; Huang, R.; Liu, Z.L.; Xiao, J.; Sun, S.G. Alloy tetrahexahedral pd-pt catalysts: Enhancing significantly the catalytic activity by synergy effect of high-index facets and electronic structure. Chem. Sci. 2012, 3, 1157–1161. [Google Scholar] [CrossRef]
- Wang, X.; Figueroa-Cosme, L.; Yang, X.; Luo, M.; Liu, J.Y.; Xie, Z.X.; Xia, Y.N. Pt-based icosahedral nanocages: Using a combination of {111} facets, twin defects, and ultrathin walls to greatly enhance their activity toward oxygen reduction. Nano Lett. 2016, 16, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.Y.; Jiang, Y.Q.; Zhang, J.W.; Zhang, L.; Chen, Q.L.; Xie, Z.X.; Zheng, L.S. Unique excavated rhombic dodecahedral ptcu3 alloy nanocrystals constructed with ultrathin nanosheets of high-energy {110} facets. J. Am. Chem. Soc. 2014, 136, 3748–3751. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Q.; Zhao, Z.P.; Fan, J.M.; Tan, Y.M.; Zheng, N.F. Amine-assisted synthesis of concave polyhedral platinum nanocrystals having {411} high-index facets. J. Am. Chem. Soc. 2011, 133, 4718–4721. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Ren, Z.; Wang, R.M.; Wang, N.; Cao, X. Platinum catalyzed growth of nipt hollow spheres with an ultrathin shell. J. Mater. Chem. 2011, 21, 1925–1930. [Google Scholar] [CrossRef]
- Shan, A.X.; Chen, Z.C.; Li, B.Q.; Chen, C.P.; Wang, R.M. Monodispersed, ultrathin nipt hollow nanospheres with tunable diameter and composition via a green chemical synthesis. J. Mater. Chem. A 2015, 3, 1031–1036. [Google Scholar] [CrossRef]
- Shan, A.X.; Cheng, M.; Fan, H.S.; Chen, Z.C.; Wang, R.M.; Chen, C.P. Nipt hollow nanocatalyst: Green synthesis, size control and electrocatalysis. Prog. Nat. Sci. 2014, 24, 175–178. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, W.; Wang, R.M. Double-layered nipt nanobowls with ultrathin shell synthesized in water at room temperature. Crystengcomm 2012, 14, 5151–5154. [Google Scholar] [CrossRef]
- Fan, H.; Cheng, M.; Wang, Z.; Wang, R. Layer-controlled pt-ni porous nanobowls with enhanced electrocatalytic performance. Nano Res. 2016, 10, 187–198. [Google Scholar] [CrossRef]
- Liu, J.L.; Xia, T.Y.; Wang, S.G.; Yang, G.; Dong, B.W.; Wang, C.; Ma, Q.D.; Sun, Y.; Wang, R.M. Oriented-assembly of hollow fept nanochains with tunable catalytic and magnetic properties. Nanoscale 2016, 8, 11432–11440. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, S.G.; Wang, R.M. Well-aligned copt hollow nanochains synthesized in water at room temperature. J. Phys. Chem. C 2012, 116, 5352–5357. [Google Scholar] [CrossRef]
- Bu, L.Z.; Ding, J.B.; Guo, S.J.; Zhang, X.; Su, D.; Zhu, X.; Yao, J.L.; Guo, J.; Lu, G.; Huang, X.Q. A general method for multimetallic platinum alloy nanowires as highly active and stable oxygen reduction catalysts. Adv. Mater. 2015, 27, 7204–7212. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Chen, Q.; Zhang, J.; Li, H.; Jiang, Y.; Shen, S.; Fu, G.; Lu, B.A.; Xie, Z.; Zheng, L. Platinum-nickel alloy excavated nano-multipods with hexagonal close-packed structure and superior activity towards hydrogen evolution reaction. Nat. Commun. 2017, 8, 15131. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Li, H.; Zhan, C.; Zhang, J.; Wang, W.; Xu, B.; Lu, F.; Jiang, Y.; Xie, Z.; Zheng, L. Monocrystalline platinum-nickel branched nanocages with enhanced catalytic performance towards the hydrogen evolution reaction. Nanoscale 2018, 10, 5072–5077. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Guo, S.; Zhang, X.; Shen, X.; Su, D.; Lu, G.; Zhu, X.; Yao, J.; Guo, J.; Huang, X. Surface engineering of hierarchical platinum-cobalt nanowires for efficient electrocatalysis. Nat. Commun. 2016, 7, 11850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, G.R.; Bai, J.; Jiang, J.X.; Lee, J.M.; Chen, Y. Polyethyleneimine functionalized platinum superstructures: Enhancing hydrogen evolution performance by morphological and interfacial control. Chem. Sci. 2017, 8, 8411–8418. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, K.D.; Ruditskiy, A.; Peng, H.-C.; Qin, D.; Xia, Y. Bimetallic nanocrystals: Syntheses, properties, and applications. Chem. Rev. 2016, 116, 10414–10472. [Google Scholar] [CrossRef] [PubMed]
- Sui, S.; Wang, X.Y.; Zhou, X.T.; Su, Y.H.; Riffatc, S.; Liu, C.J. A comprehensive review of pt electrocatalysts for the oxygen reduction reaction: Nanostructure, activity, mechanism and carbon support in pem fuel cells. J. Mater. Chem. A 2017, 5, 1808–1825. [Google Scholar] [CrossRef]
- Kaito, T.; Tanaka, H.; Mitsumoto, H.; Sugawara, S.; Shinohara, K.; Ariga, H.; Uehara, H.; Takakusagi, S.; Asakura, K. In situ x-ray absorption fine structure analysis of ptco, ptcu, and ptni alloy electrocatalysts: The correlation of enhanced oxygen reduction reaction activity and structure. J. Phys. Chem. C 2016, 120, 11519–11527. [Google Scholar] [CrossRef]
- Ogihara, Y.; Yano, H.; Matsumoto, T.; Tryk, D.; Iiyama, A.; Uchida, H. In situ ftir analysis of co-tolerance of a pt-fe alloy with stabilized pt skin layers as a hydrogen anode catalyst for polymer electrolyte fuel cells. Catalysts 2016, 7, 8. [Google Scholar] [CrossRef]
- Zhao, X.; Takao, S.; Higashi, K.; Kaneko, T.; Samjeskè, G.; Sekizawa, O.; Sakata, T.; Yoshida, Y.; Uruga, T.; Iwasawa, Y. Simultaneous improvements in performance and durability of an octahedral ptnix/c electrocatalyst for next-generation fuel cells by continuous, compressive, and concave pt skin layers. ACS Catal. 2017, 7, 4642–4654. [Google Scholar] [CrossRef]
- Beermann, V.; Gocyla, M.; Kühl, S.; Padgett, E.; Schmies, H.; Goerlin, M.; Erini, N.; Shviro, M.; Heggen, M.; Dunin-Borkowski, R.E.; et al. Tuning the electrocatalytic oxygen reduction reaction activity and stability of shape-controlled pt–ni nanoparticles by thermal annealing—Elucidating the surface atomic structural and compositional changes. J. Am. Chem. Soc. 2017, 139, 16536–16547. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Carlton, C.E.; Suntivich, J.; Xu, Z.; Shao-Horn, Y. Oxygen reduction activity and stability trends of bimetallic pt0.5m0.5 nanoparticle in acid. J. Phys. Chem. C 2015, 119, 3971–3978. [Google Scholar] [CrossRef]
- Jia, Q.; Li, J.; Caldwell, K.; Ramaker, D.E.; Ziegelbauer, J.M.; Kukreja, R.S.; Kongkanand, A.; Mukerjee, S. Circumventing metal dissolution induced degradation of pt-alloy catalysts in proton exchange membrane fuel cells: Revealing the asymmetric volcano nature of redox catalysis. ACS Catal. 2016, 6, 928–938. [Google Scholar] [CrossRef]
- Jia, Q.; Liang, W.; Bates, M.K.; Mani, P.; Lee, W.; Mukerjee, S. Activity descriptor identification for oxygen reduction on platinum-based bimetallic nanoparticles: In situ observation of the linear composition–strain–activity relationship. ACS Nano 2015, 9, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Naohara, H.; Cai, Y.; Choi, Y.M.; Liu, P.; Vukmirovic, M.B.; Wang, J.X.; Adzic, R.R. Core-protected platinum monolayer shell high-stability electrocatalysts for fuel-cell cathodes. Angew. Chem.-Int. Ed. 2010, 49, 8602–8607. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.B.; Yang, H. Platinum-based oxygen reduction electrocatalysts. Acc. Chem. Res. 2013, 46, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Chen, S.; Sheng, W.; Yabuuchi, N.; Kim, Y.-T.; Mitani, T.; Vescovo, E.; Shao-Horn, Y. Roles of surface steps on pt nanoparticles in electro-oxidation of carbon monoxide and methanol. J. Am. Chem. Soc. 2009, 131, 15669–15677. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Kim, D.Y.; Zhang, H.; Xia, Y. Platinum concave nanocubes with high-index facets and their enhanced activity for oxygen reduction reaction. Angew. Chem. Int. Ed. 2011, 50, 2773–2777. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Long, W.; Wang, L.; Yuan, R.; Ignaszak, A.; Fang, B.; Wilkinson, D.P. Unlocking the door to highly active orr catalysts for pemfc applications: Polyhedron-engineered pt-based nanocrystals. Energy Environ. Sci. 2018, 11, 258–275. [Google Scholar] [CrossRef]
- Sun, X.; Jiang, K.; Zhang, N.; Guo, S.; Huang, X. Crystalline control of {111} bounded pt3cu nanocrystals: Multiply-twinned pt3cu icosahedra with enhanced electrocatalytic properties. ACS Nano 2015, 9, 7634–7640. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, J.; Peng, Z.; Yang, S.; Wagner, F.T.; Yang, H. Truncated octahedral pt3ni oxygen reduction reaction electrocatalysts. J. Am. Chem. Soc. 2010, 132, 4984–4985. [Google Scholar] [CrossRef] [PubMed]
- Mourdikoudis, S.; Liz-Marzán, L.M. Oleylamine in nanoparticle synthesis. Chem. Mater. 2013, 25, 1465–1476. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, J. A general strategy for preparation of pt 3d-transition metal (co, fe, ni) nanocubes. J. Am. Chem. Soc. 2009, 131, 18543–18547. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.I.; Xie, S.; Shao, M.; Odell, J.H.; Lu, N.; Peng, H.C.; Protsailo, L.; Guerrero, S.; Park, J.; Xia, X.; et al. Synthesis and characterization of 9 nm pt-ni octahedra with a record high activity of 3.3 a/mg(pt) for the oxygen reduction reaction. Nano Lett. 2013, 13, 3420–3425. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Gan, L.; Li, H.H.; Yu, S.H.; Heggen, M.; Strasser, P. Octahedral ptni nanoparticle catalysts: Exceptional oxygen reduction activity by tuning the alloy particle surface composition. Nano Lett. 2012, 12, 5885–5889. [Google Scholar] [CrossRef] [PubMed]
- Kakade, B.A.; Tamaki, T.; Ohashi, H.; Yamaguchi, T. Highly active bimetallic pdpt and copt nanocrystals for methanol electro-oxidation. J. Phys. Chem. C 2012, 116, 7464–7470. [Google Scholar] [CrossRef]
- Kang, Y.; Pyo, J.B.; Ye, X.; Gordon, T.R.; Murray, C.B. Synthesis, shape control, and methanol electro-oxidation properties of pt–zn alloy and pt3zn intermetallic nanocrystals. ACS Nano 2012, 6, 5642–5647. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Liu, J.; Wang, S.; Wang, C.; Sun, Y.; Gu, L.; Wang, R. Enhanced catalytic activities of nipt truncated octahedral nanoparticles toward ethylene glycol oxidation and oxygen reduction in alkaline electrolyte. ACS Appl. Mater. Interfaces 2016, 8, 10841–10849. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Qi, L.; You, H.; Gross, A.; Li, J.; Yang, H. Icosahedral platinum alloy nanocrystals with enhanced electrocatalytic activities. J. Am. Chem. Soc. 2012, 134, 11880–11883. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Cao, Z.; Du, G.; Kuang, Q.; Huang, J.; Xie, Z.; Zheng, L. Excavated octahedral pt-co alloy nanocrystals built with ultrathin nanosheets as superior multifunctional electrocatalysts for energy conversion applications. Nano Energy 2017, 39, 582–589. [Google Scholar] [CrossRef]
- Xu, H.; Song, P.; Wang, J.; Du, Y. Shape-controlled synthesis of platinum–copper nanocrystals for efficient liquid fuel electrocatalysis. Langmuir 2018, 34, 7981–7988. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.Y.; Wu, H.B.; Wang, X.; Lou, X.W. One-pot synthesis of cubic ptcu3 nanocages with enhanced electrocatalytic activity for the methanol oxidation reaction. J. Am. Chem. Soc. 2012, 134, 13934–13937. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Kang, Y.; Huo, Z.; Zhu, Z.; Huang, W.; Xin, H.L.; Snyder, J.D.; Li, D.; Herron, J.A.; Mavrikakis, M.; et al. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science 2014, 343, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Becknell, N.; Son, Y.; Kim, D.; Li, D.; Yu, Y.; Niu, Z.; Lei, T.; Sneed, B.T.; More, K.L.; Markovic, N.M.; et al. Control of architecture in rhombic dodecahedral pt–ni nanoframe electrocatalysts. J. Am. Chem. Soc. 2017, 139, 11678–11681. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Bu, L.; Guo, S.; Zhao, Z.; Zhu, E.; Huang, Y.; Huang, X. Morphology and phase controlled construction of pt-ni nanostructures for efficient electrocatalysis. Nano Lett. 2016, 16, 2762–2767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, X.; Jiang, G.; Zhu, H.; Guo, S.; Su, D.; Lu, G.; Sun, S. Tuning nanoparticle structure and surface strain for catalysis optimization. J. Am. Chem. Soc. 2014, 136, 7734–7739. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Carlton, C.E.; Kongkanand, A.; Kukreja, R.S.; Theobald, B.R.; Gan, L.; O’Malley, R.; Strasser, P.; Wagner, F.T.; Shao-Horn, Y. Record activity and stability of dealloyed bimetallic catalysts for proton exchange membrane fuel cells. Energy Environ. Sci. 2015, 8, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Hao, Y.; Su, D.; Doan-Nguyen, V.V.T.; Wu, Y.; Li, J.; Sun, S.; Murray, C.B. Monodisperse core/shell ni/fept nanoparticles and their conversion to ni/pt to catalyze oxygen reduction. J. Am. Chem. Soc. 2014, 136, 15921–15924. [Google Scholar] [CrossRef] [PubMed]
- Shan, A.; Chen, C.; Zhang, W.; Cheng, D.; Shen, X.; Yu, R.; Wang, R. Giant enhancement and anomalous temperature dependence of magnetism in monodispersed nipt2 nanoparticles. Nano Res. 2017, 10, 3238–3247. [Google Scholar] [CrossRef]
- Ghosh, T.; Vukmirovic, M.B.; DiSalvo, F.J.; Adzic, R.R. Intermetallics as novel supports for pt monolayer o2 reduction electrocatalysts: Potential for significantly improving properties. J. Am. Chem. Soc. 2010, 132, 906–907. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.F.; Choi, S.I.; Lu, N.; Roling, L.T.; Herron, J.A.; Zhang, L.; Park, J.; Wang, J.G.; Kim, M.J.; Xie, Z.X.; et al. Atomic layer-by-layer deposition of pt on pd nanocubes for catalysts with enhanced activity and durability toward oxygen reduction. Nano Lett. 2014, 14, 3570–3576. [Google Scholar] [CrossRef] [PubMed]
- Oezaslan, M.; Hasché, F.; Strasser, P. Pt-based core–shell catalyst architectures for oxygen fuel cell electrodes. J. Phys. Chem. Lett. 2013, 4, 3273–3291. [Google Scholar] [CrossRef]
- Fu, S.F.; Zhu, C.Z.; Song, J.H.; Engelhard, M.H.; He, Y.; Du, D.; Wang, C.M.; Lin, Y.H. Three-dimensional ptni hollow nanochains as an enhanced electrocatalyst for the oxygen reduction reaction. J. Mater. Chem. A 2016, 4, 8755–8761. [Google Scholar] [CrossRef]
- Serrà, A.; Gómez, E.; Vallés, E. Facile electrochemical synthesis, using microemulsions with ionic liquid, of highly mesoporous copt nanorods with enhanced electrocatalytic performance for clean energy. Int. J. Hydrogen Energy 2015, 40, 8062–8070. [Google Scholar] [CrossRef]
- Guo, L.; Huang, L.B.; Jiang, W.J.; Wei, Z.D.; Wan, L.J.; Hu, J.S. Tuning the branches and composition of ptcu nanodendrites through underpotential deposition of cu towards advanced electrocatalytic activity. J. Mater. Chem. A 2017, 5, 9014–9021. [Google Scholar] [CrossRef]
- Li, H.-H.; Fu, Q.-Q.; Xu, L.; Ma, S.-Y.; Zheng, Y.-R.; Liu, X.-J.; Yu, S.-H. Highly crystalline ptcu nanotubes with three dimensional molecular accessible and restructured surface for efficient catalysis. Energy Environ. Sci. 2017, 10, 1751–1756. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, P.; Feng, G.; Xia, D.; Zhang, J. Crossed ptcocu alloy nanocrystals with high-index facets as highly active catalyst for methanol oxidation reaction. Adv. Mater. Interfaces 2018, 5, 1800297. [Google Scholar] [CrossRef]
- Zhang, P.; Dai, X.; Zhang, X.; Chen, Z.; Yang, Y.; Sun, H.; Wang, X.; Wang, H.; Wang, M.; Su, H.; et al. One-pot synthesis of ternary pt–ni–cu nanocrystals with high catalytic performance. Chem. Mater. 2015, 27, 6402–6410. [Google Scholar] [CrossRef]
- Moseley, P.; Curtin, W.A. Computational design of strain in core–shell nanoparticles for optimizing catalytic activity. Nano Lett. 2015, 15, 4089–4095. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Zhang, N.; Guo, S.; Zhang, X.; Li, J.; Yao, J.; Wu, T.; Lu, G.; Ma, J.Y.; Su, D.; et al. Biaxially strained ptpb/pt core/shell nanoplate boosts oxygen reduction catalysis. Science 2016, 354, 1410–1414. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Heggen, M.; Rudi, S.; Strasser, P. Core-shell compositional fine structures of dealloyed pt(x)ni(1-x) nanoparticles and their impact on oxygen reduction catalysis. Nano Lett. 2012, 12, 5423–5430. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Caldwell, K.; Strickland, K.; Ziegelbauer, J.M.; Liu, Z.; Yu, Z.; Ramaker, D.E.; Mukerjee, S. Improved oxygen reduction activity and durability of dealloyed ptco x catalysts for proton exchange membrane fuel cells: Strain, ligand, and particle size effects. ACS Catal. 2015, 5, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Mayrhofer, K.J.J.; Juhart, V.; Hartl, K.; Hanzlik, M.; Arenz, M. Adsorbate-induced surface segregation for core–shell nanocatalysts. Angew. Chem. Int. Ed. 2009, 48, 3529–3531. [Google Scholar] [CrossRef] [PubMed]
- Prabhudev, S.; Bugnet, M.; Zhu, G.Z.; Bock, C.; Botton, G.A. Surface segregation of fe in pt-fe alloy nanoparticles: Its precedence and effect on the ordered-phase evolution during thermal annealing. ChemCatChem 2015, 7, 3655–3664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-C.; Tian, X.-C.; Zhang, B.-W.; Huang, L.; Zhu, F.-C.; Qu, X.-M.; Liu, L.; Liu, S.; Jiang, Y.-X.; Sun, S.-G. Engineering phase and surface composition of pt 3 co nanocatalysts: A strategy for enhancing co tolerance. Nano Energy 2017, 34, 224–232. [Google Scholar] [CrossRef]
- Wang, R.; Tao, J.; Du, K.; Wang, Y.; Ge, B.; Li, F.; Liu, W.; Wu, L.; Liu, H.; Zhang, Y.; et al. Transmission electron microscopy. In Progress in Nanoscale Characterization and Manipulation; Wang, R., Wang, C., Zhang, H., Tao, J., Bai, X., Eds.; Springer: Singapore, 2018; pp. 69–203. [Google Scholar]
- Zhang, L.; Shi, W.; Zhang, B. A review of electrocatalyst characterization by transmission electron microscopy. J. Energy Chem. 2017, 26, 1117–1135. [Google Scholar] [CrossRef]
- Gocyla, M.; Kuehl, S.; Shviro, M.; Heyen, H.; Selve, S.; Dunin-Borkowski, R.E.; Heggen, M.; Strasser, P. Shape stability of octahedral ptni nanocatalysts for electrochemical oxygen reduction reaction studied by in situ transmission electron microscopy. ACS Nano 2018, 12, 5306–5311. [Google Scholar] [CrossRef] [PubMed]
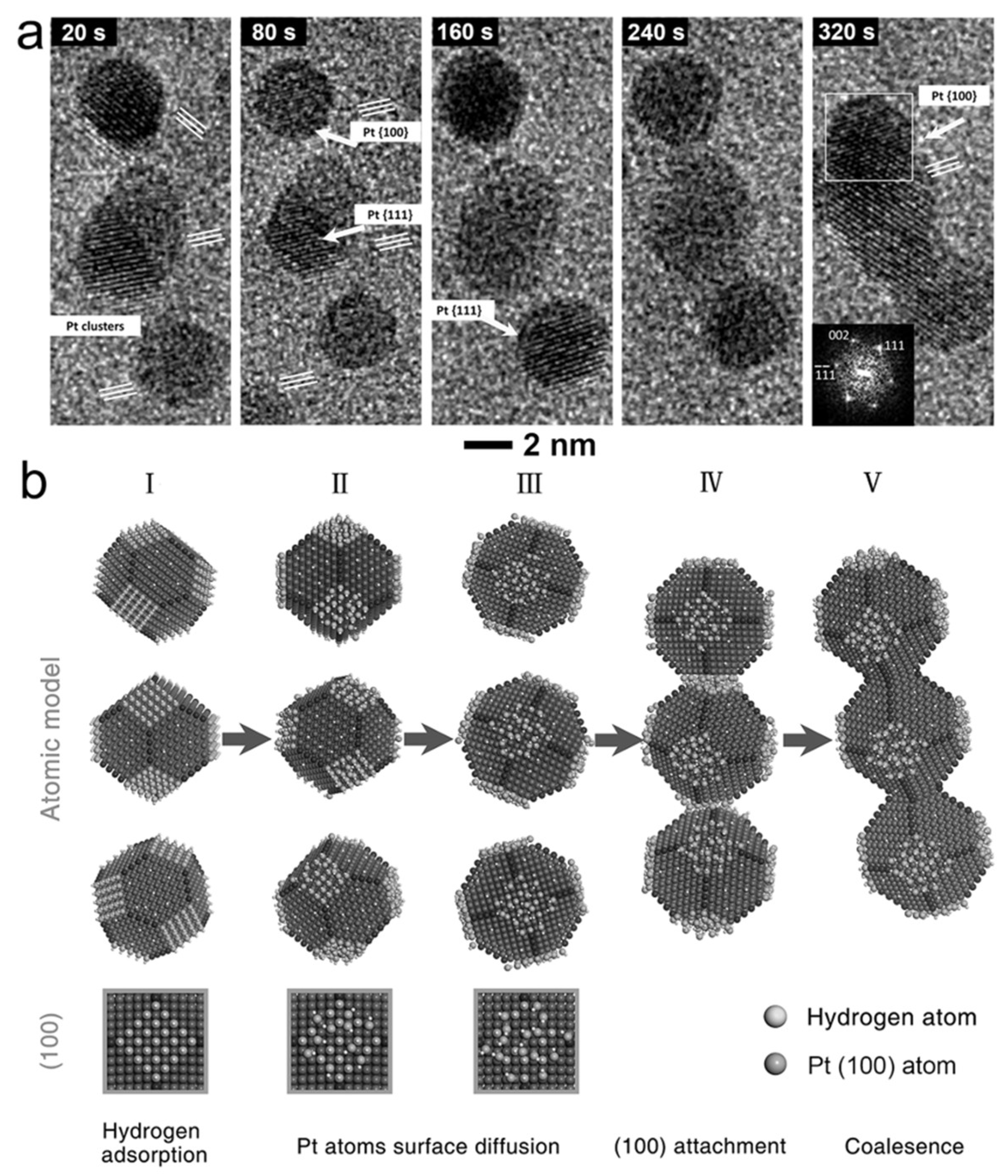
- Ma, Y.; Gao, W.; Shan, H.; Chen, W.; Shang, W.; Tao, P.; Song, C.; Addiego, C.; Deng, T.; Pan, X.; et al. Platinum-based nanowires as active catalysts toward oxygen reduction reaction: In situ observation of surface-diffusion-assisted, solid-state oriented attachment. Adv. Mater. 2017, 29, 1703460. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, W.; Sun, Q.; Wang, S.; Sun, K.; Schwank, J.; Wang, R. In situ tracing of atom migration in pt/nipt hollow spheres during catalysis of co oxidation. Chem. Commun. 2014, 50, 1804–1807. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.B.; Wang, R.M.; Liu, J.Y. Stability investigation of a high number density pt-1/fe2o3 single-atom catalyst under different gas environments by haadf-stem. Nanotechnology 2018, 29, 204002. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Engelhard, M.H.; Shao, Y.; Wang, C. Revealing the dynamics of platinum nanoparticle catalysts on carbon in oxygen and water using environmental tem. ACS Catal. 2017, 7, 7658–7664. [Google Scholar] [CrossRef]
- Hodnik, N.; Dehm, G.; Mayrhofer, K.J. Importance and challenges of electrochemical in situ liquid cell electron microscopy for energy conversion research. Acc. Chem. Res. 2016, 49, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Wood, J.D.; Chen, K.S.; Cho, E.; Hersam, M.C. In situ thermal decomposition of exfoliated two-dimensional black phosphorus. J. Phys. Chem. Lett. 2015, 6, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Becknell, N.; Kang, Y.; Chen, C.; Resasco, J.; Kornienko, N.; Guo, J.; Markovic, N.M.; Somorjai, G.A.; Stamenkovic, V.R.; Yang, P. Atomic structure of pt3ni nanoframe electrocatalysts by in situ x-ray absorption spectroscopy. J. Am. Chem. Soc. 2015, 137, 15817–15824. [Google Scholar] [CrossRef] [PubMed]
- Petkov, V.; Maswadeh, Y.; Lu, A.; Shan, S.; Kareem, H.; Zhao, Y.; Luo, J.; Zhong, C.-J.; Beyer, K.; Chapman, K. Evolution of active sites in pt-based nanoalloy catalysts for the oxidation of carbonaceous species by combined in situ infrared spectroscopy and total x-ray scattering. ACS Appl. Mater. Interfaces 2018, 10, 10870–10881. [Google Scholar] [CrossRef] [PubMed]
- Avanesian, T.; Dai, S.; Kale, M.J.; Graham, G.W.; Pan, X.; Christopher, P. Quantitative and atomic-scale view of co-induced pt nanoparticle surface reconstruction at saturation coverage via dft calculations coupled with in situ tem and ir. J. Am. Chem. Soc. 2017, 139, 4551–4558. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, V.; Mun, B.S.; Mayrhofer, K.J.J.; Ross, P.N.; Markovic, N.M.; Rossmeisl, J.; Greeley, J.; Nørskov, J.K. Changing the activity of electrocatalysts for oxygen reduction by tuning the surface electronic structure. Angew. Chem. Int. Ed. 2006, 45, 2897–2901. [Google Scholar] [CrossRef] [PubMed]
- Greeley, J.; Mavrikakis, M. Alloy catalysts designed from first principles. Nat. Mater. 2004, 3, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, V.R.; Mun, B.S.; Arenz, M.; Mayrhofer, K.J.J.; Lucas, C.A.; Wang, G.; Ross, P.N.; Markovic, N.M. Trends in electrocatalysis on extended and nanoscale pt-bimetallic alloy surfaces. Nat. Mater. 2007, 6, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, V.R.; Fowler, B.; Mun, B.S.; Wang, G.; Ross, P.N.; Lucas, C.A.; Marković, N.M. Improved oxygen reduction activity on pt3ni(111) via increased surface site availability. Science 2007, 315, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jang, J.-H.; Roh, C.-W.; Yang, S.; Kim, J.; Lim, J.; Yoo, S.J.; Lee, H. Gram-scale synthesis of highly active and durable octahedral ptni nanoparticle catalysts for proton exchange membrane fuel cell. Appl. Catal. B Environ. 2018, 225, 530–537. [Google Scholar] [CrossRef]
- Godínez-Salomón, F.; Mendoza-Cruz, R.; Arellano-Jimenez, M.J.; Jose-Yacaman, M.; Rhodes, C.P. Metallic two-dimensional nanoframes: Unsupported hierarchical nickel–platinum alloy nanoarchitectures with enhanced electrochemical oxygen reduction activity and stability. ACS Appl. Mater. Interfaces 2017, 9, 18660–18674. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, S.; Shi, F.; Chen, K.; Nie, Y.; Wang, Y.; Wu, R.; Li, J.; Zhang, Y.; Ding, W.; et al. Structural evolution of solid pt nanoparticles to a hollow ptfe alloy with a pt-skin surface via space-confined pyrolysis and the nanoscale kirkendall effect. Adv. Mater. 2016, 28, 10673–10678. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Jiang, P.; Chen, J.; Chen, Q. Nanoporous ptfe nanoparticles supported on n-doped porous carbon sheets derived from metal-organic frameworks as highly efficient and durable oxygen reduction reaction catalysts. ACS Appl. Mater. Interfaces 2017, 9, 32106–32113. [Google Scholar] [CrossRef] [PubMed]
- Dhavale, V.M.; Kurungot, S. Cu–pt nanocage with 3-d electrocatalytic surface as an efficient oxygen reduction electrocatalyst for a primary zn–air battery. ACS Catal. 2015, 5, 1445–1452. [Google Scholar] [CrossRef]
- Luo, S.; Tang, M.; Shen, P.K.; Ye, S. Atomic-scale preparation of octopod nanoframes with high-index facets as highly active and stable catalysts. Adv. Mater. 2017, 29, 1601687. [Google Scholar] [CrossRef] [PubMed]
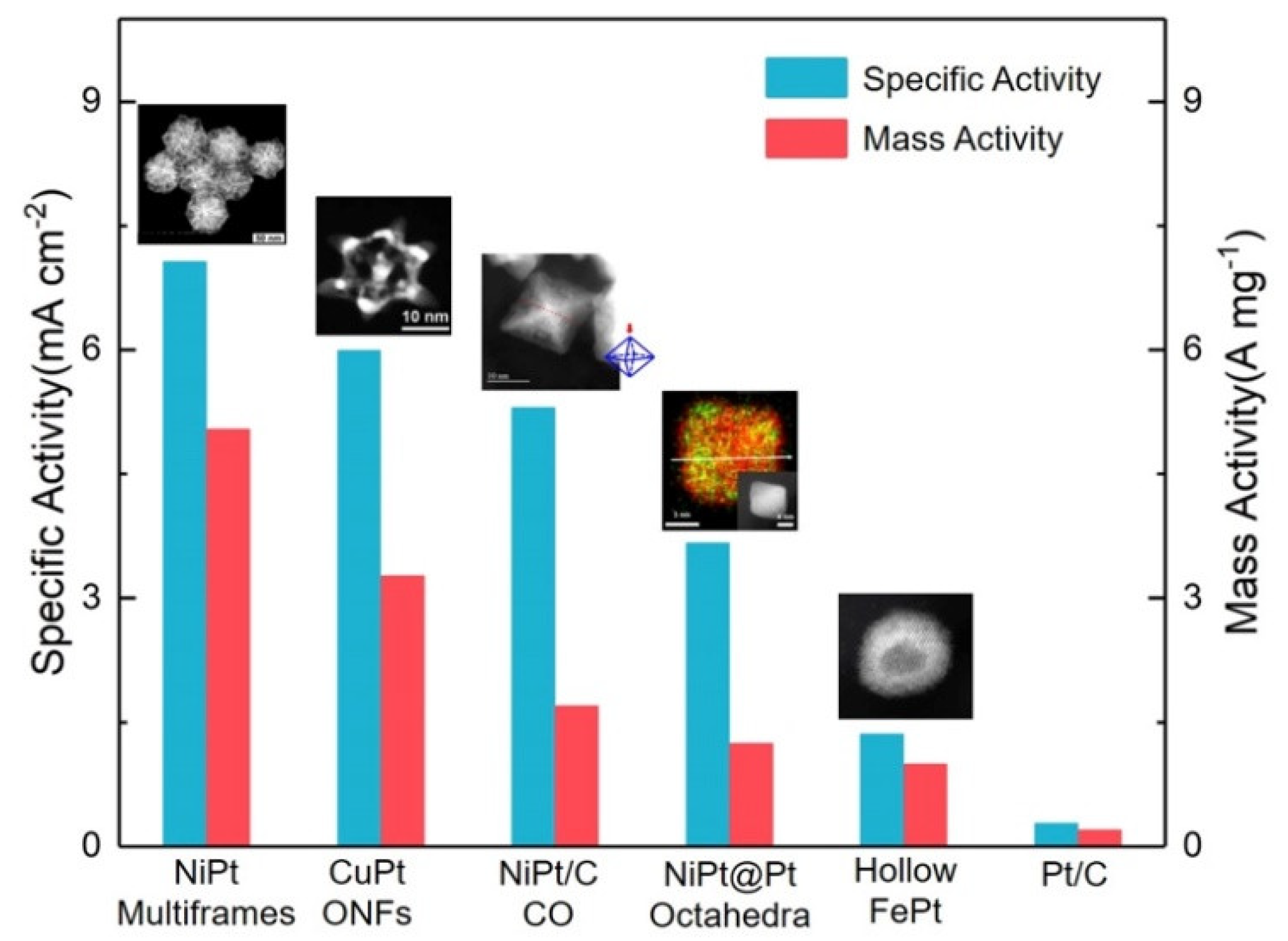
- Kwon, H.; Kabiraz, M.K.; Park, J.; Oh, A.; Baik, H.; Choi, S.I.; Lee, K. Dendrite-embedded platinum-nickel multiframes as highly active and durable electrocatalyst toward the oxygen reduction reaction. Nano Lett. 2018, 18, 2930–2936. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.N.; Tiwari, R.N.; Singh, G.; Kim, K.S. Recent progress in the development of anode and cathode catalysts for direct methanol fuel cells. Nano Energy 2013, 2, 553–578. [Google Scholar] [CrossRef]
- Tian, X.L.; Wang, L.; Deng, P.; Chen, Y.; Xia, B.Y. Research advances in unsupported pt-based catalysts for electrochemical methanol oxidation. J. Energy Chem. 2017, 26, 1067–1076. [Google Scholar] [CrossRef]
- Lu, Y.Z.; Jiang, Y.Y.; Wu, H.B.; Chen, W. Nano-ptpd cubes on graphene exhibit enhanced activity and durability in methanol electrooxidation after co stripping-cleaning. J. Phys. Chem. C 2013, 117, 2926–2938. [Google Scholar] [CrossRef]
- Yin, A.X.; Min, X.Q.; Zhang, Y.W.; Yan, C.H. Shape-selective synthesis and facet-dependent enhanced electrocatalytic activity and durability of monodisperse sub-10 nm pt-pd tetrahedrons and cubes. J. Am. Chem. Soc. 2011, 133, 3816–3819. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.Y.; Liu, J.L.; Wang, S.G.; Wang, C.; Sun, Y.; Wang, R.M. Nanomagnetic copt truncated octahedrons: Facile synthesis, superior electrocatalytic activity and stability for methanol oxidation. Sci. China-Mater. 2017, 60, 57–67. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, Z.; Jia, Y.; Wang, M.; Qi, W.; Pang, Y.; Yi, J.; Zhang, Y.; Li, Z.; Zhang, Z. Unique cu@cupt core-shell concave octahedron with enhanced methanol oxidation activity. ACS Appl. Mater. Interfaces 2017, 9, 36817–36827. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Shen, P.K. Concave platinum–copper octopod nanoframes bounded with multiple high-index facets for efficient electrooxidation catalysis. ACS Nano 2016, 11, 11946–11953. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, T.; He, T.; Ni, B.; Yuan, Q.; Wang, X. Composition-driven shape evolution to cu-rich ptcu octahedral alloy nanocrystals as superior bifunctional catalysts for methanol oxidation and oxygen reduction reaction. Nanoscale 2018, 10, 4670–4674. [Google Scholar] [CrossRef] [PubMed]
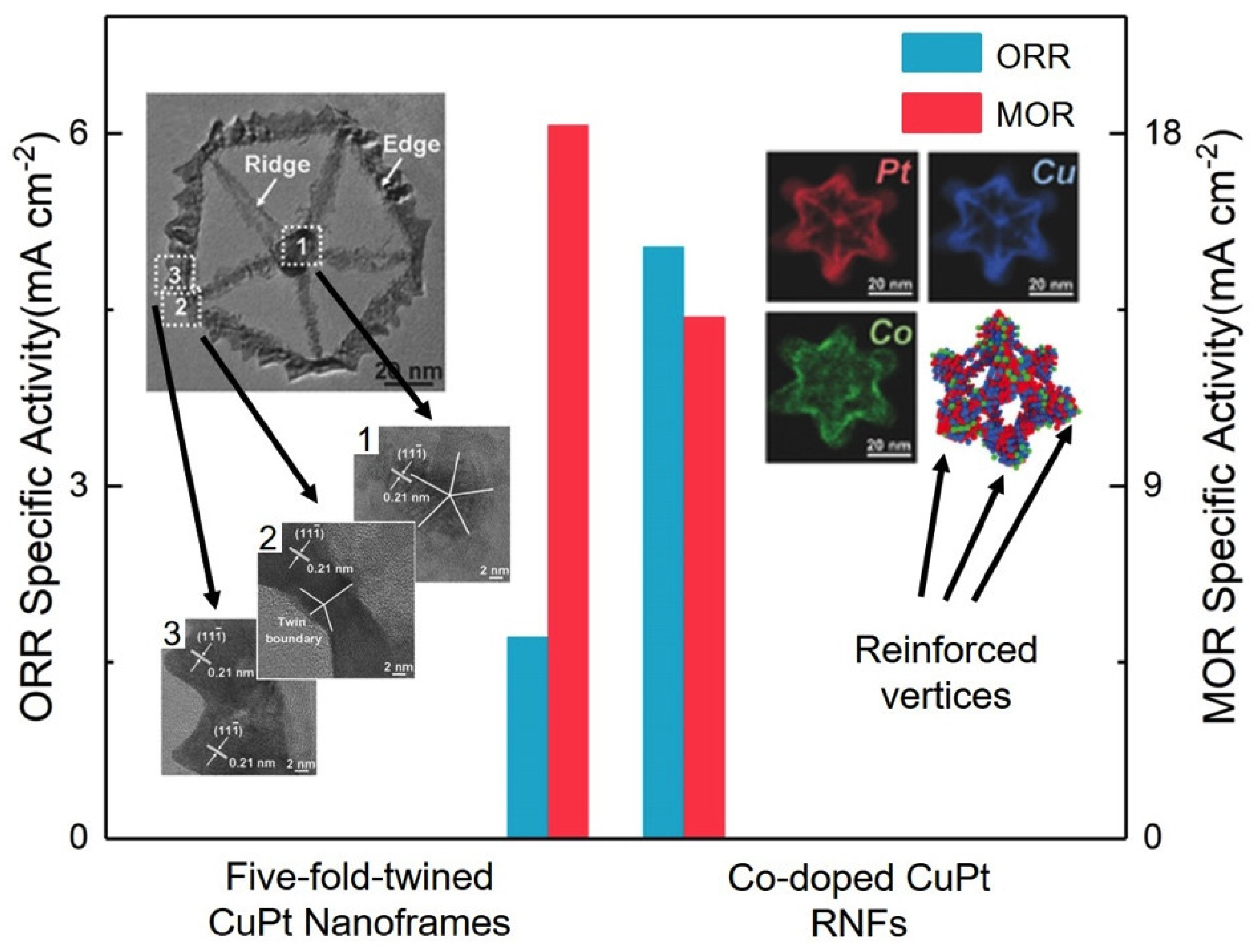
- Zhang, Z.C.; Luo, Z.M.; Chen, B.; Wei, C.; Zhao, L.; Chen, J.Z.; Zhang, X.; Lai, Z.C.; Fan, Z.X.; Tan, C.L.; et al. One-pot synthesis of highly anisotropic five-fold-twinned ptcu nanoframes used as a bifunctional electrocatalyst for oxygen reduction and methanol oxidation. Adv. Mater. 2016, 28, 8712–8717. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Jun, M.; Kim, H.Y.; Oh, A.; Park, J.; Baik, H.; Joo, S.H.; Lee, K. Vertex-reinforced ptcuco ternary nanoframes as efficient and stable electrocatalysts for the oxygen reduction reaction and the methanol oxidation reaction. Adv. Funct. Mater. 2018, 28, 1706440. [Google Scholar] [CrossRef]
- Liu, T.; Wang, K.; Yuan, Q.; Shen, Z.; Wang, Y.; Zhang, Q.; Wang, X. Monodispersed sub-5.0 nm ptcu nanoalloys as enhanced bifunctional electrocatalysts for oxygen reduction reaction and ethanol oxidation reaction. Nanoscale 2017, 9, 2963–2968. [Google Scholar] [CrossRef] [PubMed]
- Mun, Y.; Shim, J.; Kim, K.; Han, J.W.; Kim, S.-K.; Ye, Y.; Hwang, J.; Lee, S.; Jang, J.; Kim, Y.-T.; et al. Direct access to aggregation-free and small intermetallic nanoparticles in ordered, large-pore mesoporous carbon for an electrocatalyst. RSC Adv. 2016, 6, 88255–88264. [Google Scholar] [CrossRef]
- Huang, H.; Hu, X.; Zhang, J.; Su, N.; Cheng, J. Facile fabrication of platinum-cobalt alloy nanoparticles with enhanced electrocatalytic activity for a methanol oxidation reaction. Sci. Rep. 2017, 7, 45555. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Hwang, S.; Pan, Y.T.; Chen, K.; He, Y.; Karakalos, S.; Zhang, H.; Spendelow, J.S.; Su, D.; Wu, G. Ordered pt3co intermetallic nanoparticles derived from metal-organic frameworks for oxygen reduction. Nano Lett. 2018, 18, 4163–4171. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.; Li, J.; Jiang, G.; Cano, Z.P.; Ma, Z.; Zhong, C.; Su, D.; Chen, Z. Metal-organic frameworks derived platinum-cobalt bimetallic nanoparticles in nitrogen-doped hollow porous carbon capsules as a highly active and durable catalyst for oxygen reduction reaction. Appl. Catal. B Environ. 2018, 225, 496–503. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Cheng, T.; Guo, H.; Sun, B.; Wang, Y. Preparation and application in assembling high-performance fuel cell catalysts of colloidal ptcu alloy nanoclusters. J. Power Sources 2018, 395, 66–76. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, R.; Li, X.; Chen, W. Ptni nanocrystals supported on hollow carbon spheres: Enhancing the electrocatalytic performance through high-temperature annealing and electrochemical co stripping treatments. ACS Appl. Mater. Interfaces 2017, 9, 29623–29632. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, Y.; Liu, J.; Wang, F. Adding refractory 5d transition metal w into ptco system: An advanced ternary alloy for efficient oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 10700–10709. [Google Scholar] [CrossRef]
- Duan, S.; Wang, R. Bimetallic nanostructures with magnetic and noble metals and their physicochemical applications. Prog. Nat. Sci. Mater. Int. 2013, 23, 113–126. [Google Scholar] [CrossRef]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Shan, A.; Wang, R. Low Pt Alloyed Nanostructures for Fuel Cells Catalysts. Catalysts 2018, 8, 538. https://doi.org/10.3390/catal8110538
Huang S, Shan A, Wang R. Low Pt Alloyed Nanostructures for Fuel Cells Catalysts. Catalysts. 2018; 8(11):538. https://doi.org/10.3390/catal8110538
Chicago/Turabian StyleHuang, Shuoyuan, Aixian Shan, and Rongming Wang. 2018. "Low Pt Alloyed Nanostructures for Fuel Cells Catalysts" Catalysts 8, no. 11: 538. https://doi.org/10.3390/catal8110538





