Abstract
New ligand platforms of the type p- or m-Ph{-CR(3,6-tBu2Flu)(Cp)}2 (para-, R = Me (2a), H (2b); meta-, R = Me (2c)) were synthesized via nucleophilic addition of the 3,6-tBu2-fluorenyl-anion onto the parent phenylene-bridged difulvenes (1a–c). The corresponding discrete homodinuclear zirconium and hafnium bis(dichloro ansa-metallocene) complexes, Ph[{-CR(3,6-tBu2Flu)(Cp)}MCl2]2 (p-, R = Me (3a-Zr2, 3a-Hf2), R = H (3b-Zr2); m-, R = Me (3c-Zr2), were prepared by salt metathesis reactions. An attempt to generate in situ a heterodinuclear complex 3a-Zr-Hf was also undertaken. For the first time, Atmospheric Pressure PhotoIonization (APPI) mass-spectrometric data were obtained for all dinuclear compounds and found to be in excellent agreement with the simulated ones. Preliminary studies on the catalytic performances of these dinuclear complexes, upon activation with MAO, in ethylene homopolymerization and ethylene/1-hexene copolymerization revealed a few differences as compared to those of the monometallic analogues. In particular, slightly lower molecular weights and a greater formation of short methyl and ethyl branches were obtained with the dinuclear systems.
1. Introduction
The development and applications of multinuclear group 4 metal α-olefin polymerization catalysts have increased dramatically in the past decade [1,2]. The interest in this area has primarily been driven by the potential to exploit intermetallic cooperativity/synergism between the two or more proximal metal centers to eventually enhance the performance of polymerization systems. For instance, several studies on dinuclear catalysts suggest that catalyst activity [3,4,5,6,7,8,9,10,11,12,13] and molecular weight, [3,7,10,14] tacticity, [13,14,15], or comonomer incorporation [8,16,17,18,19,20] of/in the resulting polymers can be greater than those of the corresponding mononuclear analogues that have isostructural catalytic sites.
Group 4 metal catalysts based on one-carbon-bridged ansa-cyclopentadienyl-fluorenyl platforms, {R2C(Cp/Flu)}2−, hold a unique position in α-olefin polymerization thanks to their high catalytic activity, excellent control, and remarkable stereospecificity [21,22,23,24,25,26,27,28,29,30,31,32,33]. However, only a few examples of dinuclear systems of this type have been reported in the literature (Scheme 1) [9,34,35,36,37].
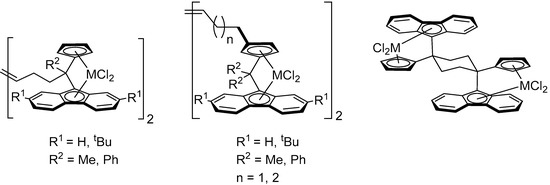
Scheme 1.
Examples of group 4 dinuclear bis(dichloro ansa-metallocene) complexes that incorporate {R2C(Cp/Flu)} ligand platforms [8,9].
We herein report on the synthesis of dinuclear group 4 bis(Cp/Flu-metallocene) complexes, linked at the C1-bridge by a para- or meta-phenylene moiety [3,18,19,20,38], as well as their characterization by NMR spectroscopy and advanced Atmospheric Pressure PhotoIonization (APPI) mass-spectrometric methods. The catalytic performances of the synthesized complexes, after their activation with MAO, were preliminarily investigated in homogeneous ethylene homopolymerization and ethylene/1-hexene copolymerization, and compared to those of the mononuclear ansa-metallocene complexes as a reference.
2. Results and Discussion
2.1. Synthesis of Proligands
An efficient and scalable synthesis via nucleophilic addition of the (3,6-di-tert-butyl)fluorenyl anion onto fulvenes is regularly utilized to prepare one-carbon-bridged R2C{Cp/Flu}H2 proligands [21,22,23,24,25,26,27,28]. This methodology was here extended to three different bis(fulvene) platforms (1a–c), which were prepared from cyclopentadiene and the corresponding aromatic diketones or dialdehydes (Scheme 2), and isolated in good yields as yellow or orange solids.
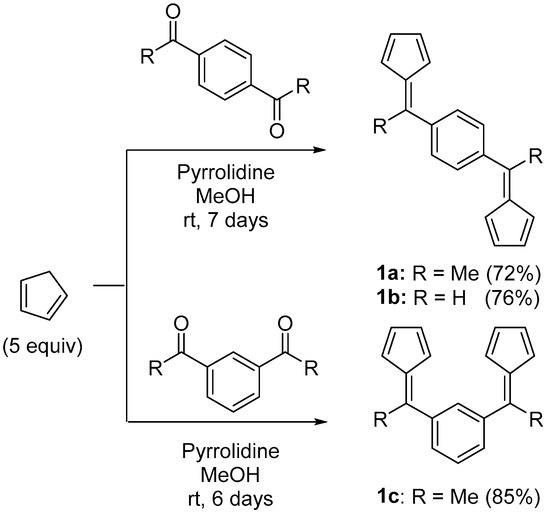
Scheme 2.
Synthesis of the para- and meta-phenylene-bridged bis(fulvenes) 1a–c.
To prepare the targeted p- and m-Ph{-CR(3,6-tBu2FluH)(CpH)}2 proligands 2a–c, bis(fulvenes) 1a–c were subsequently reacted with two equiv. of the {3,6-tBu2Flu}− anion in THF (Scheme 3). After workup, the corresponding para-bridged proligands 2a,b were isolated in good yields; synthesis/recovery of the meta-bridged proligand 2c proved somehow to be more difficult (22% isolated yield). This lower yield may be due to a larger steric hindrance in the final meta-phenylene-bridged product imposed by the two very bulky {Cp/Flu} moieties as compared to the para analogues.
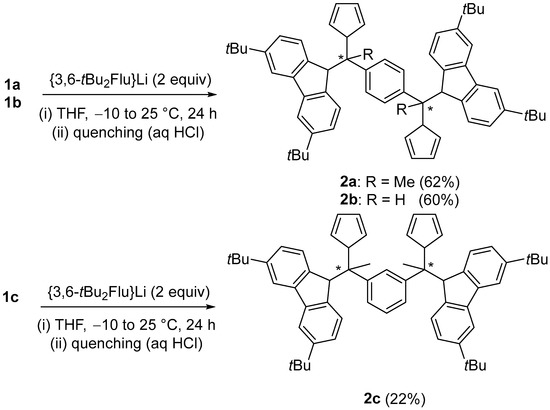
Scheme 3.
Synthesis of the proligands 2a–c.
These proligands are stable at room temperature in solution and in the solid state, and their structures were authenticated by 1H and 13C NMR spectroscopy (Figures S9, S10, S12, S13, S15, and S16) and ESI-mass spectrometry (Figures S11, S14, and S17). The NMR data for these compounds appeared to be complicated by the presence of two stereogenic centers in the molecules, resulting in the existence of pairs of diastereoisomers in each case, and also of their different tautomers (i.e., isomers of C=C bonds within the CpH rings). The ESI-MS measurements data showed clearly the expected molecular [M + H]+ ions at m/z 815.55, 787.52 for 2a,b and at 853.51 ([M + K]+) for 2c.
2.2. Synthesis of Group 4 Dinuclear Bis(dichloro ansa-metallocene) Complexes
In order to prepare the corresponding group 4 bis(dichloro ansa-metallocene) complexes, standard salt-metathesis reactions between the ligand tetraanions, generated in situ in Et2O, and MCl4 salts (2 equiv.), were used (Scheme 4). Thus, the homodinuclear bis(dichloro ansa-zirconocenes) 3a-c-Zr2 and bis(dichloro ansa-hafnocene) 3a-Hf2 were isolated in good yields as red and yellow solids, respectively.
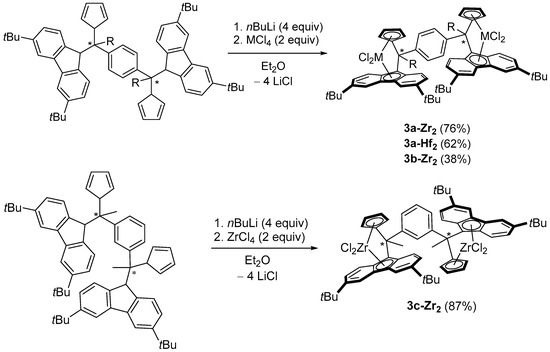
Scheme 4.
Synthesis of the group 4 homodinuclear bis(dichloro ansa-metallocene) complexes.
As 3a-c-Zr2 and 3a-Hf2 were derived from diastereomeric mixtures of proligands, two diastereomers for each of these compounds were anticipated, featuring Cs-/Ci-symmetries for the para-phenylene-bridged complexes 3a,b-M2 and Cs-/C1-symmetries for the meta-phenylene-bridged complex 3c-Zr2 (Scheme 5). Accordingly, the 1H and 13C NMR spectra of the crude 3a-Zr2 (Figures S24–S26), 3a-Hf2 (Figures S28 and S29, respectively), and 3c-Zr2 (Figures S35 and S36, respectively) complexes displayed two sets of resonances corresponding to the two diastereomers. Unexpectedly, only one set of resonances assigned to a single diastereoisomer of either Cs- or Ci-symmetry was observed in the 1H NMR spectrum of 3b-Zr2 (Figure S31). As this compound was isolated in a lower yield than the other ones, one cannot discard that only one diastereoisomer was recovered in the workup.
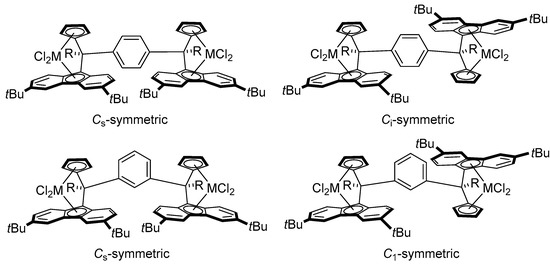
Scheme 5.
Possible diastereoisomers of the phenylene-bridged bis(dichloro ansa-metallocene) complexes.
Unfortunately, all attempts to grow single-crystals of these complexes suitable for X-ray diffraction studies have failed so far. However, the identity of these bis(dichloro ansa-metallocene) compounds was confirmed unambiguously by mass spectrometry (vide infra).
In order to obtain a better clue about the possible structures of the dinuclear bis(metallocenes), the corresponding geometries of the two Cs- and Ci-symmetric isomers of 3a-Zr2 (Figure S68; see the Experimental Section for details) and the two Cs- and C1-symmetric isomers of 3c-Zr2 (Figure S69) were modeled by DFT computations. It is noteworthy that the optimized geometries of the isomers belonging to both dinuclear systems 3a-Zr2 and 3c-Zr2 featured relatively long Zr…Zr intermetallic distances of 10.5–10.8 Å and 9.2–9.8 Å, respectively. Also, the respective orientations of the metallocenic fragments in these structures resulted in the coordination sites, represented by the chlorine ligands, pointing in opposite directions. Such an orientation of the metallocenic moieties in both para- and meta-phenylene-bridged systems may not be favorable to the mutual approach of the two metal centers in dinuclear active species derived thereof during polymerization (vide infra). Note, however, that the above observations were made on the most stable neutral isomers as determined by DFT, and they do not necessarily reflect the proximity that can be reached from dynamic conformations in those species. Also, the behavior of the active cationic species associated with counterionic moieties may be quite different.
In an attempt to synthesize a hetero-bis(metallocene) incorporating both zirconium and hafnium metals, a similar salt-metathesis protocol as that utilized for the synthesis of the homo-bis(dichloro ansa-metallocenes) 3a-Zr2 and 3a-Hf2 was probed using 1 equiv. of each of the metal precursors ZrCl4 and HfCl4 (Scheme 6). In this case, as anticipated, a statistical 1:1:2 mixture of the homodinuclear 3a-Zr2 and 3a-Hf2 complexes and the heterodinuclear 3a-Zr/Hf complex was obtained, as revealed by 1H NMR spectroscopy of the crude sample. No single-crystal suitable for X-ray diffraction studies has been grown thus far. Due to the complexity of the mixture and the obvious difficulties associated with regular elemental and spectroscopic analyses, only its mass-spectrometric characterization was performed (vide infra).
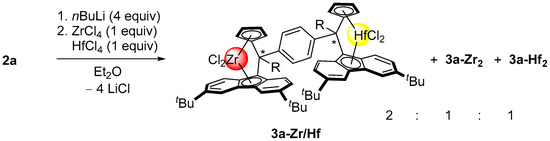
Scheme 6.
Attempted synthesis of the heterodinuclear bis(metallocene) 3a-Zr/Hf.
2.3. Synthesis of Mononuclear Ansa-metallocene Analogues
For comparative studies of mass-spectrometric analyses and of catalytic properties of the bis(metallocene)s in α-olefin polymerization, their mononuclear analogues were also synthesized (Scheme 7). The complexes 3a’,b’-Zr and 3b’-Hf were isolated in good yields and characterized by 1H and 13C NMR spectroscopic studies, X-ray diffraction (for 3a’-Zr; Figure S67), and APPI mass-spectrometry (vide infra).
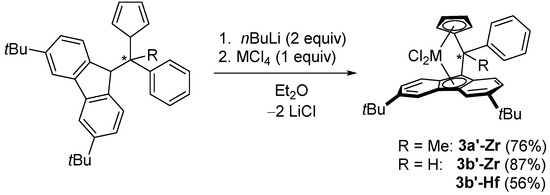
Scheme 7.
Synthesis of the mononuclear metallocene analogues.
2.4. Mass Spectrometric Studies of Mononuclear and Dinuclear Bis(dichloro ansa-metallocene) Complexes
Atmospheric Pressure PhotoIonization (APPI) was chosen instead of the more common electrospray (ESI), as APPI is very efficient for the ionization of aromatic molecules that do not contain polar groups; also, it allows for the use of dry toluene as a solvent to preserve the rather sensitive metallocene complexes [39,40]. The APPI mass spectra of the mononuclear complexes 3a’-Zr, 3b’-Zr, and 3b’-Hf are summarized in Figure 1. In each case, the corresponding intact species was detected as a M+• molecular ion. A free ligand was also observed in the spectra at m/z 444.3 and m/z 430.3, as well as the C21H26 moiety at m/z 278.
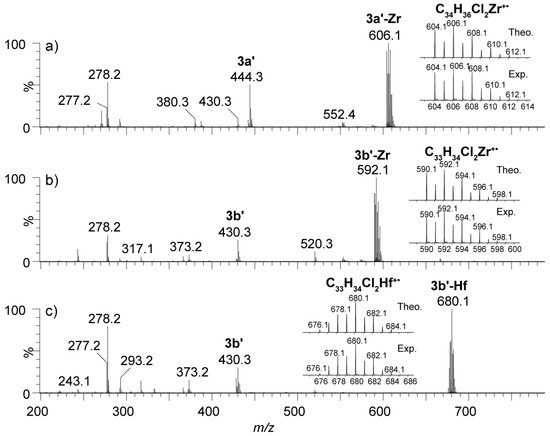
Figure 1.
The APPI(+) mass spectra of the mononuclear complexes 3a’-Zr (a), 3b’-Zr (b), and 3b’-Hf (c). The zoomed areas showcase the theoretical and experimental isotopic clusters. For each isotopic distribution, only the most intense isotope peak is labelled.
By analogy, the M+• molecular ions were identified for the dinuclear bis(zirconocene) 3a-b-Zr2 compound and the dinuclear bis(hafnocene) 3a-Hf2 compound (Figure 2). The accurate masses and isotopic distributions (m/z 1130.2074, 1102.1754, and 1310.2865, respectively) are in very good agreement (<5 ppm) with those expected theoretically based on the corresponding ions’ molecular formula (m/z 1130.2013, 1102.1700, and 1310.2850, respectively). In addition to the dinuclear species 3a,b-Zr2 and 3a-Hf2, molecular ions derived from the monometallated fragments, i.e., 3a,b-Zr and 3a-Hf, were detected in each case at m/z 972.3776, 944.3398, and 1062.4139, respectively. These ions were most likely not generated by gas-phase fragmentation, as they were not produced by collision-induced dissociation of the dinuclear molecular ions. They, however, may have been produced by partial degradation (e.g., hydrolysis) during the sample handling or in the atmosphere source that may contain traces of water.
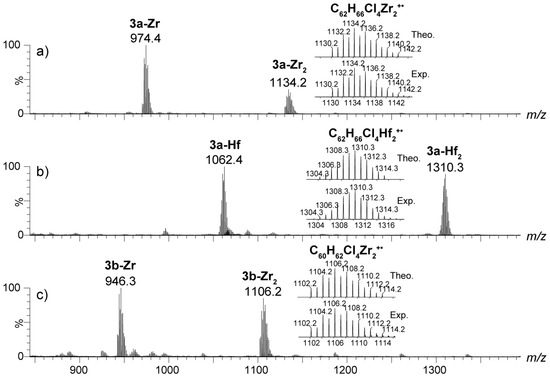
Figure 2.
The APPI(+) mass spectra of the dinuclear bis(metallocene) 3a-Zr2 (a), 3a-Hf2 (b), and 3b-Zr2 (c) complexes. The zoomed areas showcase the theoretical and experimental isotopic clusters. For each isotopic distribution, only the most intense isotope peak is labelled.
For the mixture containing the heterodinuclear bis(dichloro ansa-metallocene) compound 3a-Zr/Hf and its homodinuclear counterparts 3a-Zr2 and 3a-Hf2, a set of five isotopic distributions was observed in the APPI mass-spectrum (Figure 3). Besides the distributions corresponding to the homodinuclear 3a-Zr2 and 3a-Hf2 and their monometallated versions, i.e., 3a-Zr and 3a-Hf, respectively, an isotopic distribution at m/z 1222.2463 was identified and unequivocally assigned to 3a-Zr/Hf. APPI should present a low ionization discrimination for these species, so their relative abundance should be representative of their actual amount in the sample, although the most air-sensitive molecules may present a lower abundance because of higher degradation. This possibly accounts for the observed lower intensity of peaks arising from 3a-Hf2.
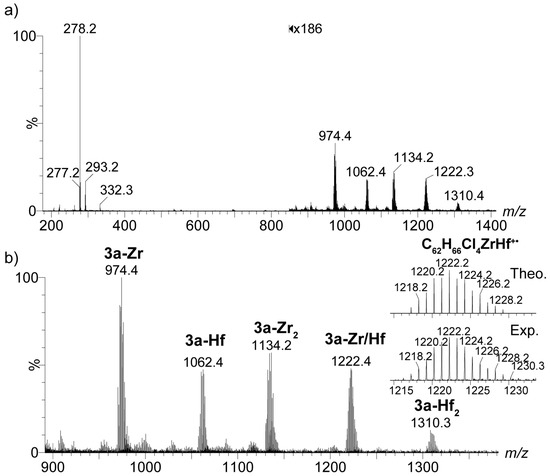
Figure 3.
(a) The APPI(+) mass spectrum of the crude reaction mixture resulting from metallation of the proligand 3a by a 1:1 mixture of ZrCl4 and HfCl4; (b) The enlargement of the m/z 900–1350 area. The zoomed areas showcase the theoretical and experimental isotopic clusters of the dinuclear bis(dichloro ansa-metallocene) complexes 3a-Zr-Hf. For each isotopic distribution, only the most intense isotope peak is labelled.
2.5. Polymerization Catalysis
The dinuclear bis(dichloro ansa-metallocene) complexes 3a-M2, 3b-Zr2, and 3c-Zr2, and their mononuclear analogues 3a,b’-Zr and 3b’-Hf, in combination with methylalumoxane (MAO), were evaluated in homogeneous ethylene polymerization (Table 1) and ethylene/1-hexene copolymerization (Table 2). Each polymerization experiment was repeated independently twice under the same conditions (toluene, 5.5 bar of ethylene, 60 °C), which revealed good reproducibility in terms of productivity (polymer yield) and physicochemical properties (Tm, Mw, polydispersity index (PDI)) of the isolated polymer.

Table 1.
Homopolymerization of ethylene a.

Table 2.
Ethylene/1-hexene copolymerization a.
In general, no significant or limited difference was observed for the experiments involving dinuclear bis(metallocene) with respect to those using the corresponding mononuclear analogues (Table 1; compare entries 1–2/3–4, 5–6/7–8, and 10–11/12, respectively). Indeed, all of the systems that were produced with similar productivities used polyethylene samples exhibiting quite similar molecular weight distributions and Tm values. A slight drop in productivity was observed for the meta-bridged system 3c-Zr2 (entry 9), also yielding a lower molecular weight polyethylene (PE) as compared to the para-bridged congeners 3a,b-Zr2. Interestingly, as established by 13C NMR spectroscopy, 3b-Zr2 induced the formation of short branches (methyl and, to a lesser extent, ethyl) to a significantly greater extent than its mononuclear analogue and any other dinuclear system; the same observation was made in ethylene/1-hexene copolymerization (vide infra). This may presumably arise from a chain-walking mechanism.
The Hf-based systems 3a-Hf2 and 3b’-Hf appeared to be ca. 3-fold less productive (entries 10–12) than their Zr-based counterparts while affording much higher molecular weight PEs. The addition of BHT (entry 11) to scavenge the excess “free” AlMe3 [41] present in MAO did not affect the productivity of this system, and also resulted in an insoluble polymer (likely due to the formation of high molecular weight polyethylene (HMWPE) because of the absence of transfer to AlMe3).
Also, no strikingly different results were obtained upon using the different mono- and dinuclear compounds in ethylene/1-hexene copolymerization (Table 2). The Zr-based systems, both di- and mononuclear, afforded a very narrow range of productivities. It is of note that, in the para-bridged series 3a,b-Zr2 and 3a’b’-Zr, the dinuclear bis(metallocenes) gave slightly but significantly lower molecular weight copolymers and narrower dispersities (compare entries 1–2/3–4 and 5–6/7–8, respectively) than their respective mononuclear analogues. Conversely, the meta-bridged system 3c-Zr2 afforded a higher molecular weight copolymer than its mononuclear analogue (compare entries 3/4 and 9). Again, higher molecular weight copolymers were obtained with Hf-based catalysts, but with lower productivities than their Zr counterparts (entries 10–12). Also, a somewhat higher incorporation of 1-hexene in copolymers was achieved with the Hf-based catalysts.
3. Materials and Methods
3.1. General Considerations
All experiments were performed under a dry argon atmosphere, using a glovebox or standard Schlenk techniques. THF and Et2O were distilled prior to use from sodium benzophenone ketyl. Hexane and heptane were distilled from CaH2 and stored over 3 Ǻ molecular sieves. Deuterated solvents (benzene-d6, toluene-d8 > 99.5% D; Euriso-top, Saint-Aubin, France) were distilled from Na/K alloy or CaH2 (for CD2Cl2) and stored over 3 Ǻ molecular sieves. CDCl3 (99.8% D, Euriso-top) was used as received. Cyclopentadiene (Acros, Geel, Belgium) was freshly distilled prior to use. 3,6-Di-tert-butyl-fluorene and MAO were generously provided by Total Raffinage-Chimie. The fulvene precursors, (1-(cyclopenta-2,4-dien-1-ylidene)ethyl)benzene and (cyclopenta-2,4-dien-1-ylidenemethyl)benzene [42], were prepared following protocols published in the literature. ZrCl4 and HfCl4 were used as received (anhydrous, Strem Chemicals, Bischheim, France). 1-Hexene (Fisher Chemical, Illkirch, France) was distilled and stored over 3 Ǻ molecular sieves under argon. Ethylene (N35, Air Liquide, Paris, France) was used without further purification.
3.2. Instruments and Measurements
The NMR spectra of air- and moisture-sensitive compounds were recorded on Bruker AM-400 and AM-500 spectrometers in Teflon-valved NMR tubes at room temperature. 1H and 13C chemical shifts are reported in ppm versus SiMe4 and were determined using residual solvent signal. Coupling constants are given in Hertz. Assignments of signals were carried out using 1D (1H, 13C{1H}, JMOD) and 2D (COSY, HMBC, HMQC) NMR experiments. Elemental analyses were performed on a Carlo Erba 1108 Elemental Analyzer instrument at the London Metropolitan University by Stephen Boyer or on a Flash EA1112 CHNS Thermo Electron apparatus at CRMPO, Rennes, and were the average of a minimum of two independent measurements.
The 13C{1H} NMR and GPC analyses of polymer samples were performed in the research center of Total Research and Technologies in Feluy (Feluy, Belgium). The 13C{1H} NMR analyses were run on a 500 MHz Bruker Avance III with a 10 mm cryoprobe HTDUL in trichlorobenzene/C6D6 (2 mL/0.5 mL). The GPC analyses were performed in 1,2,4-trichlorobenzene at 135 °C using PS standards for calibration. Differential scanning calorimetry (DSC) analyses were performed on a Setaram DSC 131 apparatus under a continuous flow of helium and using aluminum capsules. Glass transition and melting temperatures were measured during the second heating (10 °C·min−1).
The ESI (ElectroSpray Ionization) mass spectra of organic compounds, including proligands, were recorded at CRMPO-Scanmat (Rennes, France) on an Orbitrap Thermo Fisher Scientific Q-Exactive instrument with an ESI source in positive mode by direct introduction at 5–10 µg·mL−1. Samples were prepared in CH2Cl2 at 10 µg·mL−1.
The ASAP (Atmospheric Solids Analysis Probe) mass spectra of proligands were recorded at the CRMPO-Scanmat (Rennes, France) on a Q-TOF Bruker Maxis 4G instrument with an APCI source in positive mode at desorption temperatures of 255 and 300 °C.
3.3. APPI Mass Spectrometric Characterization of Metal Complexes
The mass spectra of metal complexes were recorded on a hybrid quadrupole time-of-flight instrument (Waters, Synapt G2, Manchester, England) equipped with an APPI source. The instrument was operated in the positive ion mode. The ionization experimental conditions were set as follows: desolvation gas flow, 700 L h−1; source temperature, 120 °C; probe temperature, 400 °C; sampling cone, 20 V; extraction cone, 3 V. The time-of-flight was operated in the ‘resolution mode’ yielding a resolving power of about 20,000. The samples were prepared in a glove box using dried toluene in 1.5 mL glass vials with a final concentration of 20 µM. For analysis, the sample was taken with a dry syringe stored in an oven. The solution was directly infused into the source using a syringe pump at a flow rate of 200 µL h−1. Data were acquired over the m/z 50–2000 range for 2–5 min. Note that the given accurate masses are given with Water Mass Lynx 4.1 that do not take into account the mass of the electron removed during ionization. All given masses are monoisotopic values. In the mass spectra, only the highest abundant isotope is labelled.
3.4. 1,4-Bis(1-(cyclopenta-2,4-dien-1-ylidene)ethyl)benzene (1a)
In a 250 mL round bottom flask equipped with a magnetic stirring bar and an argon inlet, freshly cracked cyclopentadiene (12.36 mL, 148.0 mmol) and 1,4-diacetylbenzene (4.82 g, 30.0 mmol) were dissolved in methanol (200 mL). To this solution, pyrrolidine (7.5 mL, 89.0 mmol) was added at 0 °C. The reaction mixture was stirred at room temperature for 7 days. After neutralization with glacial acetic acid (7.5 mL) and separation of the organic phase, volatiles were evaporated under vacuum to give a yellow powder (5.51 g, 21.3 mmol, 72%). 1H NMR (CDCl3, 400 MHz, 25 °C): δ 7.35 (s, 4H, CH-Ar), 6.59 (dt, 3J = 5.2, 4J = 1.6, 2H, CH-Cp), 6.51 (dt, 3J = 5.2, 4J = 1.6, 2H, CH-Cp), 6.43 (dt, 3J = 5.2, 4J = 1.6, 2H, CH-Cp), 6.16 (dt, 3J = 5.2, 4J = 1.6, 2H, CH-Cp), 2.50 (s, 6H, CH3). 13C NMR (CDCl3, 125 MHz, 25 °C): δ 149.0, 143.8, 142.0 (Cq), 132.1, 131.9 (CH-Cp), 129.0 (CH-Ar), 123.7, 121.2 (CH-Cp), 22.6 (CH3). ESI-MS (m/z): 259.15 ([M + H]+), 258.15 ([M]). Anal. calcd. for C20H18 (258.36): C 92.98, H 7.02; found: C 93.27, H 6.53.
3.5. 1,4-Bis(cyclopenta-2,4-dien-1-ylidenemethyl)benzene (1b)
Using a protocol similar to that described above for 1a, 1b was prepared from cyclopentadiene (30.7 mL, 373.0 mmol), 1,3-terephthalaldehyde (10.0 g, 74.5 mmol), and pyrrolidine (9.3 mL, 112.0 mmol), and isolated as an orange powder (13.03 g, 56.7 mmol, 76%). 1H NMR (CDCl3, 400 MHz, 25 °C): δ 7.63 (s, 4H, CH-Ar), 7.20 (s, 2H, CH-methine), 6.69 (m, 4H, CH-Cp), 6.52 (d, 3J = 5.0, 2H, CH-Cp), 6.32 (d, 3J = 5.0, 2H, CH-Cp). 13C NMR (CDCl3, 125 MHz, 25 °C): δ 146.2, 137.6, (Cq), 137.2, 136.1 (CH-Cp), 131.5 (=CH), 131.1 (CH-Ph), 127.4, 120.3 (CH-Cp). Anal. calcd. for C18H14 (230.30): C 93.87, H 6.13; found: C 93.98, H 6.56.
3.6. 1,3-Bis(1-(cyclopenta-2,4-dien-1-ylidene)ethyl)benzene (1c)
Using a protocol similar to that described above for 1a, 1c was prepared from cyclopentadiene (30.0 mL, 363.0 mmol), 1,3-diacetylbenzene (11.0 g, 68.0 mmol), and pyrrolidine (17.0 mL, 204.0 mmol), and isolated as an orange powder (14.9 g, 51.0 mmol, 85%). 1H NMR (CDCl3, 400 MHz, 25 °C): δ 7.41 (broad m, 4H, CH-Ar), 6.66 (dt, 3J = 5.2, 4J = 1.8, 2H, CH-Cp), 6.60 (dt, 3J = 5.2, 4J = 1.6, 2H, CH-Cp), 6.51 (dt, 3J = 5.2, 4J = 1.6, 2H, CH-Cp), 6.21 (dt, 3J = 5.2, 4J = 1.6, 2H, CH-Cp), 2.58 (s, 6H, CH3). 13C NMR (CDCl3, 125 MHz, 25 °C): δ 149.2, 143.8, 141.9 (Cq), 132.2, 131.9 (CH-Cp), 129.8, 129.2, 127.6 (CH-Ar), 123.6, 121.2 (CH-Cp), 22.7 (CH3). ESI-MS (m/z): 259.15 ([M + H]+). Anal. calcd. for C20H18 (258.36): C 92.98, H 7.02; found: C 93.02, H 7.13.
3.7. 1,4-Ph((Me)C-(3,6-tBu2FluH)(CpH))2 (2a)
In a Schlenk flask, to a solution of 3,6-di-tert-butyl-fluorene (2.17 g, 7.8 mmol) in THF (100 mL), was added n-BuLi (3.13 mL of a 2.5 M solution in hexane, 7.8 mmol). This solution was added dropwise to a solution of 1a (1.00 g, 3.9 mmol) in THF (100 mL) at −10 °C over 10 min. After completion of the addition, the reaction mixture was stirred for 2 days at room temperature. The mixture was hydrolyzed with 10% aqueous hydrochloric acid (20 mL), the organic phase was dried over sodium sulfate, and volatiles were evaporated in vacuo. The solid residues was washed with pentane (200 mL) and dried under reduced in vacuo to afford a white powder (1.96 g, 2.4 mmol, 62%). 1H NMR (CDCl3, 400 MHz, 25 °C) (mixtures of tautomers): δ 7.76 (m, 4H, CH-Ph), 7.66 (m, 4H, CH-Flu), 7.25–7.08 (m, 4H, CH-Flu), 7.01 (m, 1H, CH-Flu), 6.87 (t, 3J = 9.0, 1H, CH-Flu), 6.62 (t, 3J = 9.0, 1H, CH-Flu), 6.54–6.41 (m, 6H, CH-Flu + CH-Cp), 6.18 (m, 1H, CH-Cp), 4.96 (s, 2H, H-Flu), 3.19–2.99 (m, 4H, CH2-Cp), 1.38 (m, 36H, CH3-tBu), 1.12–1.07 (m, 6H, CH3-bridge). 13C NMR (CDCl3, 125 MHz, 25 °C): δ 156.4, 156.3, 156.2 (Cq-Cbridge), 153.8 (Cq), 150.2, 150.2, 150.1, 150.1 (Cq-C-tBu), 145.5, 145.4 (Cq), 142.8, 142.8, 142.6, 142.5, 142.3, 142.2 (Cq), 134.5, 134.4, 134.3 (CH-Flu), 133.9, 133.8 (CH-Flu), 132.2, 131.9 (CH-Cp), 127.8, 127.8, 127.7, 127.5 (CH-Cp), 126.3 (CH-Cp), 125.7, 125.6, 125.5 (CH-Cp), 123.7, 123.6, 123.5, 123.4 (CH-Flu), 115.9, 115.9 (CH-Ph), 68.1 (Cq), 55.4, 55.4, 55.3 (CH), 54.0, 53.9 (CH), 47.5, 47.4 (Cq), 46.4, 46.3 (Cq), 42.1, 41.0 (CH2-Cp), 34.9, 34.8 (Cq), 31.8 (CH3-tBu). ESI-MS (m/z): 815.55 ([M + H]+), 814.54 ([M]). Anal. calcd. for C62H70 (815.22): C 91.35, H 8.65; found: C 91.68, H 8.78.
3.8. 1,4-Ph((H)C-(3,6-tBu2FluH)(CpH))2 (2b)
Using a protocol similar to that described above for 2a, compound 2b was prepared from 3,6-di-tert-butyl-fluorene (4.83 g, 17.4 mmol), n-BuLi (7.0 mL of a 2.5 M solution in hexane, 17.4 mmol), 1b (2.00 g, 8.7 mmol), and isolated as a white powder (4.12 g, 5.2 mmol, 60%). 1H NMR (CDCl3, 400 MHz, 25 °C) (mixture of tautomers): δ 7.72 (m, 4H, CH-Ar), 7.10 (m, 8H, CH-Cp), 7.02 (m, 1H, CH-Cp), 6.88 (m, 1H, CH-Cp), 6.7 (m, 3H, CH-Cp), 6.45 (m, 2H, CH-Cp), 6.33 (m, 1H, CH-Cp), 6.18 (m, 1H, CH-Cp), 5.96 (m, 1H, CH-Cp), 4.52 (m, 2H, CH-Flu), 4.03 (m, 2H, CH-bridge), 2.96 (m, 3H, CH2-Cp), 1.36 (s, 36H, CH3-tBu). 13C NMR (CDCl3, 125 MHz, 25 °C): δ 150.87, 150.69, 150.25, 150.21, 150.19, 150.17, 150.15, 150.13, 150.11, 150.09, 150.05 (Cq-C-tBu), 148.39, 148.34 (Cq), 143.57, 143.55, 143.44, 143.40, 143.37, 143.32 (Cq), 141.69, 141.65, 141.48, 141.45, 141.42, 141.39 (Cq), 140.67 (Cq), 134.50, 134.47, 134.41 (CH-Flu), 133.89, 133.81 (CH-Flu), 132.42, 131.43, 131.40, 131.37 (CH-Cp), 129.25, 129.22, 129.19, 129.16, 128.96 (CH-Cp), 128.90, 128.87, 128.82, 128.80, 128.72, 128.67, 128.62 (CH-Cp), 125.45, 125.33, 125.31, 125.28, 125.23, 125.19, 125.16 (CH-Cp), 123.64, 123.61, 123.57, 123.55, 123.51, 123.46, 123.41 (CH-Flu), 116.12, 116.07 (CH-Ph), 51.50, 51.46, 51.44, 51.22, 51.14, 51.12 (CH-bridge), 50.44, 50.39, 50.30, 50.20, 50.16 (CH), 43.15, 43.12, 41.14, 41.12 (CH2-Cp), 34.90, 34.88 (Cq), 31.80 (CH3-tBu). Mp: 240 °C. ESI-MS (m/z): 787.52 ([M + H]+), 786.51 ([M]). Anal. calcd. for C60H66 (787.17): C 91.55, H 8.45; found: C 91.68, H 8.90.
3.9. 1,3-Ph(MeC-(3,6-tBu2FluH)(CpH))2 (2c)
Using a protocol similar to that described above for 2a, compound 2c was prepared from 3,6-di-tert-butyl-fluorene (2.17 g, 7.8 mmol), n-BuLi (3.13 mL of a 2.5 M solution in hexane, 7.8 mmol), and 1c (1.00 g, 3.9 mmol). The final product 2c was isolated as a white powder (469 mg, 0.58 mmol, 15%). 1H NMR (CDCl3, 500 MHz, 25 °C): δ 7.80 (m, 1H, CH-Flu), 7.72–7.64 (m, 4H, CH-Ph), 7.53–7.30 (m, 4H, CH-Flu), 7.11 (m, 2H, CH-Flu), 7.04–6.99 (m, 1H, CH-Flu), 6.92–6.70 (m, 4H, CH-Flu), 6.49–6.34 (m, 4H, CH-Cp), 6.26 (m, 1H, CH-Cp), 6.13–6.07 (m, 1H, CH-Cp), 4.84 (m, 2H, CH-Flu), 3.01 (m, 4H, CH2-Cp), 1.36–1.30 (s, 36H, CH3-tBu), 1.19–1.06 (m, 6H, CH3-bridge). 13C NMR (CDCl3, 125 MHz, 25 °C): δ 156.3, 156.2 (Cq-Cbridge), 153.9 (Cq), 150.2, 150.1, 150.0, 149.9 (Cq-C-tBu), 142.8, 142.4, 142.1, 142.1 (Cq), 134.5, 134.4, 134.3 (CH-Flu), 133.8, 133.6 (CH-Flu), 132.0, 131.9 (CH-Cp), 128.0, 127.9, 127.8 (CH-Cp), 126.4, 126.3 (CH-Cp), 125.9, 125.8, 125.7, 125.6, 125.5 (CH-Cp), 123.6, 123.6, 123.5, 123.4, 123.4 (CH-Flu), 115.9, 115.9, 115.8 (CH-Ph), 66.0 (Cq), 55.5, 55.4 (CH), 54.2, 54.1 (CH), 48.0, 47.9 (Cq), 47.1, 47.0 (Cq), 42.0, 41.9, 41.0 (CH2-Cp), 34.9, 34.8 (Cq), 31.7 (CH3-tBu). ESI-MS (m/z): 853.51 ([M + K]+), 837.54 ([M + Na]+), 815.55 ([M + H]+). Anal. calcd. for C62H70 (815.22): C 91.35, H 8.65; found: C 91.55, H 8.69.
3.10. PhMeC-(3,6-tBu2FluH)(CpH) (2a’)
Using a protocol similar to that described above for 2a, compound 2a’ was prepared from 3,6-di-tert-butyl-fluorene (0.97 g, 3.5 mmol), n-BuLi (1.4 mL of a 2.5 M solution in hexane, 3.5 mmol), and (1-(cyclopenta-2,4-dien-1-ylidene)ethyl)benzene (0.59 g, 3.5 mmol), and isolated as a white powder (0.65 g, 1.4 mmol, 40%). 1H NMR (CDCl3, 500 MHz, 25 °C): δ 7.75 (dd, J = 7.1, 2.0, 1H, CH-Flu), 7.72 (t, J = 2.2, 1H, CH-Flu), 7.63 (d, J = 7.8, 2H, CH-Ph), 7.39 (td, J = 7.5, 4.4, 2H, CH-Ph), 7.30 (tt, J = 7.2, 1.4, 1H, CH), 7.14 (ddd, J = 13.8, 8.1, 2.0, 1H, CH-Flu), 7.00–6.88 (m, 2H, CH-Flu), 6.71 (d, J = 8.2, 1H, CH-Cp), 6.55 6.48 (m, 2H, CH-Flu), 6.39 (dd, J = 5.3, 1.5, 1H, CH-Cp), 6.24–6.18 (m, 1H, CH-Cp), 6.00 (dd, J = 13.9, 8.1, 1H, CH-Cp), 4.91 (d, J = 3.5, 1H, CH-Flu), 3.06 (dd, J = 7.1, 1.6, 1H, CH2-Cp), 1.45–1.29 (m, 20H, CH3-tBu), 1.07 (m, 3H, CH3-bridge). 13C NMR (CDCl3, 125 MHz, 25 °C): δ 156.04 (Cq), 153.70 (Cq), 150.20, 150.14, 150.08, 150.06 (Cq), 147.74, 147.17 (Cq), 142.84, 142.79, 142.34, 142.31, 142.20, 142.19, 142.15, 142.08 (Cq), 134.18, 134.01 (CH), 132.07, 131.96 (CH), 128.38 (CH), 127.89, 127.65, 127.57 (CH), 126.47, 126.32, 126.27 (CH), 125.66, 125.47, 125.45, 125.31 (CH), 123.67, 123.61, 123.54, 123.45 (CH), 115.93, 115.87, 115.81, 115.79 (CH), 55.56, 54.30 (CH-Flu), 47.73, 46.59, 42.01, 40.98 (CH2-Cp), 34.89, 34.87, 34.81, 31.93, 31.87, 31.77, 31.72, 31.62 (CH3-tBu), 18.48, 18.40 (CH3). ESI-MS (m/z): 447.30 ([M + H]+), 277.19 ([3,6-di-tert-butyl-fluorene]+). Anal. calcd. for C34H38 (446.67): C 91.42, H 8.58; found: C 91.54, H 8.71.
3.11. Ph(H)C-(3,6-tBu2FluH)(CpH) (2b’)
Using a protocol similar to that described above for 2a, compound 2b’ was prepared from 3,6-di-tert-butyl-fluorene (4.70 g, 16.9 mmol), n-BuLi (6.8 mL of a 2.5 M solution in hexane, 16.9 mmol), and (cyclopenta-2,4-dien-1-ylidenemethyl)benzene (2.60 g, 16.9 mmol), and isolated as a white powder (0.70 g, 1.62 mmol, 10%). 1H NMR (CDCl3, 400 MHz, 25 °C): δ 7.71 (m, 2H, CH-Flu), 7.24–7.12 (m, 6H, CH-Ph + CH-Flu), 7.04 (m, 1H, CH-Flu), 6.89 (d, J = 8.1, 1H, CH-Flu), 6.68–6.01 (m, 4H, CH-Cp), 4.54 (m, 1H, CH-bridge), 4.06 (m, 1H, CH-Flu), 3.12–2.89 (m, 2H, CH2-Cp), 1.39–1.32 (m, 18H, CH3-tBu). 13C NMR (CDCl3, 125 MHz, 25 °C): 150.72, 150.22, 150.15, 150.09 (Cq), 148.38 (Cq), 143.51, 143.22, 142.76 (Cq), 141.45, 141.39 (Cq), 134.34, 134.04 (CH), 132.42, 131.47 (CH), 129.28, 129.09, 129.04, 128.64, 128.54, 128.28, 128.21 (CH), 126.52, 125.34, 125.28, 125.20, 125.13 (CH), 123.68, 123.62, 123.56 (CH), 116.13, 116.09, 116.06 (CH), 51.54, 51.48, 50.61, 50.40 (CH), 43.16, 41.18 (CH2-Cp), 34.91, 34.88, 31.88, 31.78, 31.76 (CH3-tBu). ESI-MS (m/z): 433.29 ([M + H]+). Anal. calcd. for C33H36 (432.64): C 91.61, H 8.39; found: C 91.92, H 8.84.
3.12. 1,4-Ph{MeC-(3,6-tBu2Flu)(Cp)ZrCl2}2 (3a-Zr)
To a solution of 2a (0.500 g, 0.61 mmol) in diethyl ether (50 mL) was added under stirring n-BuLi (0.98 mL of a 2.0 M solution in hexane, 2.45 mmol). The solution was kept overnight at room temperature. ZrCl4 (0.286 g, 1.23 mmol) was added to the reaction mixture with a bent finger. The resulting red mixture was stirred at room temperature overnight. Then, the mixture was evaporated in vacuo and CH2Cl2 (20 mL) was added. The resulting solution was filtered, and volatiles were evaporated in vacuo to give a red powder (0.528 g, 0.46 mmol, 76%). 1H NMR (CD2Cl2, 500 MHz, 25 °C): δ 8.20–8.10 (m, 3H, CH-Flu), 8.01–7.88 (4H, CH-Flu), 7.87–7.66 (4H, CH-Flu + CH-Ph), 7.58–7.49 (2H, CH-Ph), 7.40–7.06 (4H, 2H, CH-Flu), 6.63–6.56 (1H, CH-Cp), 6.46–6.28 (4H, CH-Cp), 6.00–5.77 (3H, CH-Cp), 2.68–2.57 (m, 6H, CH3), 1.52–1.43 (d, 36H, CH3-tBu). 13C NMR (CD2Cl2, 125 MHz, 25 °C): δ 158.3 (Cq-tBu), 150.3 (Cq-tBu), 149.7 (Cq-Ph), 145.3 (Cq-Flu), 129.6–128.8 (CH-Ph), 128.1 (Cq-Flu), 127.1 (CH-Flu), 126.8 (CH-Flu), 126.2 (Cq-Flu), 126.4 (CH-Flu), 125.8 (CH-Flu), 123.2 (CH-Cp), 123.1 (CH-Cp), 122.7 (CH-Cp), 120.3 (CH-Cp), 119.8 (CH-Cp), 117.5 (CH-Cp), 112.4 (Cq-Cp), 103.7–103.5 (CH-Cp), 102.5 (CH-Cp), 102.4 (CH-Cp), 77.4 (C-Flu), 53.8–53.4 (CH3-bridge), 31.7–30.8 (CH3-tBu). APPI-MS (m/z): 1030.20 ([M]+), 970.36 ([M–ZrCl2]+), 810.51 ([M–Zr2Cl4]+). Anal. calcd for C62H66Cl4Zr2 (1135,45): C 65.58, H 5.86; found: C 65.42, H 6.00.
3.13. 1,4-Ph{MeC-(3,6-tBu2Flu)(Cp)HfCl2}2 (3a-Hf)
Using a protocol similar to that described above for 3a-Zr, compound 3a-Hf was prepared from 1,4-bis(cyclopenta-2,4-dien-1-yl(3,6-di-tert-butyl-fluoren-9-yl)ethyl)benzene (0.500 g, 0.61 mmol), n-BuLi (0.98 mL of a 2.5 M solution in hexane, 2.45 mmol), and HfCl4 (0.380 g, 1.23 mmol). The compound was recovered as an orange-yellow powder (0.520 g, 0.38 mmol, 62%). 1H NMR (toluene-d8, 500 MHz, 25 °C): δ 8.33–8.10 (m, 4H, CH-Flu), 8.00 (s, 2H, CH-Flu), 7.77–7.23 (m, 7H, CH-Flu + CH-Ph), 6.79 (d, J = 9.2, 1H, CH-Flu), 6.34 (d, J = 9.3, 1H, CH-Flu), 6.17–5.85 (m, 4H, CH-Cp), 5.65–5.30 (m, 4H, CH-Cp), 2.30 (s, 6H, CH3), 1.70–1.17 (m, 36H, CH3-tBu). 13C NMR (C6D6, 125 MHz, 25 °C): δ 150.02, 149.75, 149.71, 146.71, 132.18 (Cq), 130.29, 129.93, 129.53, 129.40, 129.22, 128.76, 128.55, 128.45, 128.36, 128.25, 128.16, 128.06, 127.72, 127.43, 127.16, 126.79, 126.43, 125.25, 125.02, 123.49, 123.19, 122.97, 122.61, 122.47, 122.43, 122.34, 121.41, 121.20, 120.39, 120.16, 119.88, 119.74, 119.34, 116.98, 116.79, 115.48, 114.77, 101.70, 101.51, 100.27, 79.57, 78.89 (C-Flu), 49.95, 49.64 (CH3), 35.46, 35.42, 35.37, 35.32, 35.27, 35.11, 32.21, 32.18, 32.05, 32.02, 32.00, 31.97, 31.96, 31.47, 29.92, 29.78, 27.45 (CH3-tBu). APPI-MS (m/z): 1310.3046 ([M]+•), 1062.4287 ([M–HfCl2]+). Anal. calcd for C62H66Cl4Hf2 (1310.2850): C 56.85, H 5.08; found: C 56.73, H 5.16.
3.14. 1,4-Ph{(H)C-(3,6-tBu2Flu)(Cp)ZrCl2}2 (3b-Zr)
Using a protocol similar to that described above for 3a-Zr, compound 3b-Zr was prepared from 2b (0.660 g, 0.84 mmol), n-BuLi (1.37 mL of a 2.0 M solution in hexane, 3.37 mmol), and ZrCl4 (0.392 g, 1.68 mmol). The compound was isolated as a red powder (0.350 g, 0.32 mmol, 38%). 1H NMR (CD2Cl2, 500 MHz, 25 °C): δ 8.16 (d, 3J = 4.0, 2H, CH-Flu), 7.98 (s, 4H, CH-Ph), 7.67 (m, 4H, CH-Flu), 7.27 (dd, 3J = 9.0, 4J = 1.6, 2H, CH-Flu), 6.69 (d, 3J = 9.0, 2H, CH-Flu), 6.64 (s, 2H, CH-ansa), 6.36 (dq, 3J = 2.5, 4H, CH-Cp), 5.84 (dq, 3J = 2.5, 4H, CH-Cp), 1.47 (d, 36H, CH3-tBu). 13C NMR (CD2Cl2, 125 MHz, 25 °C): δ 150.4 (C-Flu-tBu), 137.6 (Cq), 128.2 (CH-Ph), 127.8 (CH-Ph), 127.5 (CH-Flu), 122.8 (Cq), 121.9 (CH-Flu), 120.3 (Cq), 119.6 (CH-Flu), 119.6 (CH-Cp), 119.5 (CH-Cp), 117.6 (CH-Cp), 106.2 (Cq), 104.9 (CH-Cp), 101.9 (CH-Cp), 73.9 (C-Flu), 41.7 (CH-bridge), 31.2 (CH3-tBu). APPI-MS (m/z): 1102.1766 ([M]+•), 942.3300 ([M–ZrCl2]+), 782.8 ([M–Zr2Cl4]+). Anal. calcd. for C60H62Cl4Zr2 (1102.16997): C 65.08, H 5.64; found: C 65.75, H 6.01.
3.15. 1,3-Ph{MeC-(3,6-tBu2Flu)(Cp)ZrCl2}2 (3c-Zr)
Using a protocol similar to that described above for 3a-Zr, compound 3c-Zr was prepared from 2c (0.520 g, 0.64 mmol), n-BuLi (1.0 mL of a 2.5 M solution in hexane, 2.55 mmol), and ZrCl4 (0.300 g, 1.27 mmol). The product was isolated as a red powder (0.630 g, 0.56 mmol, 87%). 1H NMR (C6D6, 500 MHz, 25 °C): δ 8.43–8.23 (m, 4H, CH-Flu), 8.05 (m, 2H, CH-Flu), 7.70 (dt, J = 7.8, 1.4, 1H, CH-Flu), 7.63–7.25 (m, 6H, CH-Flu + CH-Ph), 7.06–6.90 (m, 2H, CH-Ph), 6.47 (d, J = 9.1, 1H, CH-Ph), 6.28–6.23 (m, 1H, CH-Flu), 6.23–6.15 (m, 1H, CH-Cp), 6.13–6.04 (m, 1H, CH-Cp), 5.90–5.84 (m, 1H, CH-Cp), 5.74 (m, 1H, CH-Cp), 5.67–5.57 (m, 2H, CH-Cp), 5.55–5.36 (m, 1H, CH-Cp), 5.14 (d, J = 2.7, 1H, CH-Cp), 2.55–2.13 (m, 6H, CH3), 1.51–1.29 (m, 36H, CH3-tBu). 13C NMR (C6D6, 125 MHz, 25 °C): δ 150.36, 150.10, 150.00, 149.79, 148.63, 148.55 (Cq), 131.99, 130.20, 129.34, 129.25, 129.19, 127.55, 127.27, 127.05, 126.53, 124.83, 124.53, 124.50, 123.91, 123.86, 123.84, 123.60, 123.52, 123.27, 123.22, 123.19, 123.00, 122.88, 122.85, 122.77, 122.60, 122.56, 120.56, 120.31, 120.21, 120.13, 120.10, 120.05, 119.90, 119.73, 117.86, 117.77, 117.30, 112.98, 112.63, 112.46, 104.92, 103.75, 103.57, 102.37, 101.31, 101.26, 79.19, 78.67, 78.63 (C-Flu), 50.49, 50.18, 50.09 (CH3-bridge), 35.22, 35.18, 35.16, 35.08, 32.00, 31.97, 31.95, 31.92, 31.85, 31.78, 31.73, 31.70, 31.36, 30.95, 29.73 (CH3-tBu), 18.14. APPI-MS (m/z): 1130.2240. Anal. calcd for C62H66Cl4Zr2 (1130.2013): C 65.53, H 5.94; found: C 66.03, H 6.64.
3.16. {Ph(Me)C-(3,6-tBu2Flu)(Cp)}ZrCl2 (3a’-Zr)
Using a protocol similar to that described above for 3a-Zr, compound 3b-Zr was prepared from 2a’ (0.400 g 0.89 mmol), n-BuLi (0.72 mL of a 2.5 M solution in hexane, 1.79 mmol), and ZrCl4 (0.209 g, 0.89 mmol). The compound was isolated as a red powder (0.410 g, 0.67 mmol, 76%). 1H NMR (toluene-d8, 500 MHz, 25 °C): δ 8.07 (ddd, J = 7.0, 1.9, 0.7, 2H, CH-Flu), 7.43 (dt, J = 7.8, 1.7, 1H, CH-Ph), 7.39 (dd, J = 9.3, 0.8, 1H, CH-Flu), 7.24 (dt, J = 7.8, 1.7, 1H, CH-Ph), 7.22–7.16 (m, 2H, CH-Ph), 7.08–6.96 (m, 3H, CH-Flu), 6.88 (m, 1H, CH-Cp), 6.65 (m, 1H, CH-Cp), 6.12–5.76 (m, 2H, CH-Cp), 5.32 (t, J = 2.7, 2H), 2.01 (s, 3H, CH3-bridge), 1.25 (m, 18H, CH3-tBu). 13C NMR (toluene-d8, 125 MHz, 25 °C): δ 150.05 (C-Flu-tBu), 149.74 (C-Flu-tBu), 147.28 (Cq-Ph), 129.45, 129.19 (CH-Ph), 128.25 (CH-Flu), 127.29 (CH-Ph), 127.23 (CH-Flu), 125.72 (CH-Cp), 125.41 (CH-Cp), 123.65, 123.55, 123.25 (CH-Cp), 122.93, 122.68 (CH-Cp), 120.25 (CH-Cp), 119.94, 119.39, 117.46 (CH-Flu), 112.95 (Cq-Cp), 103.58, 102.30 (CH-Cp), 78.99 (C-Flu), 49.94 (Cq-tBu), 35.15, 35.10 (Cq-tBu), 31.77, 31.07 (CH3-tBu). APPI-MS (m/z): 604.1239 ([M]+•), 444.2664 ([M–ZrCl2]+. Anal. calcd for C34H36Cl2Zr (604.1241): C 67.30, H 5.98; found: C 67.82, H 6.09.
3.17. {Ph(H)C-(3,6-tBu2Flu)(Cp)}ZrCl2 (3b’-Zr)
Using a protocol similar to that described above for 3a-Zr, compound 3b’-Zr was prepared from 2b’ (0.430 g, 0.99 mmol), n-BuLi (0.81 mL of a 2.5 M solution in hexane, 1.99 mmol), and ZrCl4 (0.230 g, 0.99 mmol). The product was isolated as a red powder (0.540 g, 0.86 mmol, 87%). 1H NMR (C6D6, 500 MHz, 25 °C): δ 8.24 (s, 2H, CH-Flu), 7.55 (d, J = 7.4, 2H, CH-Ph), 7.42 (d, J = 9.0, 1H, CH-Ph), 7.24 (m, 3H, CH-Ph), 6.99 (d, J = 8.8, 1H, CH-Flu), 6.92 (d, J = 9.1, 1H, CH-Flu), 6.51 (d, J = 9.0, 1H, CH-Flu), 6.17–6.05 (m, 2H, CH-Flu), 5.80 (s, 1H, CH), 5.48 (t, J = 2.8, 1H, CH-Cp), 5.29 (t, J = 2.8, 1H, CH-Cp), 1.39 (s, 18H, CH3-tBu). 13C NMR (C6D6, 125 MHz, 25 °C): δ 150.08 (C-Flu-tBu), 149.89 (C-Flu-tBu), 138.64 (C-Flu), 128.78, 128.38 (C-tBu), 127.96, 127.21 (CH-Flu), 122.77, 122.31, 122.24, 121.54 (CH-Cp), 119.95, 119.76, 119.74, 119.51, 117.01 (CH-Flu), 106.86, 105.16, 101.87 (CH-Cp), 73.63 (C-Flu), 41.81 (Cq), 34.98, 34.85 (Cq), 31.57, 31.49 (CH3-tBu). APPI-MS (m/z): 590.1115 ([M]+•), 430.2659 ([M–ZrCl2]+. Anal. calcd for C33H34Cl2Zr (590.1085): C 66.87, H 5.78; found: C 67.11, H 5.97.
3.18. {Ph(H)C-(3,6-tBu2Flu)(Cp)}HfCl2 (3b’-Hf)
This compound was prepared as described above for 3a starting from 2b’ (0.090 g, 0.20 mmol), n-BuLi (0.16 mL of a 2.5 M solution in hexane, 0.41 mmol), and HfCl4 (0.060 g, 0.2 mmol). The product was isolated as an orange powder (0.076 g, 0.86 mmol, 56%). 1H NMR (C6D6, 500 MHz, 25 °C): δ 8.22 (dt, J = 9.0, 1.9, 2H, CH-Flu), 7.56 (dt, J = 8.0, 1.4, 2H, CH-Flu), 7.40 (dd, J = 9.0, 1.7, 1H, CH-Ph), 7.28–7.20 (m, 3H, CH-Ph), 7.04 (d, J = 9.0, 1H), 6.91 (dd, J = 9.1, 1.8, 1H, CH-Ph), 6.57 (dd, J = 9.1, 1.8, 1H, CH-Ph), 6.09–6.00 (m, 2H, CH-Flu), 5.85 (s, 1H, CH-Cp), 5.44 (q, J = 2.7, 1H, CH-Cp), 5.25 (q, J = 2.8, 1H, CH-Cp), 1.39 (s, 18H, CH3-tBu). 13C NMR (C6D6, 125 MHz, 25 °C): δ 149.80, 149.60 (Cq), 139.28, 122.45, 121.64, 121.52, 120.32, 119.96, 119.92, 119.42, 118.78, 116.70, 116.43, 110.06, 103.15, 99.85, 74.22 (C-Flu), 42.10, 35.32, 35.18, 34.90, 34.84, 31.94, 31.85, 31.83, 31.79, 31.74, 31.70, 31.64, 31.52 (CH3-tBu). APPI-MS (m/z): 680.1497 ([M]+•), 430.2659 ([M–HfCl2]+), 278.2035 (C21H26, [3,6-di-tert-butyl-fluorene + H]+•). Anal. calcd. for C33H34Cl2Hf (680.1503): C 58.29, H 5.04; found: C 58.55, H 5.26.
3.19. Ethylene Homopolymerization and Ethylene/1-hexene Copolymerization
Polymerization experiments were performed in a 300 mL high-pressure glass reactor equipped with a mechanical stirrer (Pelton turbine) and externally heated with a double mantle with a circulating water bath. The reactor was filled with toluene (100 mL), 1-hexene comonomer (when relevant; typically 2.5 mL), and MAO (0.20 mL of a 30 wt-% solution in toluene) and pressurized at 5.5 bar of ethylene (Air Liquide, 99.99%). The reactor was thermally equilibrated at the desired temperature for 30 min, the ethylene pressure was decreased to 1 bar, and a solution of the catalyst precursor in toluene (ca. 2 mL) was added by syringe. The ethylene pressure was immediately increased to 5.5 bar (kept constant with a back regulator), and the solution was stirred for the desired time (typically 15 min). The temperature inside the reactor (typically 60 °C) was monitored using a thermocouple. The polymerization was stopped by venting the vessel and quenching with a 10% HCl solution in methanol (ca. 2 mL). The polymer was precipitated in methanol (ca. 200 mL), and 35% aqueous HCl (ca. 1 mL) was added to dissolve possible catalyst residues. The polymer was collected by filtration, washed with methanol (ca. 200 mL), and dried under vacuum overnight.
3.20. Crystal Structure Determination of 3a’-Zr
Diffraction data were collected at 150 K using a Bruker APEX CCD diffractometer (Bruker, Billerica, MA, USA) with graphite-monochromated MoKα radiation (λ = 0.71073 Ǻ). A combination of ω- and φ-scans was carried out to obtain at least a unique data set. The crystal structures were solved by direct methods, and the remaining atoms were located from a difference Fourier synthesis followed by full-matrix least-squares refinement based on F2 (programs SIR97 [43] and SHELXL-97 [44]). Hydrogen atoms were placed at calculated positions and forced to ride on the attached atom. All non-hydrogen atoms were refined with anisotropic displacement parameters. The locations of the largest peaks in the final difference Fourier map calculation as well as the magnitude of the residual electron densities were of no chemical significance. The main crystal and refinement data are summarized in Table S1. Crystal data, details of data collection, and structure refinement for compound 3a’-Zr (CCDC 1863222) can be obtained from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
3.21. Computational Details
All calculations were performed with the TURBOMOLE program package using density functional theory (DFT) [45,46,47,48]. The gradient-corrected density functional BP86 in combination with the resolution identity approximation (RI) [49,50] was applied for the geometry optimizations of a stationary point. The triple-ζ zeta valence quality basis set def-TZVP was used for all atoms [51].
The stationary points were characterized as energy minima (no negative Hessian eigenvalues) by vibrational frequency calculations at the same level of theory.
4. Conclusions
The synthesis of original bis(Cp/Flu) ligand systems linked at the C1-bridge through a phenylene group was developed starting from difulvene precursors. These ligand platforms were utilized for the preparation of homodinuclear zirconium and hafnium bis(dichloro ansa-metallocene) complexes via a regular salt-metathesis metallation protocol. The synthesis of a heterodinuclear zirconium/hafnium bis(dichloro ansa-metallocene) was also performed, although the desired product was generated as a statistical mixture with the corresponding homodinuclear complexes. For the first time, an advanced APPI mass-spectrometric method was applied to the characterization of dinuclear bis(ansa-metallocene) complexes and their mononuclear ansa-metallocene analogues, and relevant data were obtained.
Ethylene homopolymerization as well as ethylene/1-hexene copolymerization were conducted using the homodinuclear dichloro catalyst precursors, as well as with their mononuclear analogues, in combination with MAO. Limited cooperativity evidence has been observed with dinuclear systems so far, with, in some cases, slightly different molecular weights or a greater formation of short methyl and ethyl branches as compared to the mononuclear reference systems. The apparent lack of significant cooperative behavior observed for the dinuclear systems was substantiated by a computational analysis. Thus, the computed para- and meta-phenylene-bridged neutral dinuclear structures suggest that the two metallocenic fragments may orientate their coordination spheres in opposite directions, hence resulting in distant (>9 Å) isolated metal centers. Further investigations in our laboratories are focused on the elaboration of other polynuclear precatalysts with improved performances and identification of the nature of possible intermetallic cooperative effects.
Supplementary Materials
The following are available online at http://www.mdpi.com/2073-4344/8/11/558/s1, Figure S1: 1H NMR spectrum of 1a; Figure S2: 13C NMR spectrum of 1a; Figure S3: ASAP mass spectrum of 1a; Figure S4: 1H NMR spectrum of 1b; Figure S5: 13C NMR spectrum of 1b; Figure S6: 1H NMR spectrum of 1c; Figure S7: 13C NMR spectrum of 1c; Figure S8: ASAP mass spectrum of 1c; Figure S9: 1H NMR spectrum of 2a; Figure S10: 13C NMR spectrum of 2a; Figure S11: ASAP mass spectrum of 2a; Figure S12: 1H NMR spectrum of 2b; Figure S13: 13C NMR spectrum of 2b; Figure S14: ASAP mass spectrum of 2b; Figure S15: 1H NMR spectrum of 2c; Figure S16: 13C NMR spectrum of 2c; Figure S17: ASAP mass spectrum of 2c; Figure S18: 1H NMR spectrum of 2a’; Figure S19: 13C NMR spectrum of 2a’; Figure S20: ASAP mass spectrum of 2a’; Figure S21: 1H NMR spectrum of 2b’; Figure S22: 13C NMR spectrum of 2b’; Figure S23: ASAP mass spectrum of 2b’; Figure S24: 1H NMR spectrum of 3a-Zr2; Figure S25: HMBC spectrum of 3a-Zr2; Figure S26: HSQC spectrum of 3a-Zr2; Figure S27: APPI-IMMS of 3a-Zr2; Figure S28: 1H NMR spectrumof 3a-Hf2; Figure S29: 13C NMR spectrum of 3a-Hf2; Figure S30: APPI-IMMS of 3a-Hf2; Figure S31: 1H NMR spectrum of 3b-Zr2; Figure S32: HMBC spectrum of 3b-Zr2; Figure S33: HSQC spectrum of 3b-Zr2; Figure S34: APPI-IMMS of 3b-Zr2; Figure S35: 1H NMR spectrum of 3c-Zr2; Figure S36: 13C NMR spectrum of 3c-Zr2; Figure S37: 1H NMR spectrum of 3a’-Zr; Figure S38: 13C NMR spectrum of 3a’-Zr; Figure S39: APPI-IMMS of 3a’-Zr; Figure S40: 1H NMR spectrum of 3b’-Zr; Figure S41: 13C NMR spectrum of 3b’-Zr; Figure S42: APPI-IMMS of 3b’-Zr; Figure S43: 1H NMR spectrum of 3b’-Hf; Figure S44: 13C NMR spectrum of 3b’-Hf; Figure S45: APPI-IMMS of 3b’-Hf; Figure S46: 13C NMR spectrum of PE (Table 1, run 1); Figure S47: GPC trace of PE (Table 1, run 1) obtained with 3a-Zr2; Figure S48: 13C NMR spectrum of PE (Table 1, run 3); Figure S49: 13C NMR spectrum of PE (Table 1, run 5); Figure S50: GPC trace of PE (Table 1, run 5) obtained with 3b-Zr2; Figure S51: GPC trace of PE (Table 1, run 6) obtained with 3b-Zr2; Figure S52: GPC trace of PE (Table 1, run 7) obtained with 3b’-Zr; Figure S53: GPC trace of PE (Table 1, run 8) obtained with 3b’-Zr; Figure S54. GPC trace of PE (Table 1, run 9) obtained with 3c-Zr2; Figure S55: GPC trace of PE (Table 1, run 12) obtained with 3b’-Hf; Figure S57: GPC trace of PE/PHex (Table 2, run 1) obtained with 3a-Zr2; Figure S58: 13C NMR spectrum of PE/PHex (Table 2, run 4); Figure S59: 13C NMR spectrum of PE/PHex (Table 2, run 5); Figure S60: GPC trace of PE/PHex (Table 2, run 5) obtained with 3b-Zr2; Figure S61: GPC trace of PE/PHex (Table 2, run 6) obtained with 3b-Zr2; Figure S62. GPC trace of PE/PHex (Table 2, run 7) obtained with 3b’-Zr; Figure S63: GPC trace of PE/PHex (Table 2, run 8) obtained with 3b’-Zr; Figure S64: GPC trace of PE/PHex (Table 2, run 9) obtained with 3c-Zr2; Figure S65: GPC trace of PE/PHex (Table 2, run 10) obtained with 3a-Hf2; Figure S66: GPC trace of PE/PHex (Table 2, run 11) obtained with 3a-Hf2; Figure S67: Molecular structure of 3a’-Zr; Table S1: Summary of Crystal and Refinement Data for Compound 3a’-Zr; Figure S68: DFT-optimized structures of Cs-symmetric and Ci-symmetric isomers of 3a-Zr2; Figure S69: DFT-optimized structures of Cs-symmetric and C1-symmetric isomers of 3c-Zr2.
Author Contributions
G.S. (investigation), M.F. (investigation), L.B. (investigation), A.V. (conceptualization, project administration), A.W. (conceptualization), J.-M.B. (project administration), C.A. (data analysis), P.G. (data analysis), J.-F.C. (conceptualization, supervision, writing—review and editing), E.K. (conceptualization, investigation, supervision, writing—original draft preparation, writing—review and editing).
Funding
This research received no external funding.
Acknowledgments
This work was supported by Total S. A. and Total Research and Technologies Feluy and Gonfreville (postdoctoral and PhD grants, respectively, to G.S. and M.F.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Delferro, M.; Marks, T.J. Multinuclear Olefin Polymerization Catalysts. Chem. Rev. 2011, 111, 2450–2485. [Google Scholar] [CrossRef] [PubMed]
- McInnis, J.P.; Delferro, M.; Marks, T.J. Multinuclear Group 4 Catalysis: Olefin Polymerization Pathways Modified by Strong Metal–Metal Cooperative Effects. Acc. Chem. Res. 2014, 47, 2545–2557. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kim, S.K.; Do, Y. Biphenylene-Bridged Dinuclear Group 4 Metal Complexes: Enhanced Polymerization Properties in Olefin Polymerization. Organometallics 2005, 24, 3618–3620. [Google Scholar] [CrossRef]
- Soga, K.; Ban, H.T.; Uozumi, T. Synthesis of a dinuclear ansa-zirconocene catalyst having a biphenyl bridge and application to ethene polymerization. J. Mol. Catal. A Chem. 1998, 128, 273–278. [Google Scholar] [CrossRef]
- Deckers, P.J.W.; Hessen, B.; Teuben, J.H. Catalytic Trimerization of Ethene with Highly Active Cyclopentadienyl−Arene Titanium Catalysts. Organometallics 2002, 21, 5122–5135. [Google Scholar] [CrossRef]
- Lee, H.W.; Ahn, A.H.; Park, Y.H. Copolymerization characteristics of homogeneous and in situ supported [(CH2)5(C5H4)2][(C9H7)ZrCl2]2 catalyst. J. Mol. Catal. A Chem. 2003, 194, 19–28. [Google Scholar] [CrossRef]
- Noh, S.K.; Kim, J.; Jung, J.; Ra, C.S.; Lee, D.H.; Lee, H.B.; Lee, S.W.; Huh, W.S. Syntheses of polymethylene bridged dinuclear zirconocenes and investigation of their polymerisation activities. J. Organomet. Chem. 1999, 580, 90–97. [Google Scholar] [CrossRef]
- Noh, S.K.; Kim, S.; Yang, Y.; Lyoo, W.S.; Lee, D.H. Preparation of syndiotactic polystyrene using the doubly bridged dinuclear titanocenes. Eur. Polym. J. 2004, 40, 227–235. [Google Scholar] [CrossRef]
- Kuwabara, J.; Takeuchi, D.; Osakada, K. Zr/Zr and Zr/Fe Dinuclear Complexes with Flexible Bridging Ligands. Preparation by Olefin Metathesis Reaction of the Mononuclear Precursors and Properties as Polymerization Catalysts. Organometallics 2005, 24, 2705–2712. [Google Scholar] [CrossRef]
- Xiao, X.; Sun, J.; Li, X.; Li, H.; Wang, Y. Binuclear titanocenes linked by the bridge combination of rigid and flexible segment: Synthesis and their use as catalysts for ethylene polymerization. J. Mol. Catal. A Chem. 2007, 267, 86–91. [Google Scholar] [CrossRef]
- Li, H.; Stern, C.L.; Marks, T.J. Significant Proximity and Cocatalyst Effects in Binuclear Catalysis for Olefin Polymerization. Macromolecules 2005, 38, 9015–9027. [Google Scholar] [CrossRef]
- Li, L.; Metz, M.V.; Li, H.; Chen, M.-C.; Marks, T.J.; Liable Sands, L.; Rheingold, A.L. Catalyst/Cocatalyst Nuclearity Effects in Single-Site Polymerization. Enhanced Polyethylene Branching and α-Olefin Comonomer Enchainment in Polymerizations Mediated by Binuclear Catalysts and Cocatalysts via a New Enchainment Pathway. J. Am. Chem. Soc. 2002, 124, 12725–12741. [Google Scholar] [CrossRef] [PubMed]
- Salata, M.R.; Marks, T.J. Catalyst Nuclearity Effects in Olefin Polymerization. Enhanced Activity and Comonomer Enchainment in Ethylene + Olefin Copolymerizations Mediated by Bimetallic Group 4 Phenoxyiminato Catalysts. Macromolecules 2009, 42, 1920–1933. [Google Scholar] [CrossRef]
- Spaleck, W.; Küber, F.; Bachmann, B.; Fritze, C.; Winter, A. New bridged zirconocenes for olefin polymerization: Binuclear and hybrid structures. J. Mol. Catal. A Chem. 1998, 128, 279–287. [Google Scholar] [CrossRef]
- Salata, M.R.; Marks, T.J. Synthesis, Characterization, and Marked Polymerization Selectivity Characteristics of Binuclear Phenoxyiminato Organozirconium Catalysts. J. Am. Chem. Soc. 2008, 130, 12–13. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.K.; Byun, G.G.; Lee, C.S.; Lee, D.; Yoon, K.B.; Kang, K.S. Synthesis, characterization, and reactivities of the polysiloxane-bridged binuclear metallocenes tetramethyldisiloxanediylbis(cyclopentadienyltitanium trichloride) and hexamethyltrisiloxanediylbis(cyclopentadienyltitanium trichloride). J. Organomet. Chem. 1996, 518, 1–6. [Google Scholar] [CrossRef]
- Lee, D.H.; Yoon, K.B.; Lee, E.H.; Noh, S.K.; Byun, G.G.; Lee, C.S. Polymerizations of ethylene and styrene initiated with trisiloxane-bridged dinuclear titanium metallocene/MMAO catalyst systems. Macromol. Rapid Commun. 1995, 16, 265–268. [Google Scholar] [CrossRef]
- Linh, N.T.B.; Huyen, N.T.D.; Noh, S.K.; Lyoo, W.S.; Lee, D.H.; Kim, Y. Preparation of new dinuclear half-titanocene complexes with ortho- and meta-xylene linkages and investigation of styrene polymerization. J. Organomet. Chem. 2009, 694, 3438–3443. [Google Scholar] [CrossRef]
- Noh, S.K.; Jung, W.; Oh, H.; Lee, Y.R.; Lyoo, W.S. Synthesis and styrene polymerization properties of dinuclear half-titanocene complexes with xylene linkage. J. Organomet. Chem. 2006, 691, 5000–5006. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Zhang, H.; Xiao, X.; Lin, F. Ethylene polymerization by novel phenylenedimethylene bridged homobinuclear titanocene/MAO systems. Eur. Polym. J. 2005, 41, 1519–1524. [Google Scholar] [CrossRef]
- Alt, H.; Samuel, E. Fluorenyl complexes of zirconium and hafnium as catalysts for olefin polymerization. Chem. Soc. Rev. 1998, 27, 323–329. [Google Scholar] [CrossRef]
- Alt, H. From the lab bench to the plant: How to commercialize a metallocene catalyst? Macromol. Chem. Symp. 2001, 173, 65–76. [Google Scholar] [CrossRef]
- Razavi, A. Site selective ligand modification and tactic variation in polypropylene chains produced with metallocene catalysts. Coord. Chem. Rev. 2006, 250, 155–169. [Google Scholar] [CrossRef]
- Alt, H.G.; Köppl, A. Effect of the Nature of Metallocene Complexes of Group IV Metals on Their Performance in Catalytic Ethylene and Propylene Polymerization. Chem. Rev. 2000, 100, 1205–1222. [Google Scholar] [CrossRef] [PubMed]
- Coates, G.W. Precise Control of Polyolefin Stereochemistry Using Single-Site Metal Catalysts. Chem. Rev. 2000, 100, 1223–1252. [Google Scholar] [CrossRef] [PubMed]
- Resconi, L.; Cavallo, L.; Falt, A.; Piemontesi, F. Selectivity in Propene Polymerization with Metallocene Catalysts. Chem. Rev. 2000, 100, 1253–1346. [Google Scholar] [CrossRef] [PubMed]
- Gladysz, J.A. Frontiers in Metal-Catalyzed Polymerization: Designer Metallocenes, Designs on New Monomers, Demystifying MAO, Metathesis Déshabillé. Chem. Rev. 2000, 100, 1167–1168. [Google Scholar] [CrossRef] [PubMed]
- Price, C.J.; Irwin, L.J.; Aubry, D.A.; Miller, S.A. Fluorenyl Containing Catalysts for Stereoselective Propylene Polymerization. In Stereoselective Polymerization with Single Site Catalysts; Baugh, L.S., Canich, J.A.M., Eds.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Kirillov, E.; Marquet, N.; Razavi, A.; Belia, V.; Hampel, F.; Roisnel, T.; Gladysz, J.A.; Carpentier, J.-F. New C1-Symmetric Ph2C-Bridged Multisubstituted ansa-Zirconocenes for Highly Isospecific Propylene Polymerization: Synthetic Approach via Activated Fulvenes. Organometallics 2010, 29, 5073–5082. [Google Scholar] [CrossRef]
- Kirillov, E.; Marquet, N.; Bader, M.; Razavi, A.; Belia, V.; Hampel, F.; Roisnel, T.; Gladysz, J.A.; Carpentier, J.-F. Chiral-at-ansa-Bridged Group 4 Metallocene Complexes {(R1R2C)-(3,6-tBu2Flu)(3-R3-5-Me-C5H2)}MCl2: Synthesis, Structure, Stereochemistry, and Use in Highly Isoselective Propylene Polymerization. Organometallics 2011, 30, 263–272. [Google Scholar] [CrossRef]
- Bader, M.; Marquet, N.; Kirillov, E.; Roisnel, T.; Razavi, A.; Lhost, O.; Carpentier, J.-F. Old and New C1-Symmetric Group 4 Metallocenes {(R1R2C)-(R2′R3′R6′R7′-Flu)(3-R3-5-R4-C5H2)}ZrCl2: From Highly Isotactic Polypropylenes to Vinyl End-Capped Isotactic-Enriched Oligomers. Organometallics 2012, 32, 8375–8387. [Google Scholar] [CrossRef]
- Castro, L.; Kirillov, E.; Miserque, O.; Welle, A.; Haspeslagh, L.; Carpentier, J.-F.; Maron, L. Are Solvent and Dispersion Effects Crucial in Olefin Polymerization DFT Calculations? Some Insights from Propylene Coordination and Insertion Reactions with Group 3 and 4 Metallocenes. ACS Catal. 2015, 5, 416–425. [Google Scholar] [CrossRef]
- Castro, L.; Therukauff, G.; Vantomme, A.; Welle, A.; Haspeslagh, L.; Brusson, J.-M.; Maron, L.; Carpentier, J.-F.; Kirillov, E. A Theoretical Outlook on the Stereoselectivity Origins of Isoselective Zirconocene Propylene Polymerization Catalysts. Chem. Eur. J. 2018, 24, 10784–10792. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.E.; Jayaratne, K.C.; Yang, Q.; Martin, J.L. Highly Soluble Olefin Polymerization Catalyst Activator. U.S. Patent 20,090,170,690 A1, 2 July 2009. [Google Scholar]
- Murray, R.E.; Martin, J.L.; Jayaratne, K.C.; Yang, Q. Nano-Linked Metallocene Catalyst Compositions and tHeir Polymer Products. European Patent WO 2,009,085,124 A1, 9 July 2009. [Google Scholar]
- Inoue, Y.; Shiomura, T.; Kuono, M.; Sonobe, Y.; Mizutani, K. Method of Polymerizing an Olefin Using a Novel Transition Metal Compound. U.S. Patent 5,439,994, 8 August 1995. [Google Scholar]
- Peifer, B.; Milius, W.; Alt, H.G. Selbstimmobilisierende Metallocenkatalysatoren. J. Organomet. Chem. 1998, 553, 205–220. [Google Scholar] [CrossRef]
- Jüngling, S.; Mülhaupt, R. Cooperative effects in binuclear zirconocenes: Their synthesis and use as catalyst in propene polymerization. J. Organomet. Chem. 1993, 460, 191–195. [Google Scholar] [CrossRef]
- Farenc, M.; Assumani, B.; Afonso, C.; Giusti, P. Analysis of Metallocene using Atmospheric Pressure Chemical Ionization and Atmospheric Pressure Photo Ionization Coupled with Ion Mobility Mass Spectrometry. In Proceedings of the 64th ASMS conference on Mass Spectrometry and Allied Topics, San Atonio, TX, USA, 5–9 June 2016. [Google Scholar]
- Farenc, M.; Corilo, Y.E.; Lalli, P.M.; Riches, E.; Rodgers, R.P.; Afonso, C.; Giusti, P. Comparison of Atmospheric Pressure Ionization for the Analysis of Heavy Petroleum Fractions with Ion Mobility-Mass Spectrometry. Energy Fuels 2016, 30, 8896–8903. [Google Scholar] [CrossRef]
- Busico, V.; Cipullo, R.; Pellechia, R.; Talarico, G.; Razavi, A. Hafnocenes and MAO: Beware of Trimethylaluminum! Macromolecules 2009, 42, 1789–1791. [Google Scholar] [CrossRef]
- Coşkun, N.; Erden, I. An efficient catalytic method for fulvene synthesis. Tetrahedron 2011, 67, 8607–8614. [Google Scholar] [CrossRef] [PubMed]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Ahlrichs, R.; Bär, M.; Häser, M.; Horn, H.; Kölmel, C. Electronic structure calculations on workstation computers: The program system turbomole. Chem. Phys. Lett. 1989, 162, 165–169. [Google Scholar] [CrossRef]
- Treutler, O.; Ahlrichs, R. Efficient molecular numerical integration schemes. J. Chem. Phys. 1995, 102, 346–354. [Google Scholar] [CrossRef]
- Eichkorn, K.; Treutler, O.; Öhm, H.; Häser, M.; Ahlrichs, R. Auxiliary basis sets to approximate coulomb potentials. Chem. Phys. Lett. 1995, 242, 652–660. [Google Scholar] [CrossRef]
- TURBOMOLE V6.2 2010, A Development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989–2007, TURBOMOLE GmbH, Since 2007. Available online: http://www.turbomole.com (accessed on 1 July 2010).
- Sierka, M.; Hogekamp, A.; Ahlrichs, R. Fast evaluation of the coulomb potential for electron densities using multipole accelerated resolution of identity approximation. J. Chem. Phys. 2003, 118, 9136–9148. [Google Scholar] [CrossRef]
- Deglmann, P.; May, K.; Furche, F.; Ahlrichs, R. Nuclear second analytical derivative calculations using auxiliary basis set expansions. Chem. Phys. Lett. 2004, 384, 103–107. [Google Scholar] [CrossRef]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).