Full-Spectrum Photocatalytic Activity of ZnO/CuO/ZnFe2O4 Nanocomposite as a PhotoFenton-Like Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and Optimization
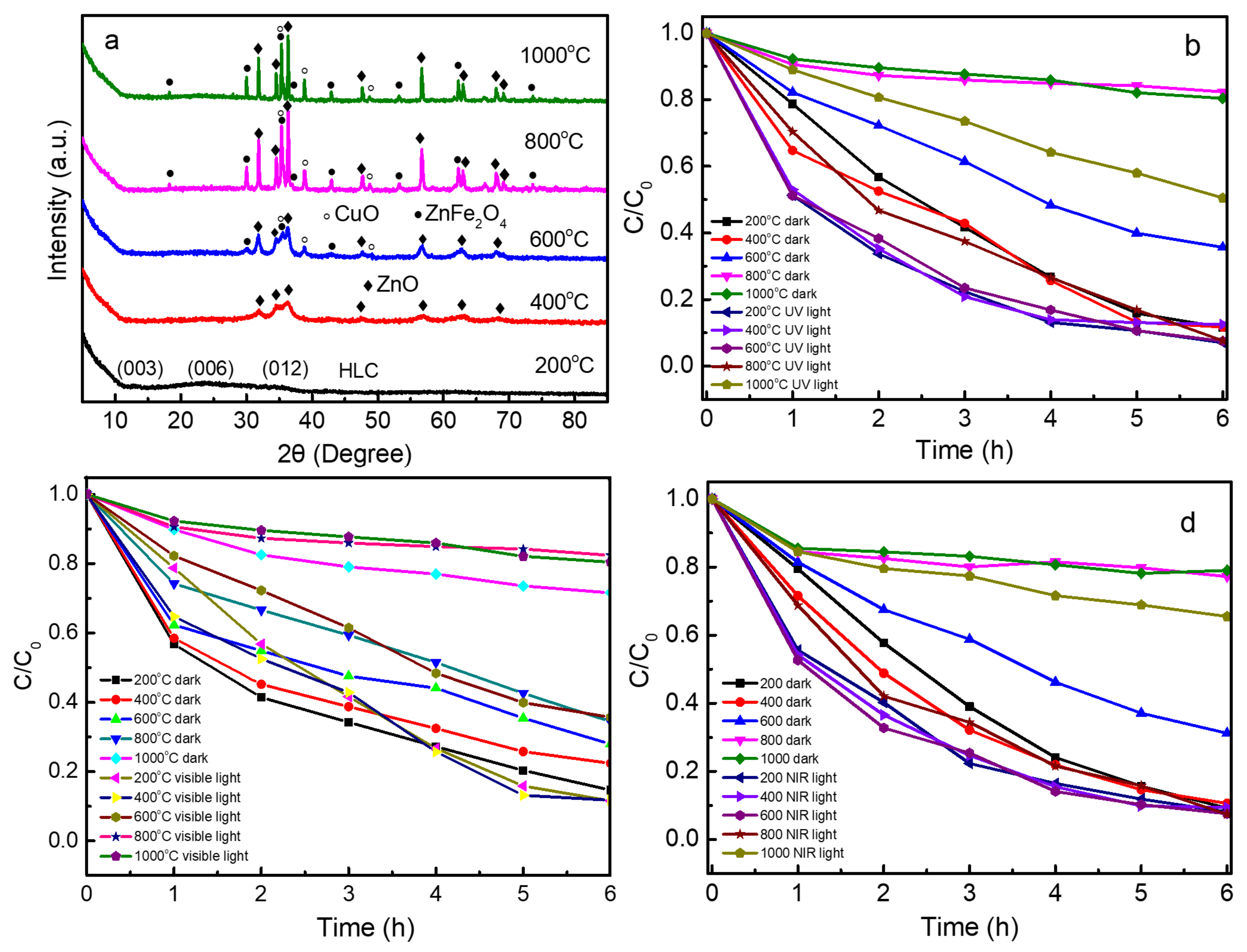
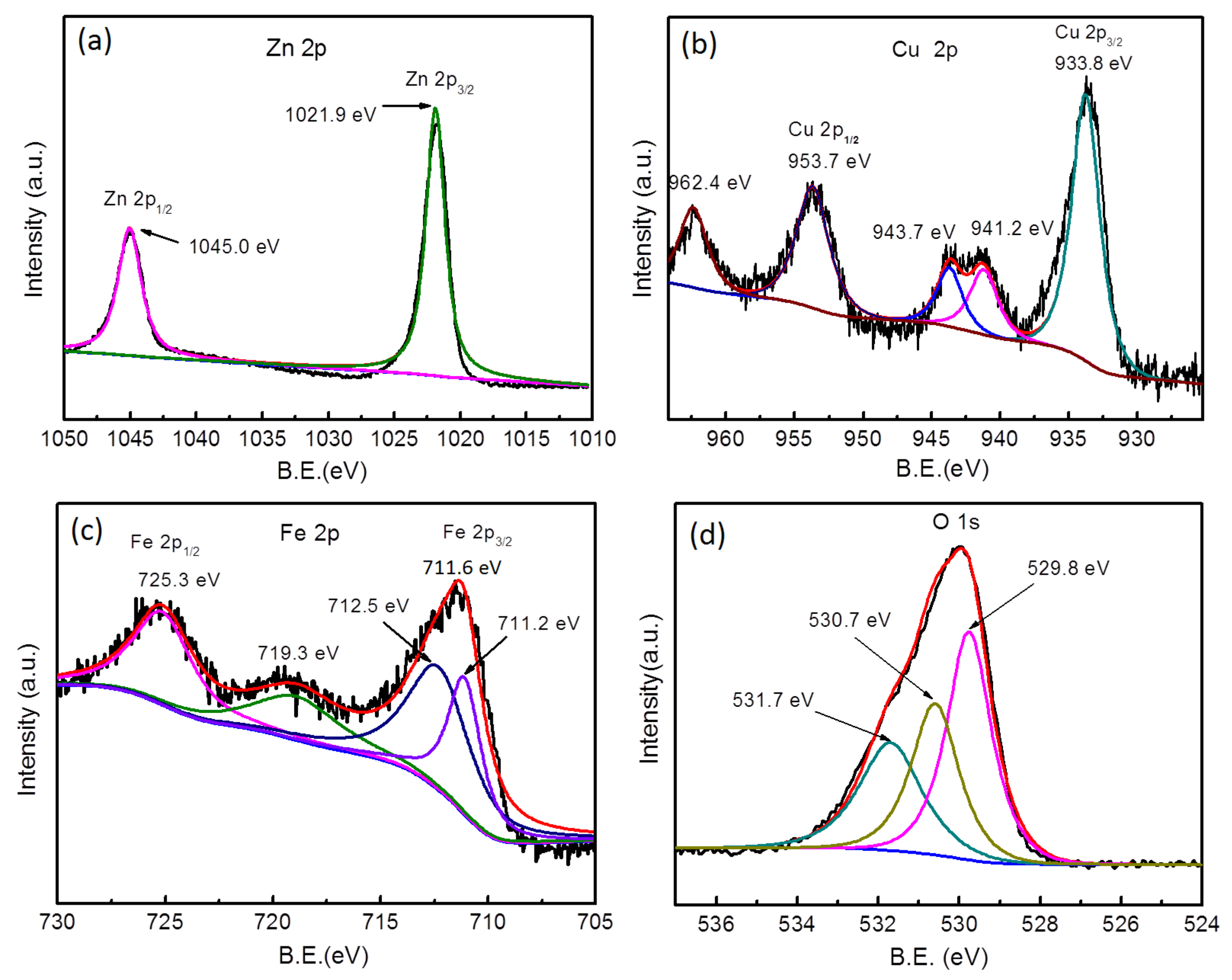
2.2. Characterization
2.3. Photo-Fenton-like Catalytic Activity
2.4. Optical Properties and Photocurrent Response
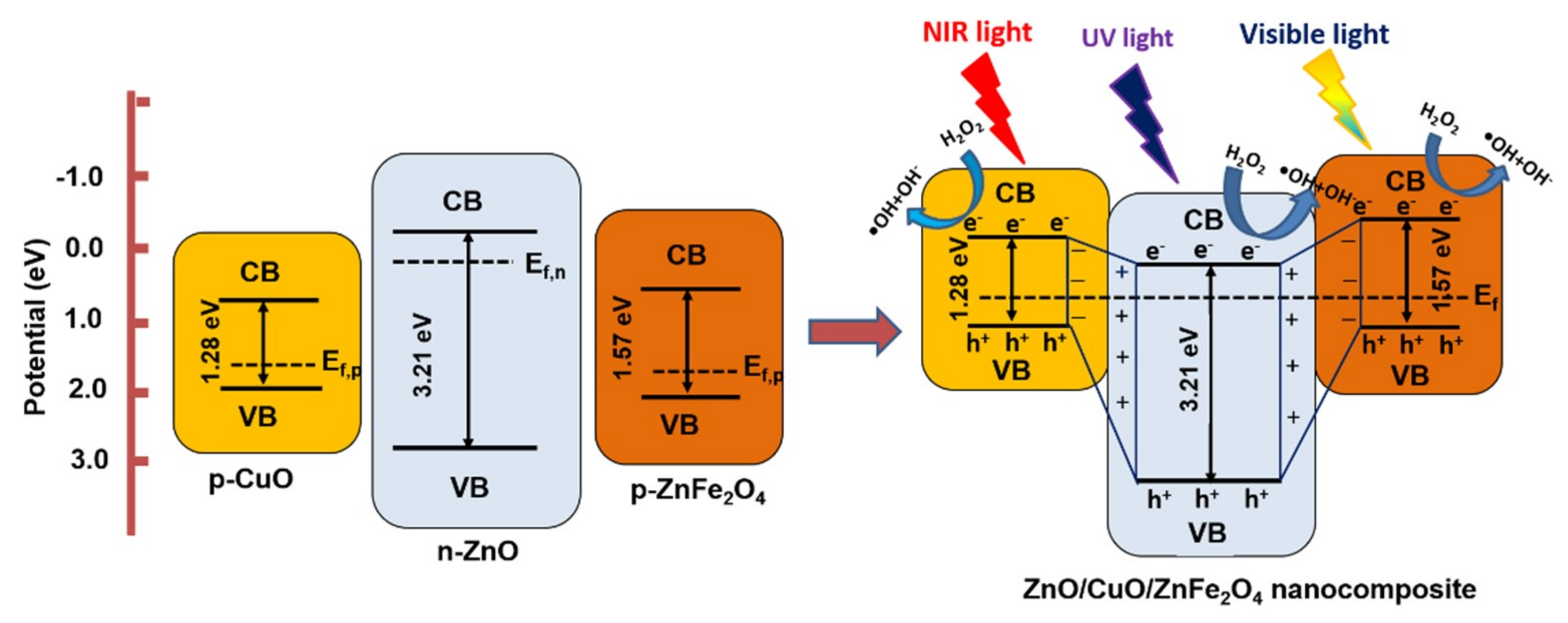
2.5. Suggested Photocatalytic Mechanism
3. Experimental
3.1. Preparation of the ZnO/CuO/ZnFe2O4 Nanocomposite
3.2. Characterization
3.3. Photocatalytic Activity Test
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wang, Q.; O’Hare, D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Luo, W.; Xia, S.; Ni, Z. Preparation of Salen–metal complexes (metal = Co or Ni) intercalated ZnCr-LDHs and their photocatalytic degradation of Rhodamine B. Catalysts 2017, 7, 143. [Google Scholar] [CrossRef]
- Prevot, V.; Tokudome, Y. 3D hierarchical and porous layered double hydroxide structures: An overview of synthesis methods and applications. J. Mater. Sci. 2017, 52, 11229–11250. [Google Scholar] [CrossRef]
- Carja, G.; Grosu, E.F.; Mureseanu, M.; Lutic, D. A family of solar light responsive photocatalysts obtained using Zn2+ Me3+ (Me = Al/Ga) LDHs doped with Ga2O3 and In2O3 and their derived mixed oxides: A case study of phenol/4-nitrophenol decomposition. Catal. Sci. Technol. 2017, 7, 5402–5412. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, T.; Li, K.; Cao, Y.; Zeng, Y. Dehydrogenation catalysts for synthesis of O-phenylphenol via Cu/Ni/Mg/Al hydrotalcite-like compounds as precursors. Catalysts 2018, 8, 186. [Google Scholar] [CrossRef]
- Chowdhury, P.R.; Bhattacharyya, K.G. Ni/Ti layered double hydroxide: Synthesis, characterization and application as a photocatalyst for visible light degradation of aqueous methylene blue. Dalton Trans. 2015, 44, 6809–6824. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Xie, L.; Li, Z.; Li, F. Ternary MgO/ZnO/In2O3 heterostructured photocatalysts derived from a layered precursor and visible-light-induced hotocatalytic activity. Chem. Eng. J. 2013, 221, 222–229. [Google Scholar] [CrossRef]
- Huo, R.; Kuang, Y.; Zhao, Z.; Zhang, F.; Xu, S. Enhanced photocatalytic performances of hierarchical ZnO/ZnAl2O4 microsphere derived from layered double hydroxide precursor spray-dried microsphere. J. Colloid Interface Sci. 2013, 407, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Mikami, G.; Grosu, F.; Kawamura, S.; Yoshida, Y.; Carja, G.; Izumi, Y. Harnessing self-supported Au nanoparticles on layered double hydroxides comprising Zn and Al for enhanced phenol decomposition under solar light. Appl. Catal. B 2016, 199, 260–271. [Google Scholar] [CrossRef]
- Nayak, S.; Pradhan, A.C.; Parida, K.M. Topotactic Transformation of solvated MgCr-LDH nanosheets to highly efficient porous MgO/MgCr2O4 nanocomposite for photocatalytic H2 evolution. Inorg. Chem. 2018, 57, 8646–8661. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Durand, P.; André, E.; Carteret, C. Enhanced photocatalytic ability of Cu, Co doped ZnAl based mixed metal oxides derived from layered double hydroxides. Colloids Surf. A 2017, 524, 43–52. [Google Scholar] [CrossRef]
- Pan, D.; Ge, S.; Zhao, J.; Shao, Q.; Guo, L.; Zhang, X.; Lin, J.; Xu, G.; Guo, Z. Synthesis, characterization and photocatalytic activity of mixed-metal oxides derived from NiCoFe ternary layered double hydroxides. Dalton Trans. 2018, 47, 9765–9778. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, S.; Chen, F.; Luo, K.; Sun, J.; Gong, C.; Yao, F.; Wang, X.; Wu, J.; Li, X.; et al. Enhanced visible-light-driven photocatalytic removal of refractory pollutants by Zn/Fe mixed metal oxide derived from layered double hydroxide. Catal. Commun. 2017, 99, 15–19. [Google Scholar] [CrossRef]
- Chen, M.; Wu, P.; Wei, Q.; Zhu, Y.; Yang, S.; Ju, L.; Zhu, N.; Lin, Z. The role of oxygen vacancy over ZnCr-layered double oxide in enhancing solar light-driven photocatalytic degradation of bisphenol. A. Environ. Chem. 2018, 15, 226–235. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, L.; Zhao, R.; Su, R.; Wang, Q.; Wang, P. Microwave-assisted synthesis of ZnNiAl-layered double hydroxides with calcination treatment for enhanced PNP photo-degradation under visible-light irradiation. J. Photochem. Photobiol. A 2018, 356, 633–641. [Google Scholar] [CrossRef]
- Goswami, K.; Ananthakrishnan, R.; Mandal, S. Facile synthesis of cation doped ZnO-ZnCo2O4 hetero-nanocomposites for photocatalytic decomposition of aqueous organics under visible light. Mater. Chem. Phys. 2018, 206, 174–185. [Google Scholar] [CrossRef]
- Gao, W.; Liu, W.; Leng, Y.; Wang, X.; Wang, X.; Hu, B.; Yu, D.; Sang, Y.; Liu, H. In2S3 nanomaterial as a broadband spectrum photocatalyst to display significant activity. Appl. Catal. B 2015, 176, 83–90. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, P.; Jiang, F.; Tian, J.; Cui, H.; Yang, J. Vanadium sulfide sub-microspheres: A new near-infrared-driven photocatalyst. J. Colloid Interface Sci. 2017, 498, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Lei, W.; Sui, X.; Liu, X.; Qi, X.; Tang, K.; Liu, G.; Liu, M. Anchoring black phosphorus quantum dots on molybdenum disulfide nanosheets: A 0D/2D nanohybrid with enhanced visible−and NIR −light photoactivity. Appl. Catal. B 2018, 238, 444–453. [Google Scholar] [CrossRef]
- Wang, X.; Wang, F.; Sang, Y.; Liu, H. Full-spectrum solar-light-activated photocatalysts for light–chemical energy conversion. Adv. Energy Mater. 2017, 7, 1700473. [Google Scholar] [CrossRef]
- Chen, J.; Liu, W.; Li, Z.; Liu, H. Thermally-assisted photodegradation of lignin by TiO2/H2O2 under visible/near-infrared light irradiation. Sci. China. Mater. 2018, 61, 382–390. [Google Scholar] [CrossRef]
- Yang, M.-Q.; Gao, M.; Hong, M.; Ho, G.W. Visible-to-NIR photon harvesting: Progressive engineering of catalysts for solar-powered environmental purification and fuel production. Adv. Mater. 2018, 1802894. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Mora, P.; Domen, K.; Hisatomi, T.; Lyu, H.; Méndez-Ramos, J.; Ruiz-Morales, J.C.; Khaidukov, N.M. Shifting the NIR into the UV-blue: Up-conversion boosted photocatalysis. Opt. Mater. 2018, 83, 315–320. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M.; Zhao, Z.; Sun, B.; Zhang, X. Visible/near-IR-light-driven TNFePc/BiOCl organic–inorganic heterostructures with enhanced photocatalytic activity. Dalton Trans. 2016, 45, 9497–9505. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, W.; Gao, W. Tuning photocatalytic activity of In2S3 broadband spectrum photocatalyst based on morphology. Appl. Surf. Sci. 2016, 368, 288–297. [Google Scholar] [CrossRef]
- Tian, J.; Sang, Y.; Yu, G.; Jiang, H.; Mu, X.; Liu, H. A Bi2WO6-based hybrid photocatalyst with broad spectrum photocatalytic properties under UV, visible, and near-infrared irradiation. Adv. Mater. 2013, 25, 5075–5080. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Liu, W.; Li, Z.; Chen, H.; Li, G.; Yu, D. Er3+-doped ZnO/ZnAl2O4 multi-phase oxides acting as near-infrared active photocatalyst. J. Mater. Sci. - Mater. Electron. 2018, 29, 8293–8302. [Google Scholar] [CrossRef]
- Chen, H.; Liu, W.; Hu, B.; Qin, Z.; Liu, H. A full-spectrum photocatalyst with strong near-infrared photoactivity derived from synergy of nano-heterostructured Er3+-doped multi-phase oxides. Nanoscale 2017, 9, 18940–18950. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, W.; Qin, Z. ZnO/ZnFe2O4 nanocomposite as a broadspectrum photo-Fenton-like photocatalyst with near-infrared activity. Catal. Sci. Technol. 2017, 7, 2236–2244. [Google Scholar] [CrossRef]
- Tougaard, S.M. Determination of the Cu 2p primary excitation spectra for Cu, Cu2O and CuO. Surf. Sci. 2014, 620, 17–22. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Pan, F.; Guo, Y.; Cheng, F.; Fa, T.; Yao, S. Synthesis of ZnFe2O4 nanomagnets by Fe-ion implantation into ZnO and post-annealing. Chin. Phys. B 2011, 20, 127501. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, X.L.; Zhang, G.H.; Xue, H.; Ma, S.Y. Blue shift of optical band gap in Er-doped ZnO thin films deposited by direct current reactive magnetron sputtering technique. Physica E 2010, 42, 1713–1716. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Liu, W.; Gao, W.; Han, J.; Liu, H.; Lucia, L.A. Pseudo-Janus Zn/Al-based nanocomposites for Cr(VI) sorption/remediation and evolved photocatalytic functionality. Chem. Eng. J. 2015, 277, 150–158. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Chen, H.; Liu, W. Full-Spectrum Photocatalytic Activity of ZnO/CuO/ZnFe2O4 Nanocomposite as a PhotoFenton-Like Catalyst. Catalysts 2018, 8, 557. https://doi.org/10.3390/catal8110557
Li Z, Chen H, Liu W. Full-Spectrum Photocatalytic Activity of ZnO/CuO/ZnFe2O4 Nanocomposite as a PhotoFenton-Like Catalyst. Catalysts. 2018; 8(11):557. https://doi.org/10.3390/catal8110557
Chicago/Turabian StyleLi, Zhenzhen, Huabin Chen, and Wenxia Liu. 2018. "Full-Spectrum Photocatalytic Activity of ZnO/CuO/ZnFe2O4 Nanocomposite as a PhotoFenton-Like Catalyst" Catalysts 8, no. 11: 557. https://doi.org/10.3390/catal8110557





