Catalytic Oxidative/Extractive Desulfurization of Model Oil using Transition Metal Substituted Phosphomolybdates-Based Ionic Liquids
Abstract
:1. Introduction
2. Results and Discussion
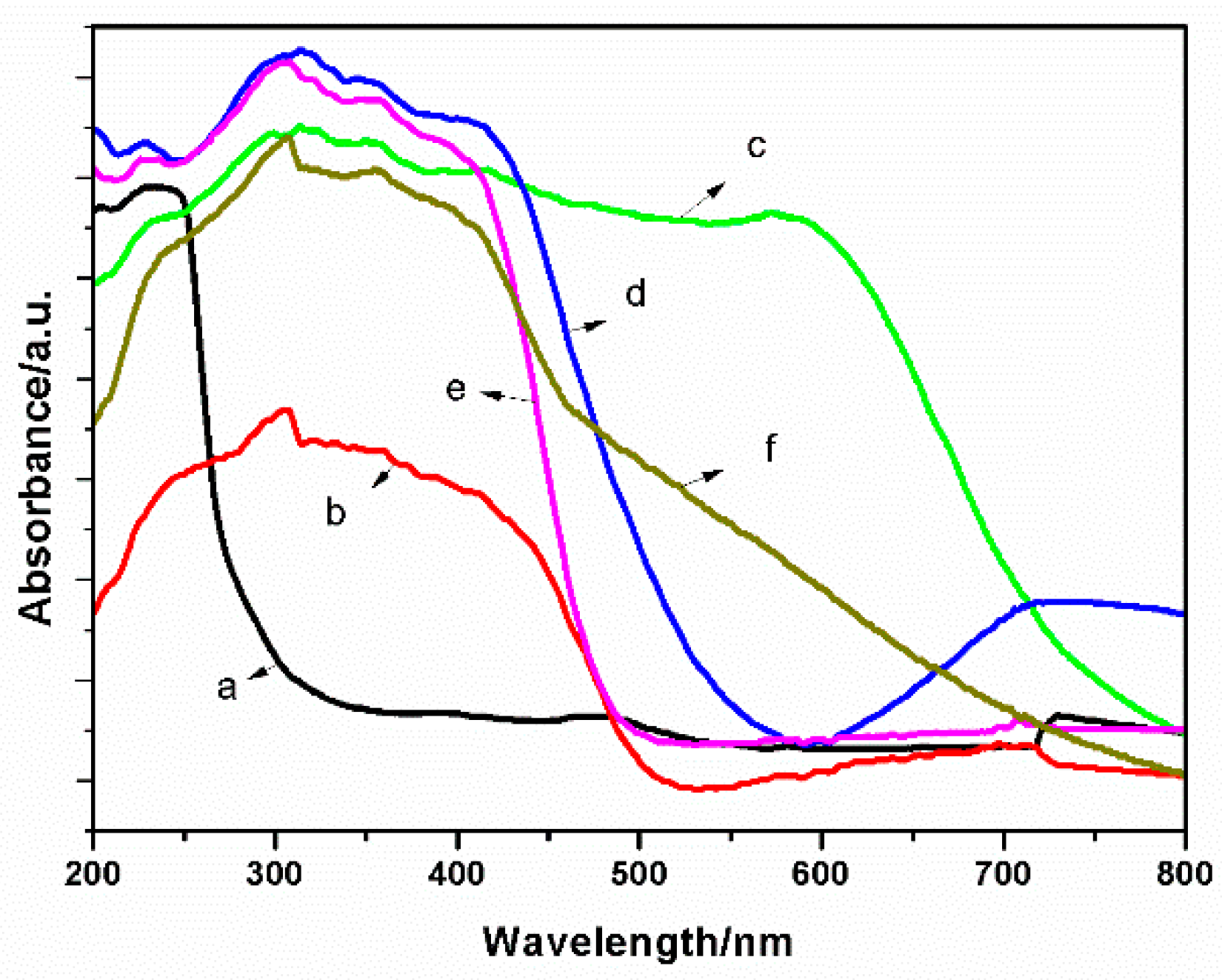
2.1. Characterization of the Catalysts
2.2. Extractive and Catalytic Oxidation Desulfurization (ECODS) of Model Sulfur Compounds
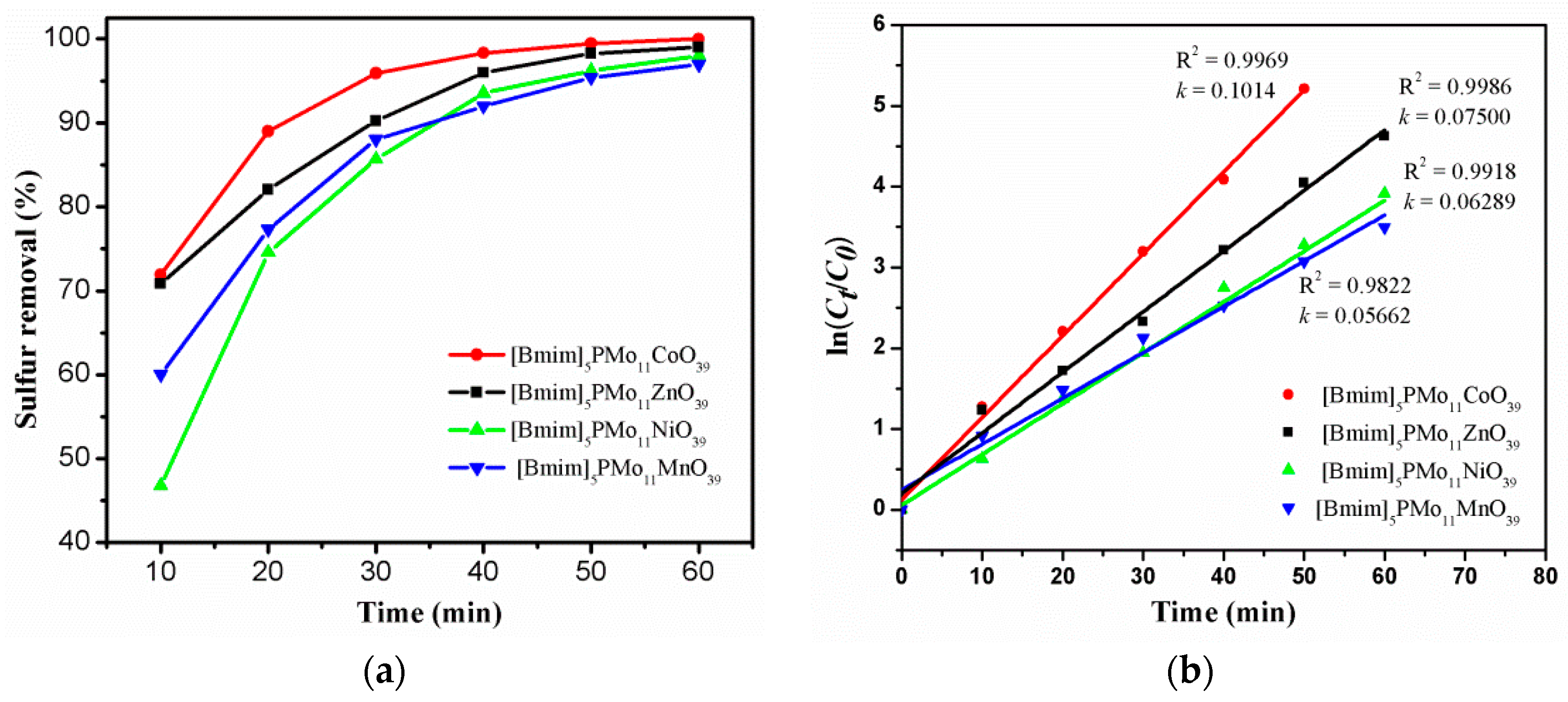
2.2.1. Desulfurization of Model Oil with [Bmim]5[PMo11M(H2O)O39] (M = Co2+, Ni2+, Zn2+, and Mn2+) as Catalysts
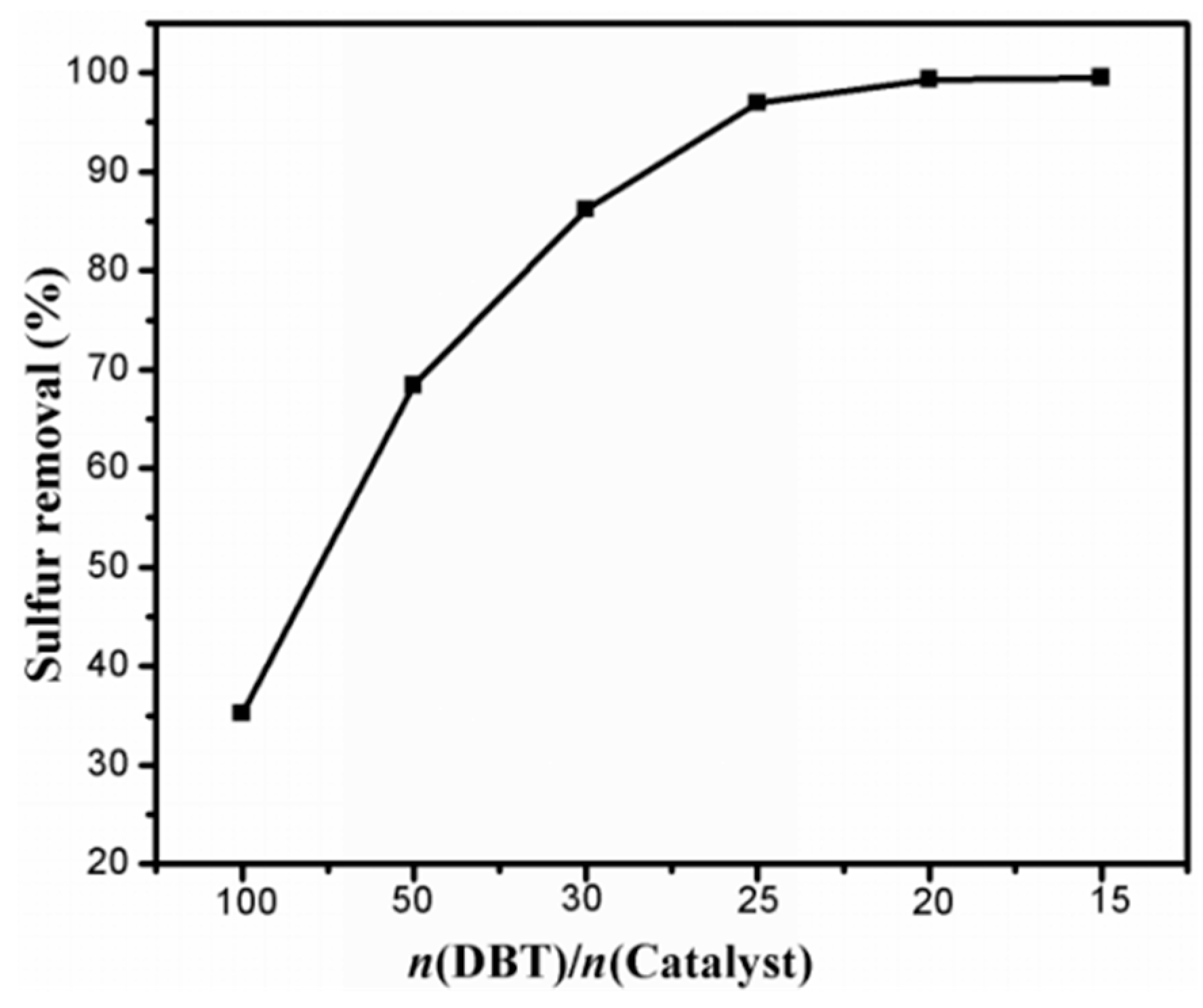
2.2.2. Influence of the Amount of Catalyst on Desulfurization
2.2.3. Influence of the H2O2/DBT Molar Ratio on Desulfurization
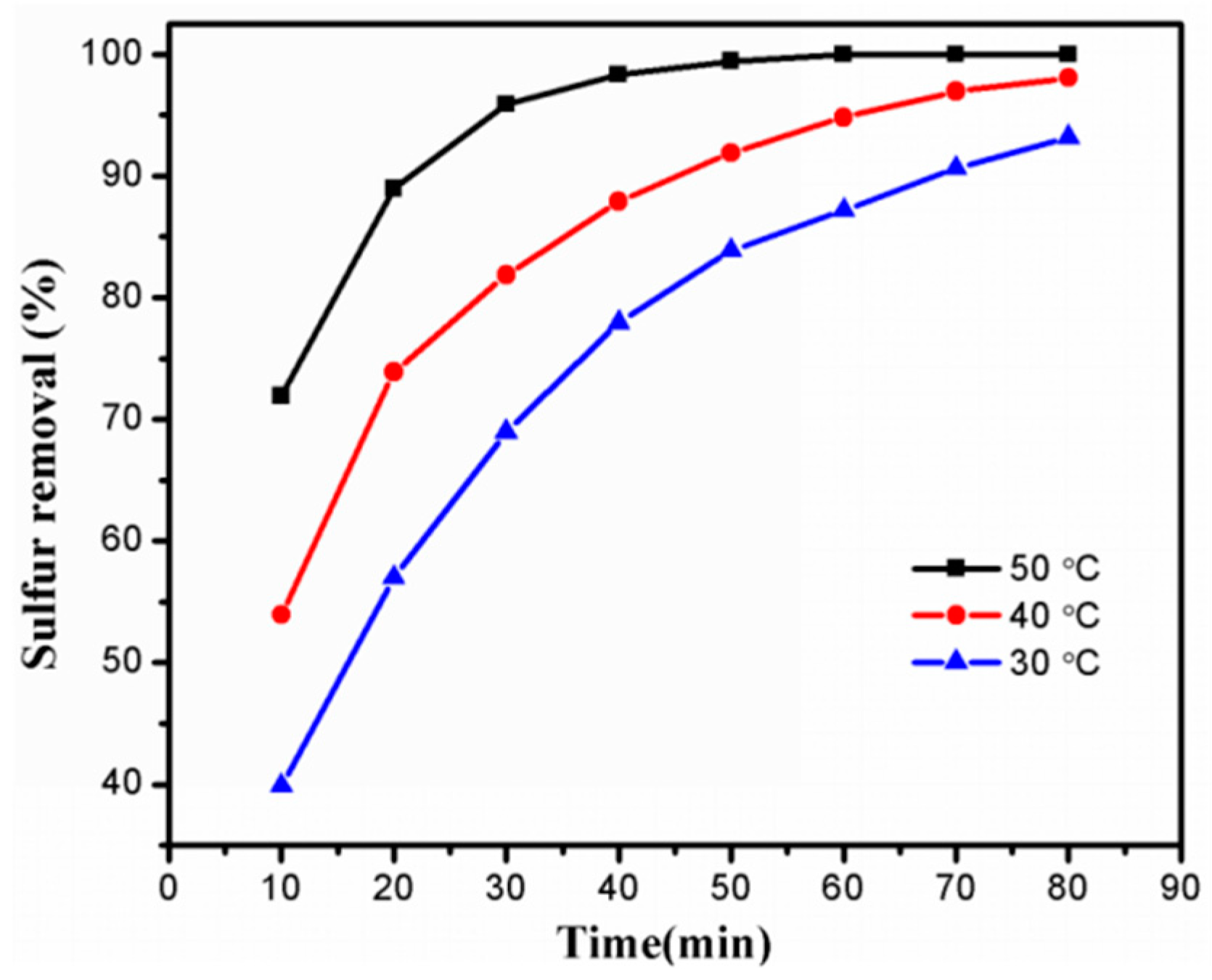
2.2.4. Influence of Temperature and Reaction Time on Desulfurization
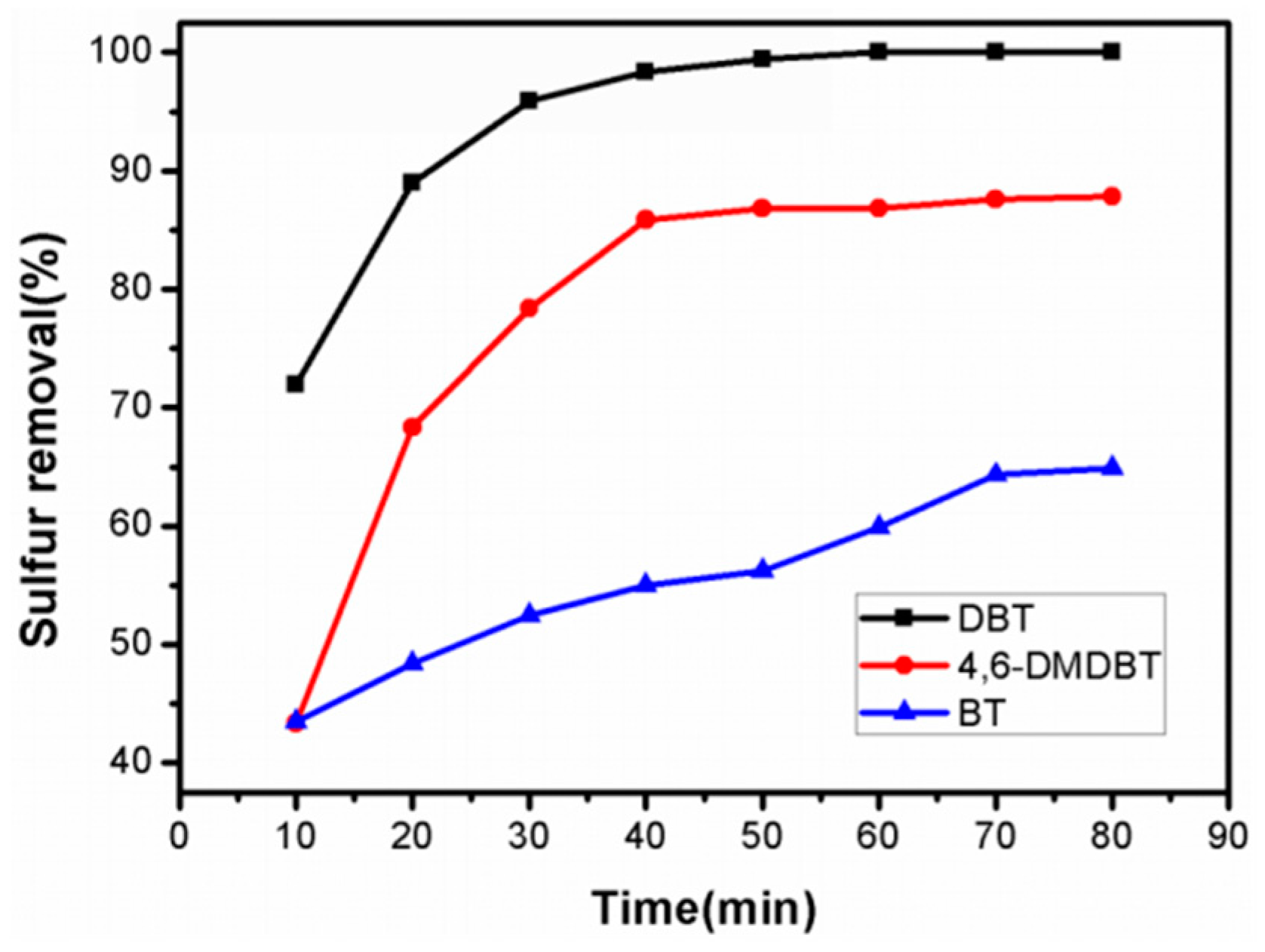
2.2.5. Catalytic Oxidation Results for 4,6-Dimethyldibenzothiophene (DMDBT) and Benzothiophene (BT)
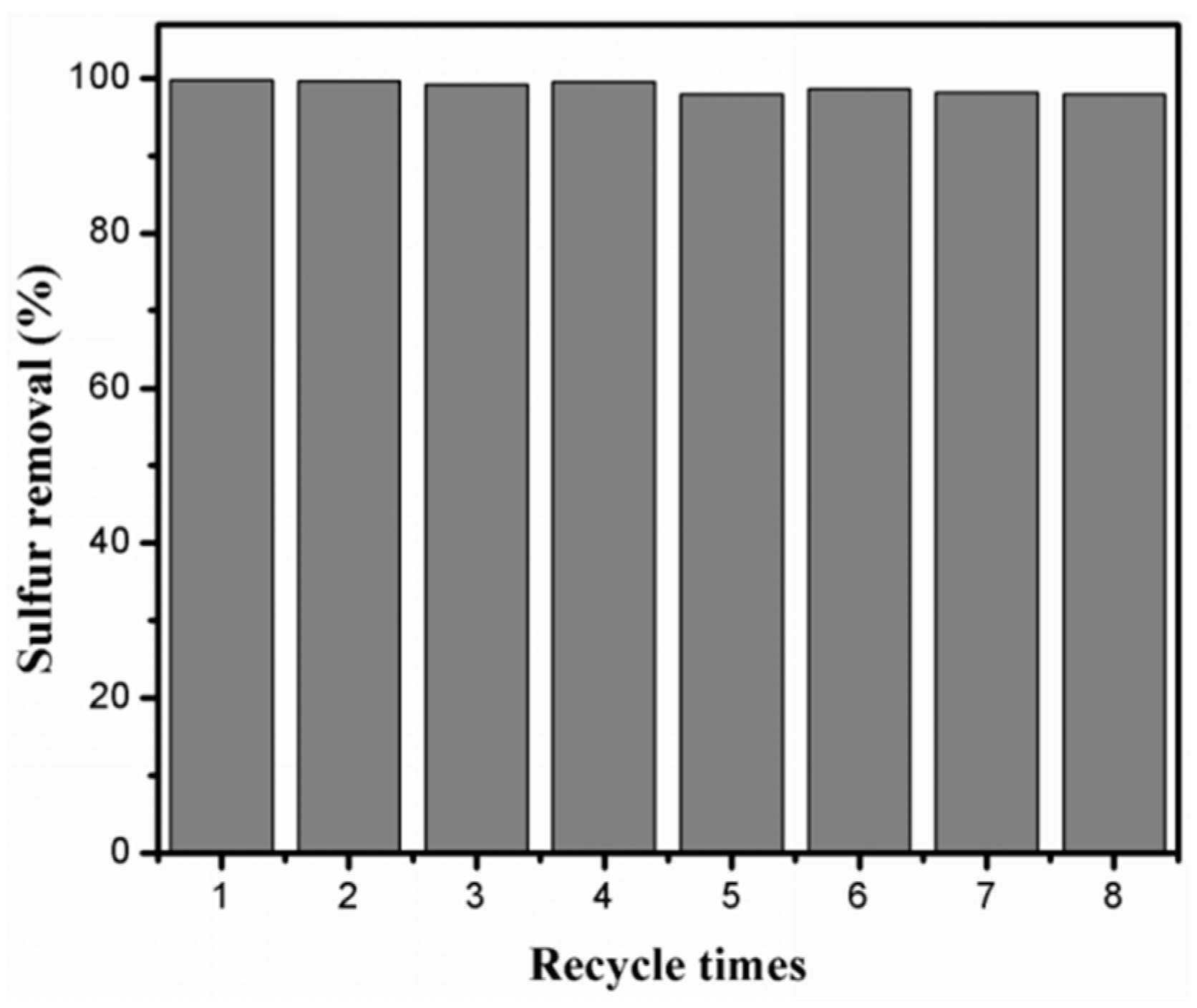
2.2.6. Recyclability of the Catalytic System
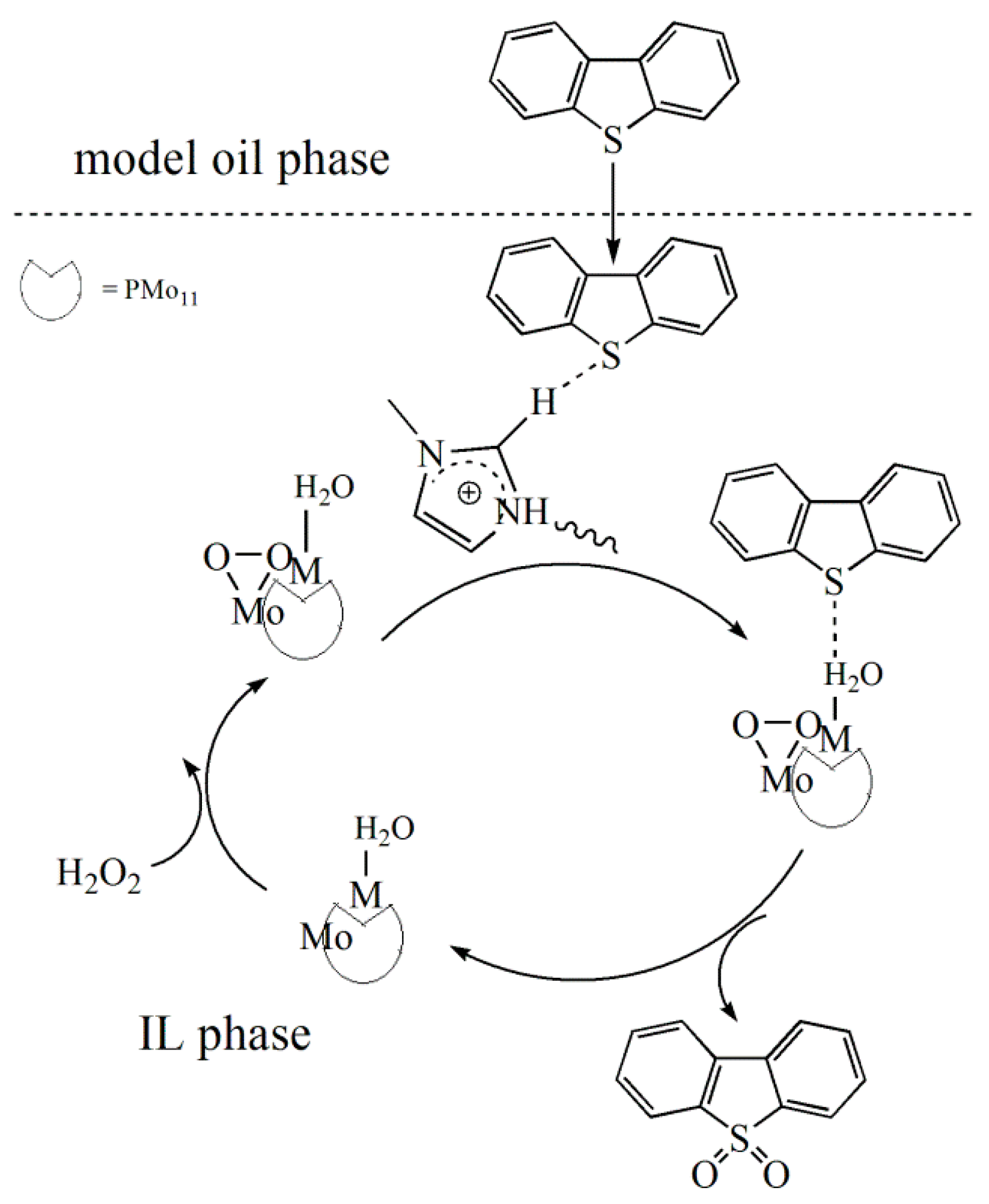
2.2.7. The Possible Mechanism
2.2.8. Comparison of the Catalytic Desulfurization Efficiency with some Related, Reported Catalysts
3. Experimental Section
3.1. Materials and Characterization
3.2. Synthesis of [Bmim]5[PMo11M(H2O)O39] (Bmim = 1-Butyl 3-Methyl Imidazolium; M = Co2+, Ni2+, Zn2+, and Mn2+)
3.3. ECODS Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Stanislaus, A.; Marafi, A.; Rana, M.S. Recent advances in the science and technology of ultra low sulfur diesel (ULSD) production. Catal. Today 2010, 153, 1–68. [Google Scholar] [CrossRef]
- Eßer, J.; Wasserscheid, P.; Jess, A. Deep desulfurization of oil refinery streams by extraction with ionic liquids. Green Chem. 2004, 6, 316–322. [Google Scholar] [CrossRef]
- Babich, I.V.; Moulij, J.A. Science and technology of novel processes for deep desulfurization of oil refinery streams: A review. Fuel 2003, 82, 607–631. [Google Scholar] [CrossRef]
- Francisco, M.; Arce, A.; Soto, A. Ionic liquids on desulfurization of fuel oils. Fluid Phase Equilib. 2010, 294, 39–48. [Google Scholar] [CrossRef]
- Rothlisberger, A.; Prin, R. Intermediates in the hydrodesulfurization of 4,6-dimethyl-dibenzothiophene over Pd/γ-Al2O3. J. Catal. 2005, 235, 229–240. [Google Scholar] [CrossRef]
- Jiang, X.; Li, H.; Zhu, W.; He, L.; Shu, H.; Lu, J. Deep desulfurization of fuels catalyzed by surfactant-type decatungstates using H2O2 as oxidant. Fuel 2009, 88, 431–436. [Google Scholar] [CrossRef]
- Ko, N.H.; Lee, J.S.; Huh, E.S.; Lee, H.; Jung, K.D.; Kim, H.S.; Cheong, M. Extractive Desulfurization Using Fe-Containing Ionic Liquids. Energy Fuels 2008, 22, 1687–1690. [Google Scholar] [CrossRef]
- Hansmeier, A.R.; Meindersma, G.W.; Haan, A.B. Desulfurization and denitrogenation of gasoline and diesel fuels by means of ionic liquids. Green Chem. 2011, 13, 1907–1913. [Google Scholar] [CrossRef]
- Soleimani, M.; Bassi, A.; Margaritis, A. Biodesulfurization of refractory organic sulfur compounds in fossil fuels. Biotechnol. Adv. 2007, 25, 570–596. [Google Scholar] [CrossRef]
- Lo, W.; Yang, H.; Wei, G. One-pot desulfurization of light oils by chemical oxidation and solvent extraction with room temperature ionic liquids. Green Chem. 2003, 5, 639–642. [Google Scholar] [CrossRef]
- Silva, G.; Voth, S.; Szymanski, P.; Prokopchuk, E.M. Oxidation of dibenzothiophene by hydrogen peroxide in the presence of bis(acetylacetonato)oxovanadium(IV). Fuel Process. Technol. 2011, 92, 1656–1661. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Jiang, Z.X.; Li, J.; Zhang, Y.N.; Lin, F.; Liu, Y.; Li, C. Catalytic oxidation of thiophene and its derivatives via dual activation for ultra-deep desulfurization of fuels. J. Catal. 2012, 287, 5–12. [Google Scholar] [CrossRef] [Green Version]
- García-Gutiérrez, J.L.; Fuentes, G.A.; Hernández-Terán, M.E.; García, P.; Murrieta-Guevara, F.; Jiménez-Cruz, F. Ultra-deep oxidative desulfurization of diesel fuel by the Mo/Al2O3-H2O2 system: The effect of system parameters on catalytic activity. Appl. Catal. A Gen. 2008, 334, 366–373. [Google Scholar] [CrossRef]
- Gui, J.Z.; Liu, D.; Sun, Z.L.; Liu, D.S.; Min, D.; Song, B.; Peng, X.L. Deep oxidative desulfurization with task-specific ionic liquids: An experimental and computational study. J. Mol. Catal. A Chem. 2010, 331, 64–70. [Google Scholar] [CrossRef]
- Zhou, A.N.; Ma, X.L.; Song, C.S. Effects of oxidative modification of carbon surface on the adsorption of sulfur compounds in diesel fuel. Appl. Catal. B Environ. 2009, 87, 190–199. [Google Scholar] [CrossRef]
- Mei, H.; Mei, B.W.; Yen, T.F. A new method for obtaining ultra-low sulfur diesel fuel via ultrasound assisted oxidative desulfurization. Fuel 2003, 82, 405–414. [Google Scholar] [CrossRef]
- Wu, Z.L.; Ondruschka, B. Ultrasound-assisted oxidative desulfurization of liquid fuels and its industrial application. Ultrason. Sonochem. 2010, 17, 1027–1032. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, D.S.; Yang, L.Y.; Li, Y.B. Photocatalytic oxidation dibenzothiophene using TS-1. Chem. Eng. J. 2010, 156, 528–531. [Google Scholar]
- Wei, Z.S.; Zeng, G.H.; Xie, Z.R. Microwave Catalytic Desulfurization and Denitrification Simultaneously on Fe/Ca-5A Zeolite Catalyst. Energy Fuels 2009, 23, 2947–2951. [Google Scholar] [CrossRef]
- Trakarnpruk, W.; Rujiraworawut, K. Oxidative desulfurization of Gas oil by polyoxometalates catalysts. Fuel Process. Technol. 2009, 90, 411–414. [Google Scholar] [CrossRef]
- Campos-Martin, J.M.; Capel-Sanchez, M.C.; Perez-Presas, P.; Fierro, J.L.G. Oxidative processes of desulfurization of liquid fuels. J. Chem. Technol. Biotechnol. 2010, 85, 879–890. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, S.; Barbosa, A.D.S.; Gomes, A.C.; Pillinger, M.; Gonçalves, I.S.; Cunha-Silva, L.; Balula, S.S. Catalytic oxidative desulfurization systems based on Keggin phosphotungstate and metal-organic framework MIL-101. Fuel Process. Technol. 2013, 116, 350–357. [Google Scholar] [CrossRef]
- Capel-Sanchez, M.C.; Perez-Presas, P.; Campos-Martin, J.M.; Fierro, J.L.G. Highly efficient deep desulfurization of fuels by chemical oxidation. Catal. Today 2010, 157, 390–396. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Yu, G.; Abro, R.; Abdeltawab, A.A.; Al-Deyab, S.S.; Chen, X. Desulfurization of fuel oils: Mutual solubility of ionic liquids and fuel oil. Fuel 2016, 173, 164–171. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, W.; Li, H.; Jiang, W.; Jiang, Y.; Huang, W.; Yan, Y. Deep oxidative desulfurization of fuels by Fenton-like reagent in ionic liquids. Green Chem. 2009, 11, 1801–1807. [Google Scholar] [CrossRef]
- Bosmann, A.; Datsevich, L.; Jess, A.; Lauter, A.; Schmitz, C.; Wasserscheid, P. Deep desulfurization of diesel fuel by extraction with ionic liquids. Chem. Commun. 2001, 23, 2494–2495. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Z.C. Novel properties of ionic liquids in selective sulfur removal from fuels at room temperature. Green Chem. 2002, 4, 376–379. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Sugawara, K. Removal of organic sulfur from hydrocarbon resources using ionic liquids. Energy Fuels 2008, 22, 3303–3307. [Google Scholar] [CrossRef]
- Nie, Y.; Li, C.; Sun, A.; Meng, H.; Wang, Z. Extractive Desulfurization of Gasoline Using Imidazolium-Based Phosphoric Ionic Liquids. Energy Fuels 2006, 20, 2083–2087. [Google Scholar] [CrossRef]
- Jiang, X.; Nie, Y.; Li, C.; Wang, Z. Imidazolium-based alkylphosphate ionic liquids—A potential solvent for extractive desulfurization of fuel. Fuel 2008, 87, 79–84. [Google Scholar] [CrossRef]
- Gao, H.S.; Luo, M.F.; Xing, J.M.; Wu, Y.; Li, Y.G.; Li, W.L.; Liu, Q.F.; Liu, H.Z. Desulfurization of Fuel by Extraction with Pyridinium-Based Ionic Liquids. Ind. Eng. Chem. Res. 2008, 47, 8384–8388. [Google Scholar] [CrossRef]
- Huang, C.; Chen, B.; Zhang, J.; Liu, Z.; Li, Y. Desulfurization of Gasoline by Extraction with New Ionic Liquids. Energy Fuels 2004, 18, 1862–1864. [Google Scholar] [CrossRef]
- Omwoma, S.; Gore, C.T.; Ji, Y.; Hu, C.; Song, Y. Environmentally benign polyoxometalate materials. Coord. Chem. Rev. 2015, 286, 17–29. [Google Scholar] [CrossRef]
- Yan, X.; Mei, P.; Xiong, L.; Gao, L.; Yang, Q.; Gong, L. Mesoporous titania–silica–polyoxometalate nanocomposite materials for catalytic oxidation desulfurization of fuel oil. Catal. Sci. Technol. 2013, 3, 1985–1992. [Google Scholar] [CrossRef]
- Huang, W.; Zhu, W.; Li, H.; Shi, H.; Zhu, G.; Liu, H.; Chen, G. Heteropolyanion-Based Ionic Liquid for Deep Desulfurization of Fuels in Ionic Liquids. Ind. Eng. Chem. Res. 2010, 49, 8998–9003. [Google Scholar] [CrossRef]
- Pope, M.T.; Müller, A. Polyoxometalate Chemistry from Topology via Self-Assembly to Applications; Springer: Dordrecht, The Netherlands, 2002; pp. 335–417. ISBN 978-0-306-47625-9. [Google Scholar]
- Hill, C.L.; Prosser-McCartha, C.M. Homogeneous catalysis by transition metal oxygen anion clusters. Coord. Chem. Rev. 1995, 143, 407–455. [Google Scholar] [CrossRef]
- Mizuno, N.; Yamaguchi, K.; Kamata, K. Epoxidation of olefins with hydrogen peroxide catalyzed by polyoxometalates. Coord. Chem. Rev. 2005, 249, 1944–1956. [Google Scholar] [CrossRef]
- Kholdeeva, O.A. Heterogeneous Catalysis Research Progress; Gunther, M.B., Ed.; Nova Science Publ.: New York, NY, USA, 2008. [Google Scholar]
- Zhu, W.; Zhu, G.; Li, H.; Chao, Y.; Chang, Y.; Chen, G.; Han, C. Oxidative desulfurization of fuel catalyzed by metal-based surfactant-type ionic liquids. J. Mol. Catal. A Chem. 2011, 347, 8–14. [Google Scholar] [CrossRef]
- Chamack, M.; Mahjoub, A.R.; Aghayan, H. Cesium salts of tungsten-substituted molybdophosphoric acid immobilized onto platelet mesoporous silica: Efficient catalysts for oxidative desulfurization of dibenzothiophene. Chem. Eng. J. 2014, 255, 686–694. [Google Scholar] [CrossRef]
- Ribeiro, S.O.; Julião, D.; Cunha-Silva, L.; Domingues, V.F.; Valença, R.; Ribeiro, J.C.; de Castro, B.; Balula, S.S. Catalytic oxidative/extractive desulfurization of model and untreated diesel using hybrid based zinc-substituted polyoxometalates. Fuel 2016, 166, 268–275. [Google Scholar] [CrossRef]
- Banisharif, F.; Dehghani, M.R.; Capel-Sanchez, M.C.; Campos-Martin, J.M. Extractive-oxidative removals of dibenzothiophene and quinoline using vanadium substituted Dawson emulsion catalyst and ionic liquid based solvents. J. Ind. Eng. Chem. 2017, 47, 348–359. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, Y.; Dong, X.; Wu, P.; Li, Y.; Hu, H.; Xue, G. Deep Oxidative Desulfurization of Refractory Sulfur Compounds with Cesium Salts of Mono-Substituted Phosphomolybdate as Efficient Catalyst. Catal. Lett. 2017, 147, 1811–1819. [Google Scholar] [CrossRef]
- Rao, G.R.; Rajkumar, T.; Varghese, B. Synthesis and characterization of 1-butyl 3-methyl imidazolium phosphomolybdate molecular salt. Solid State Sci. 2009, 11, 36–42. [Google Scholar]
- Fournier, M.; Louis, C.; Che, M.; Chaquin, P.; Masure, D. Polyoxometallates as models for oxide catalysts: Part I. An UV-visible reflectance study of polyoxomolybdates: Influence of polyhedra arrangement on the electronic transitions and comparison with supported molybdenum catalysts. J. Catal. 1989, 119, 400–414. [Google Scholar] [CrossRef]
- Mazari, T.; Marchal, C.R.; Hocine, S.; Salhi, N.; Rabia, C. Oxidation of propane over substituted Keggin phosphomolybdate salts. J. Nat. Gas Chem. 2009, 18, 319–324. [Google Scholar] [CrossRef]
- Salomon, W.; Rivière, E.; López, X.; Suaud, N.; Mialane, P.; Haouas, M.; Saad, A.; Marrot, J.; Dolbecq, A. Bicapped Keggin polyoxomolybdates: Discrete species and experimental and theoretical investigations on the electronic delocalization in a chain compound. Dalton Trans. 2018, 47, 10636–10645. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari-Siahkali, A.; Philippou, A.; Dwyer, J.; Anderson, M.W. The acidity and catalytic activity of heteropoly acid on MCM-41 investigated by MAS NMR, FTIR and catalytic tests. Appl. Catal. A Gen. 2000, 192, 57–69. [Google Scholar] [CrossRef]
- Otsuk, S.; Nonaka, T.; Takashima, N.; Qian, W.H.; Ishihara, A.; Imai, T.; Kabe, T. Oxidative Desulfurization of Light Gas Oil and Vacuum Gas Oil by Oxidation and Solvent Extraction. Energy Fuels 2000, 14, 1232–1239. [Google Scholar] [CrossRef]
- Nogueira, L.S.; Ribeiro, S.; Granadeiro, C.M.; Pereira, E.; Feio, G.; Cunha-Silva, L.; Balula, S.S. Novel polyoxometalate silica nano-sized spheres: Efficient catalysts for olefin oxidation and the deep desulfurization process. Dalton Trans. 2014, 43, 9518–9528. [Google Scholar] [CrossRef]
- Wang, J.; Yan, L.; Li, G.; Wang, X.; Ding, Y.; Suo, J. Mono-substituted Keggin-polyoxometalate complexes as effective and recyclable catalyst for the oxidation of alcohols with hydrogen peroxide in biphasic system. Tetrahedron Lett. 2005, 46, 7023–7027. [Google Scholar] [CrossRef]
- Ingle, R.H.; Raj, N.K.K. Lacunary Keggin type polyoxotungstates in conjunction with a phase transfer catalyst: An effective catalyst system for epoxidation of alkenes with aqueous H2O2. J. Mol. Catal. A Chem. 2008, 294, 8–13. [Google Scholar] [CrossRef]
- Hu, J.; Li, K.; Li, W.; Ma, F.; Guo, Y. Selective oxidation of styrene to benzaldehyde catalyzed by Schiff base-modified ordered mesoporous silica materials impregnated with the transition metal-monosubstituted Keggin-type polyoxometalates. Appl. Catal. A Gen. 2009, 364, 211–220. [Google Scholar] [CrossRef]
- García-Gutiérrez, J.L.; Fuentes, G.A.; Hernández-Terán, M.E.; Murrieta, F.; Navarrete, J.; Jiménez-Cruz, F. Ultra-deep oxidative desulfurization of diesel fuel with H2O2 catalyzed under mild conditions by polymolybdates supported on Al2O3. Appl. Catal. A Gen. 2006, 305, 15–20. [Google Scholar] [CrossRef]
- Zhen, B.; Li, H.; Jiao, Q.; Li, Y.; Wu, Q.; Zhang, Y. SiW12O40-Based Ionic Liquid Catalysts: Catalytic Esterification of Oleic Acid for Biodiesel Production. Ind. Eng. Chem. Res. 2012, 51, 10374–10380. [Google Scholar] [CrossRef]
- Yan, X.; Mei, P.; Lei, J.; Mi, Y.; Xiong, L.; Guo, L. Synthesis and characterization of mesoporous phosphotungstic acid/TiO2 nanocomposite as a novel oxidative desulfurization catalyst. J. Mol. Catal. A Chem. 2009, 304, 52–57. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, A.; Li, X.; Ma, X. Oxidative desulfurization of dibenzothiophene and diesel over [Bmim]3PMo12O40. J. Catal. 2011, 279, 269–275. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, G.; Zhao, H. Polyoxometalate as effective catalyst for the deep desulfurization of diesel oil. Catal. Today 2010, 149, 117–121. [Google Scholar] [CrossRef]
- Hsein, W.U. Contribution to chemistry of phosphomolybdic acid, phosphotungstic acid and allied substances. J. Biol. Chem. 1920, 43, 189–220. [Google Scholar]



| Sulfur Removal (%) | ||||
|---|---|---|---|---|
| Entry | Type of ILs | IL | IL + H2O2 | IL + H2O2 + catalyst |
| 1 | [Bmim]BF4 | 19.6 | 28.5 | 94.4 |
| 2 | [Omim]BF4 | 31.5 | 35.6 | 99.8 |
| 3 | [Bmim]PF6 | 15.4 | 17.2 | 76.1 |
| 4 | [Omim]PF6 | 28.3 | 30.7 | 90.5 |
| Substrates/Reference | Catalysts | Reaction Conditions | Removal of DBT (%) |
|---|---|---|---|
| DBT(1000 ppm)/[57] | [PhPyPS]PW | H2O2, H2O/EtOH, 60 °C, 2 h | 93.6 |
| DBT(500 ppm)/[58] | H3PW12O40/TiO2 | H2O2, CH3CN, 60 °C, 2 h | 95.2 |
| DBT(500 ppm)/[59] | [Bmim]3PMo12O40/SiO2 | H2O2, CH3CN, 60 °C, 2 h | 100 |
| DBT(500 ppm)/[60] | H3PW6Mo6O40 | H2O2, CH3CN, 60 °C, 60 min | 99.7 |
| DBT(1000 ppm)/[35] | HPW-TiO2-SiO2 (1:3) | H2O2, petroleum ether, 70 °C, 2 h | 100 |
| DBT(640 ppm)/[20] | Na2HPW12O40 | H2O2, CH3CN, 60 °C, 150 min | 95 |
| DBT(500 ppm)/[43] | [Bmim]5PW11Zn(H2O)O39 | H2O2, CH3CN, 50 °C, 60 min | 10 |
| DBT(500 ppm)/[43] | [Bmim]5PW11Zn(H2O)O39 | H2O2, CH3CN, 50 °C, 3 h | 100 |
| DBT(500 ppm)/[36] | [PSPy]3PW12O40·2H2O | H2O2, [Omim]BF4, 30 °C, 60 min | 77.1 |
| DBT(500 ppm)/[41] | [(CH3)N(n-C8H17)3]2[Mo2O11] | H2O2, [Omim]BF4, 30 °C, 2 h | 97.8 |
| DBT(500 ppm)/[41] | [(CH3)N(n-C8H17)3]2[W2O11] | H2O2, [Omim]BF4, 30 °C, 2 h | 85.9 |
| DBT(500 ppm)/[45] | CsPMo11M (M = Co, Ni, Mn, Zn) | H2O2, CH3CN, 60 °C, 100 min | >97 |
| DBT(500 pm)/This work | [Bmim]5PMo11Co(H2O)O39 | H2O2, [Omim]BF4, 50 °C, 60 min | 99.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, Y.; Wu, P.; Feng, C.; Xue, G. Catalytic Oxidative/Extractive Desulfurization of Model Oil using Transition Metal Substituted Phosphomolybdates-Based Ionic Liquids. Catalysts 2018, 8, 639. https://doi.org/10.3390/catal8120639
Li Y, Zhang Y, Wu P, Feng C, Xue G. Catalytic Oxidative/Extractive Desulfurization of Model Oil using Transition Metal Substituted Phosphomolybdates-Based Ionic Liquids. Catalysts. 2018; 8(12):639. https://doi.org/10.3390/catal8120639
Chicago/Turabian StyleLi, Yunlei, Yanjie Zhang, Panfeng Wu, Caiting Feng, and Ganglin Xue. 2018. "Catalytic Oxidative/Extractive Desulfurization of Model Oil using Transition Metal Substituted Phosphomolybdates-Based Ionic Liquids" Catalysts 8, no. 12: 639. https://doi.org/10.3390/catal8120639




