Palladium Supported on Carbon Nanotubes as a High-Performance Catalyst for the Dehydrogenation of Dodecahydro-N-ethylcarbazole
Abstract
:1. Introduction
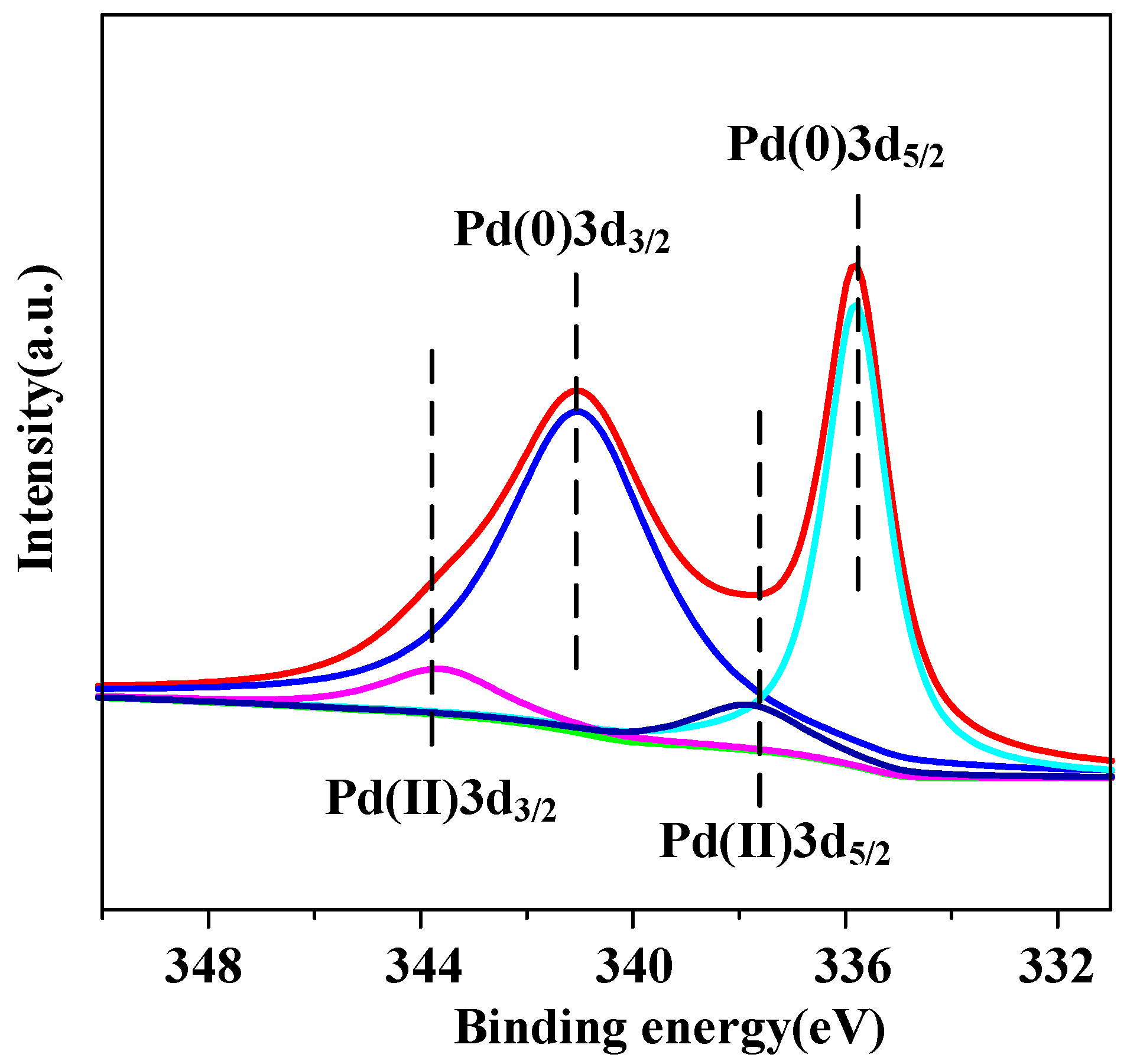
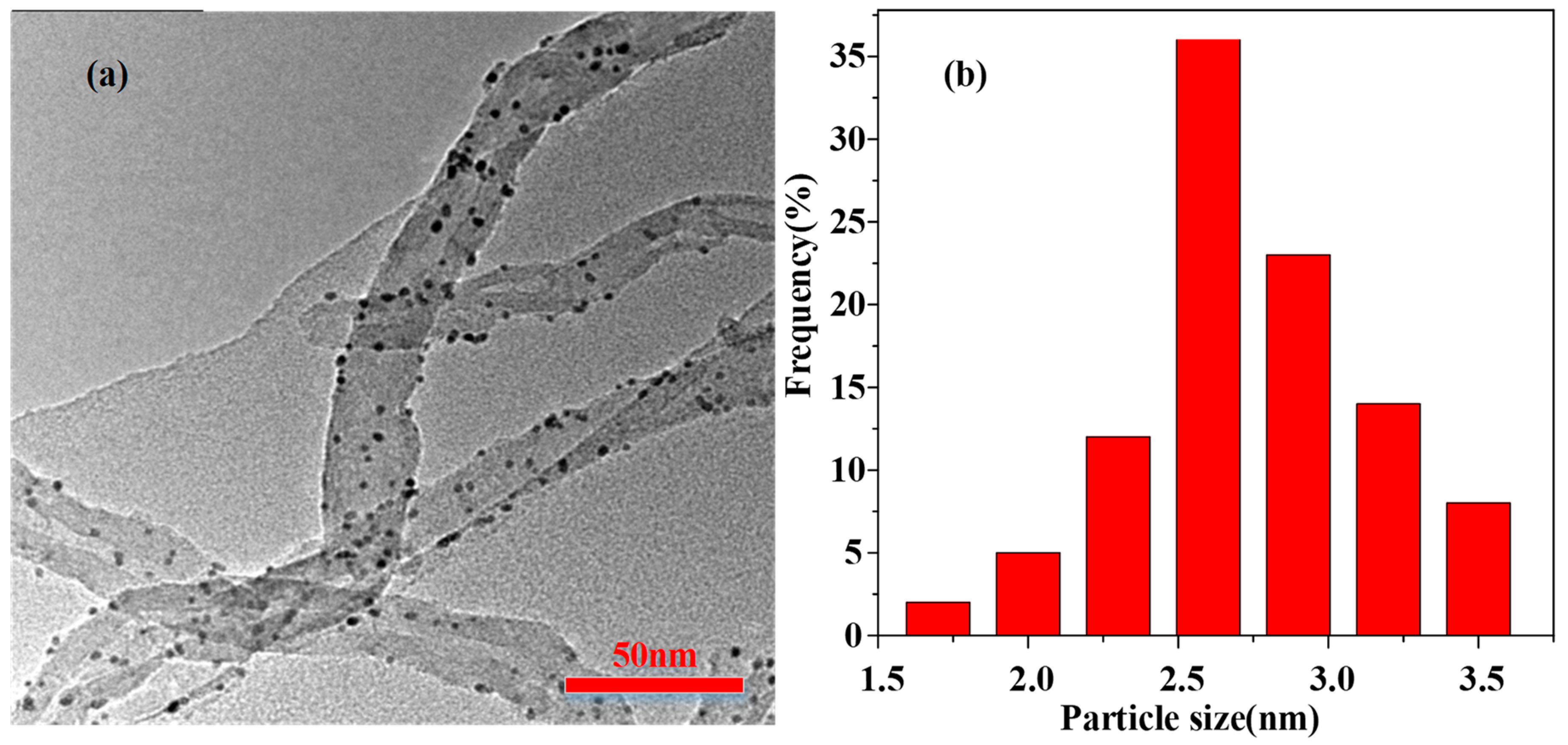
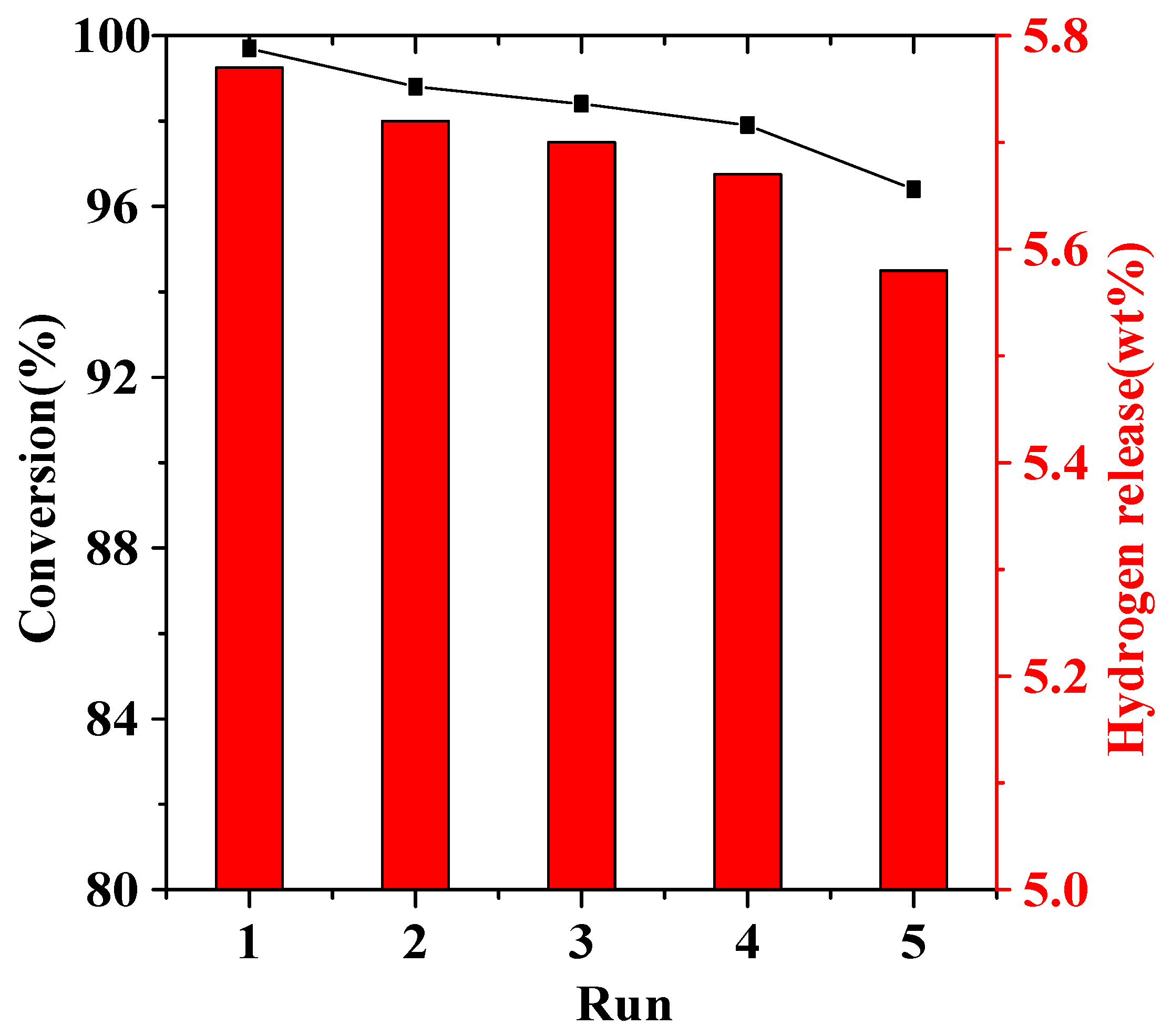
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
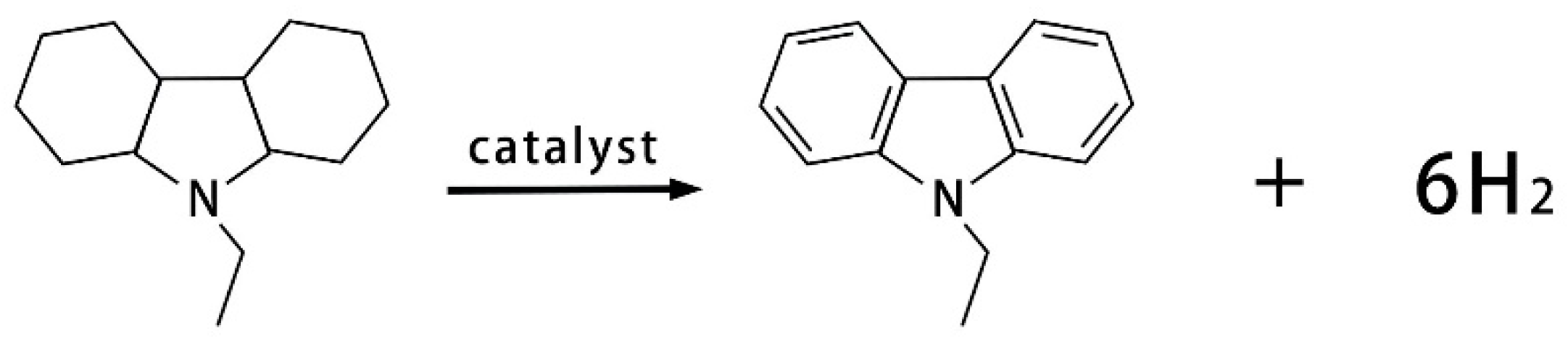
3.3. Hydrogen Generation from Dodecahydro-N-ethylcarbazole
3.4. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Turner, J.A. Sustainable Hydrogen Production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Kanezashi, M.; Tsuru, T. Catalytic Ammonia Decomposition over High-Performance Ru/Graphene Nanocomposites for Efficient COx-Free Hydrogen Production. Catalysts 2017, 7, 23. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Shao, H.; Li, W.; Lin, H. Catalysis and Downsizing in Mg-Based Hydrogen Storage Materials. Catalysts 2018, 8, 89. [Google Scholar] [CrossRef]
- Patil, S.P.; Pande, J.V.; Biniwale, R.B. Non-noble Ni–Cu/ACC bimetallic catalyst for dehydrogenation of liquid organic hydrides for hydrogen storage. Int. J. Hydrogen Energy 2013, 38, 15233–15241. [Google Scholar] [CrossRef]
- Xu, L.X.; Liu, N.; Hong, B.; Cui, P.; Cheng, D.G.; Chen, F.Q.; An, Y.; Wan, C. Nickel–platinum nanoparticles immobilized on graphitic carbon nitride as highly efficient catalyst for hydrogen release from hydrous hydrazine. RSC Adv. 2016, 6, 31687–31691. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niaz, S.; Manzoor, T.; Pandith, A.H. Hydrogen storage: Materials, methods and perspectives. Renew. Sustain. Energy Rev. 2015, 50, 457–469. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Xu, Q. Liquid organic and inorganic chemical hydrides for high-capacity hydrogen storage. Energy Environ. Sci. 2015, 8, 478–512. [Google Scholar] [CrossRef]
- Yao, F.; Li, X.; Wan, C.; Xu, L.X.; An, Y.; Ye, M.F.; Lei, Z. Highly efficient hydrogen release from formic acid using a graphitic carbon nitride-supported AgPd nanoparticle catalyst. Appl. Surf. Sci. 2017, 426, 605–611. [Google Scholar] [CrossRef]
- Xia, Z.J.; Liu, H.Y.; Lu, H.F.; Zhang, Z.K.; Chen, Y.F. Study on catalytic properties and carbon deposition of Ni-Cu/SBA-15 for cyclohexane dehydrogenation. Appl. Surf. Sci. 2017, 422, 905–912. [Google Scholar] [CrossRef]
- Bandaru, S.; English, N.J.; Phillips, A.D.; MacElroy, J.M.D. Exploring Promising Catalysts for Chemical Hydrogen Storage in Ammonia Borane: A Density Functional Theory Study. Catalysts 2017, 7, 140. [Google Scholar] [CrossRef]
- Teichmann, D.; Arlt, W.; Wasserscheid, P.; Freymann, R. A future energy supply based on Liquid Organic Hydrogen Carriers (LOHC). Energy Environ. Sci. 2011, 4, 2767–2773. [Google Scholar] [CrossRef]
- Jiang, Z.; Pan, Q.; Xu, J.; Fang, T. Current situation and prospect of hydrogen storage technology with new organic liquid. Int. J. Hydrogen Energy 2014, 39, 17442–17451. [Google Scholar] [CrossRef]
- Markiewicz, M.; Zhang, Y.Q.; Bösmann, A.; Brückner, N.; Thöming, J.; Wasserscheid, P.; Stolte, S. Environmental and health impact assessment of Liquid Organic Hydrogen Carrier (LOHC) systems—challenges and preliminary results. Energy Environ. Sci. 2015, 8, 1035–1045. [Google Scholar] [CrossRef]
- Patil, S.P.; Bindwal, A.B.; Pakade, Y.B.; Biniwale, R.B. On H2 supply through liquid organic hydrides-Effect of functional groups. Int. J. Hydrogen Energy 2017, 42, 16214–16224. [Google Scholar] [CrossRef]
- Teichmann, D.; Stark, K.; Müller, K.; Zöttl, G.; Wasserscheid, P.; Arlt, W. Energy storage in residential and commercial buildings via Liquid Organic Hydrogen Carriers (LOHC). Energy Environ. Sci. 2012, 5, 9044–9054. [Google Scholar] [CrossRef]
- Wan, C.; An, Y.; Chen, F.Q.; Cheng, D.G.; Wu, F.Y.; Xu, G.H. Kinetics of N-ethylcarbazole hydrogenation over a supported Ru catalyst for hydrogen storage. Int. J. Hydrogen Energy 2013, 38, 7065–7069. [Google Scholar] [CrossRef]
- Eblagon, K.M.; Tam, K.; Yu, K.M.K.; Zhao, S.L.; Gong, X.Q.; He, H.Y.; Ye, L.; Wang, L.C.; Ramirez-Cuesta, A.J.; Tsang, S.C. Study of Catalytic Sites on Ruthenium for Hydrogenation of N-ethylcarbazole: Implications of Hydrogen Storage via Reversible Catalytic Hydrogenation. J. Phys. Chem. C 2010, 114, 9720–9730. [Google Scholar] [CrossRef]
- Preuster, P.; Papp, C.; Wasserscheid, P. Liquid Organic Hydrogen Carriers (LOHCs): Toward a Hydrogen-free Hydrogen Economy. Acc. Chem. Res. 2017, 50, 74–85. [Google Scholar] [CrossRef]
- Sotoodeh, F.; Huber, B.J.M.; Smith, K.J. The effect of the N atom on the dehydrogenation of heterocycles used for hydrogen storage. Appl. Catal. A 2012, 419–420, 67–72. [Google Scholar] [CrossRef]
- Crabtree, R.H. Nitrogen-Containing Liquid Organic Hydrogen Carriers: Progress and Prospects. ACS Sustain. Chem. Eng. 2017, 5, 4491–4498. [Google Scholar] [CrossRef]
- Stark, K.; Emel’yanenko, V.N.; Zhabina, A.A.; Varfolomeev, M.A.; Verevkin, S.P.; Müller, K.; Arlt, W. Liquid Organic Hydrogen Carriers: Thermophysical and Thermochemical Studies of Carbazole Partly and Fully Hydrogenated Derivatives. Ind. Eng. Chem. Res. 2015, 54, 7953–7966. [Google Scholar] [CrossRef]
- Papp, C.; Wasserscheid, P.; Libuda, J.; Steinrück, H.P. Liquid Organic Hydrogen Carriers: Surface Science Studies of Carbazole Derivatives. Chem. Rec. 2014, 14, 879–896. [Google Scholar] [CrossRef] [PubMed]
- Arnende, M.; Gleichweit, C.; Werner, K.; Schernich, S.; Zhao, W.; Lorenz, M.P.A.; Hofert, O.; Papp, C.; Koch, M.; Wasserscheid, P. Model Catalytic Studies of Liquid Organic Hydrogen Carriers: Dehydrogenation and Decomposition Mechanisms of Dodecahydro-N-ethylcarbazole on Pt(111). ACS Catal. 2014, 4, 657–665. [Google Scholar]
- Amende, M.; Schernich, S.; Sobota, M.; Nikiforidis, I.; Hieringer, W.; Assenbaum, D.; Gleichweit, C.; Drescher, H.J.; Papp, C.; Steinruck, H.P. Dehydrogenation Mechanism of Liquid Organic Hydrogen Carriers: Dodecahydro-N-ethylcarbazole on Pd(111). Chem. Eur. J. 2013, 19, 10854–10865. [Google Scholar] [CrossRef] [PubMed]
- Gleichweit, C.; Amende, M.; Schernich, S.; Zhao, W.; Lorenz, M.P.A.; Hofert, O.; Bruckner, N.; Wasserscheid, P.; Libuda, J.; Steinruck, H.P. Dehydrogenation of Dodecahydro-N-ethylcarbazole on Pt(111). ChemSusChem 2013, 6, 974–977. [Google Scholar] [CrossRef] [PubMed]
- Amende, M.; Gleichweit, C.; Schernich, S.; Höfert, O.; Lorenz, M.P.A.; Zhao, W.; Koch, M.; Obesser, K.; Papp, C.; Wasserscheid, P.; et al. Size and Structure Effects Controlling the Stability of the Liquid Organic Hydrogen Carrier Dodecahydro-N-ethylcarbazole during Dehydrogenation over Pt Model Catalysts. J. Phys. Chem. Lett. 2014, 5, 1498–1504. [Google Scholar] [CrossRef]
- Sobota, M.; Nikiforidis, I.; Amende, M.; Zanon, B.S.; Staudt, T.; Hofert, O.; Lykhach, Y.; Papp, C.; Hieringer, W.; Laurin, M. Dehydrogenation of Dodecahydro-N-ethylcarbazole on Pd/Al2O3 Model Catalysts. Chem. Eur. J. 2011, 17, 11542–11552. [Google Scholar] [CrossRef]
- Peters, W.; Eypasch, M.; Frank, T.; Schwerdtfeger, J.; Korner, C.; Bosmann, A.; Wasserscheid, P. Efficient hydrogen release from perhydro-N-ethylcarbazole using catalyst-coated metallic structures produced by selective electron beam melting. Energy Environ. Sci. 2015, 8, 641–649. [Google Scholar] [CrossRef] [Green Version]
- Peters, W.; Seidel, A.; Herzog, S.; Bosmann, A.; Schwieger, W.; Wasserscheid, P. Macrokinetic effects in perhydro-N-ethylcarbazole dehydrogenation and H2 productivity optimization by using egg-shell catalysts. Energy Environ. Sci. 2015, 8, 3013–3021. [Google Scholar] [CrossRef]
- Tarasov, A.L.; Tkachenko, O.P.; Kustov, L.M. Mono and Bimetallic Pt–(M)/Al2O3 Catalysts for Dehydrogenation of Perhydro-N-ethylcarbazole as the Second Stage of Hydrogen Storage. Catal. Lett. 2018, 148, 1472–1477. [Google Scholar] [CrossRef]
- Fei, S.X.; Han, B.; Li, L.L.; Mei, P.; Zhu, T.; Yang, M.; Cheng, H.S. A study on the catalytic hydrogenation of N-ethylcarbazole on the mesoporous Pd/MoO3 catalyst. Int. J. Hydrogen Energy 2017, 42, 25942–25950. [Google Scholar] [CrossRef]
- Sotoodeh, F.; Smith, K.J. Structure sensitivity of dodecahydro-N-ethylcarbazole dehydrogenation over Pd catalysts. J. Catal. 2011, 279, 36–47. [Google Scholar] [CrossRef]
- Wang, Z.H.; Tonks, I.; Belli, J.; Jensen, C.M. Dehydrogenation of N-ethyl perhydrocarbazole catalyzed by PCP pincer iridium complexes: Evaluation of a homogenous hydrogen storage system. J. Organomet. Chem. 2009, 694, 2854–2857. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, M.; Mei, P.; Li, C.G.; Li, L.L. Dehydrogenation kinetics study of perhydro-N-ethylcarbazole over a supported Pd catalyst for hydrogen storage application. Int. J. Hydrogen Energy 2016, 41, 8498–8505. [Google Scholar] [CrossRef]
- Yang, M.; Dong, Y.; Fei, S.X.; Ke, H.Z.; Cheng, H.S. A comparative study of catalytic dehydrogenation of perhydro-N-ethylcarbazole over noble metal catalysts. Int. J. Hydrogen Energy 2014, 39, 18976–18983. [Google Scholar] [CrossRef]
- Kustov, L.M.; Tarasov, A.L.; Kirichenko, O.A. Microwave-activated dehydrogenation of perhydro-N-ethylcarbazol over bimetallic Pd-M/TiO2 catalysts as the second stage of hydrogen storage in liquid substrates. Int. J. Hydrogen Energy 2017, 42, 26723–26729. [Google Scholar] [CrossRef]
- Wang, B.; Chang, T.Y.; Jiang, Z.; Wei, J.J.; Zhang, Y.H.; Yang, S.; Fang, T. Catalytic dehydrogenation study of dodecahydro-N-ethylcarbazole by noble metal supported on reduced graphene oxide. Int. J. Hydrogen Energy 2018, 43, 7317–7325. [Google Scholar] [CrossRef]
- Yazdan-Abad, M.Z.; Noroozifar, M.; Alfi, N.; Modarresi-Alam, A.R.; Saravani, H. A simple and fast method for the preparation of super active Pd/CNTs catalyst toward ethanol electrooxidation. Int. J. Hydrogen Energy 2018, 43, 12103–12109. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, S.F.; Xiang, Y.; Shen, P.K.; Liu, J.; Jiang, S.P. Carbon-Nanotubes-Supported Pd Nanoparticles for Alcohol Oxidations in Fuel Cells: Effect of Number of Nanotube Walls on Activity. ChemSusChem 2015, 8, 2956–2966. [Google Scholar] [CrossRef]
- Xia, Y.; Ye, J.R.; Cheng, D.G.; Chen, F.Q.; Zhan, X.L. Identification of a flattened Pd–Ce oxide cluster as a highly efficient catalyst for low-temperature CO oxidation. Catal. Sci. Technol. 2018, 8, 5137–5147. [Google Scholar] [CrossRef]
- Wang, B.; Yan, T.; Chang, T.Y.; Wei, J.J.; Zhou, Q.; Yang, S.; Fang, T. Palladium supported on reduced graphene oxide as a high-performance catalyst for the dehydrogenation of dodecahydro-N-ethylcarbazole. Carbon 2017, 122, 9–18. [Google Scholar] [CrossRef]
- Wang, A.Q.; Li, J.; Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2018, 2, 65–81. [Google Scholar] [CrossRef]
- Passaponti, M.; Savastano, M.; Clares, M.P.; Inclán, M.; Lavacchi, A.; Bianchi, A.; García-España, E.; Innocenti, M. MWCNTs-Supported Pd(II) Complexes with High Catalytic Efficiency in Oxygen Reduction Reaction in Alkaline Media. Inorg. Chem. 2018, 57, 14484–14488. [Google Scholar] [CrossRef] [PubMed]
- Teranishi, T.; Miyake, M. Size Control of Palladium Nanoparticles and Their Crystal Structures. Chem. Mater. 1998, 10, 594–600. [Google Scholar] [CrossRef]
- Wu, J.M.; Zeng, L.; Cheng, D.G.; Chen, F.Q.; Zhan, X.L.; Gong, J.L. Synthesis of Pd nanoparticles supported on CeO2 nanotubes for CO oxidation at low temperatures. Chin. J. Catal. 2016, 37, 83–90. [Google Scholar] [CrossRef]
- Koh, K.; Seo, J.-E.; Lee, J.H.; Goswami, A.; Yoon de, C.W.; Asefa, T. Ultrasmall palladium nanoparticles supported on amine-functionalized SBA-15 efficiently catalyze hydrogen evolution from formic acid. J. Mater. Chem. A 2014, 2, 20444–20449. [Google Scholar] [CrossRef]
- Sotoodeh, F.; Huber, B.J.M.; Smith, K.J. Dehydrogenation kinetics and catalysis of organic heteroaromatics for hydrogen storage. Int. J. Hydrogen Energy 2012, 37, 2715–2722. [Google Scholar] [CrossRef]
- Sotoodeh, F.; Zhao, L.; Smith, K.J. Kinetics of H2 recovery from dodecahydro-N-ethylcarbazole over a supported Pd catalyst. Appl. Catal. A Gen. 2009, 362, 155–162. [Google Scholar] [CrossRef]
- Wan, C.; An, Y.; Xu, G.; Kong, W. Study of catalytic hydrogenation of N-ethylcarbazole over ruthenium catalyst. Int. J. Hydrogen Energy 2012, 37, 13092–13096. [Google Scholar] [CrossRef]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.; Xu, L.; Du, L.; An, Y.; Wan, C. Palladium Supported on Carbon Nanotubes as a High-Performance Catalyst for the Dehydrogenation of Dodecahydro-N-ethylcarbazole. Catalysts 2018, 8, 638. https://doi.org/10.3390/catal8120638
Zhu M, Xu L, Du L, An Y, Wan C. Palladium Supported on Carbon Nanotubes as a High-Performance Catalyst for the Dehydrogenation of Dodecahydro-N-ethylcarbazole. Catalysts. 2018; 8(12):638. https://doi.org/10.3390/catal8120638
Chicago/Turabian StyleZhu, Mengyan, Lixin Xu, Lin Du, Yue An, and Chao Wan. 2018. "Palladium Supported on Carbon Nanotubes as a High-Performance Catalyst for the Dehydrogenation of Dodecahydro-N-ethylcarbazole" Catalysts 8, no. 12: 638. https://doi.org/10.3390/catal8120638