Modeling of a Pilot-Scale Fixed-Bed Reactor for Dehydration of 2,3-Butanediol to 1,3-Butadiene and Methyl Ethyl Ketone
Abstract
:1. Introduction
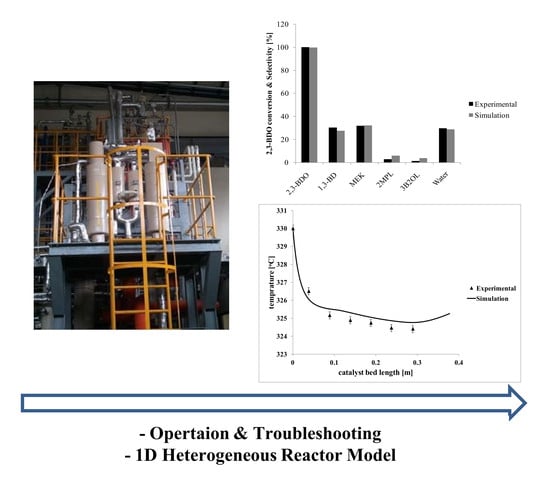
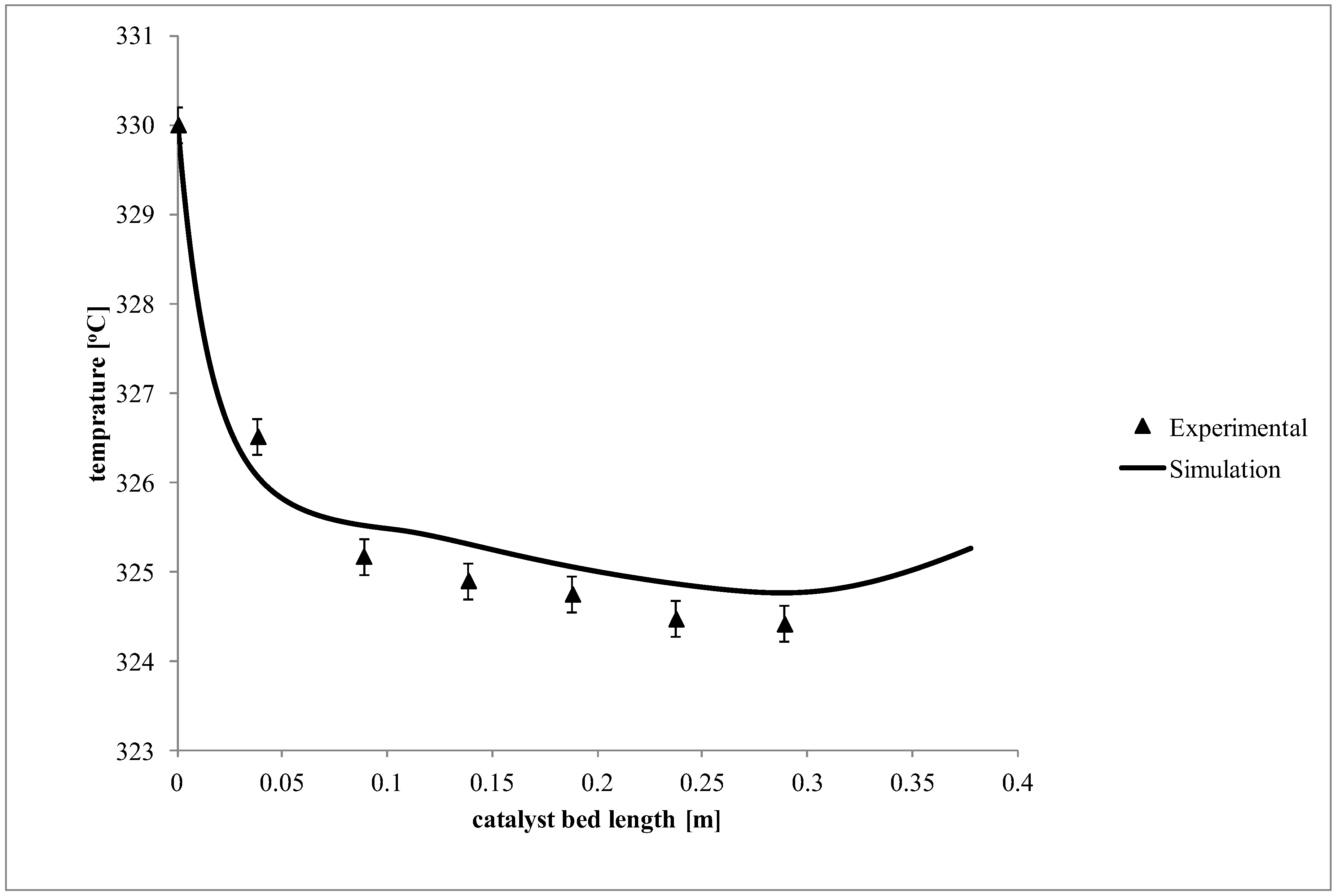
2. Results and Discussion
3. Experimental Studies
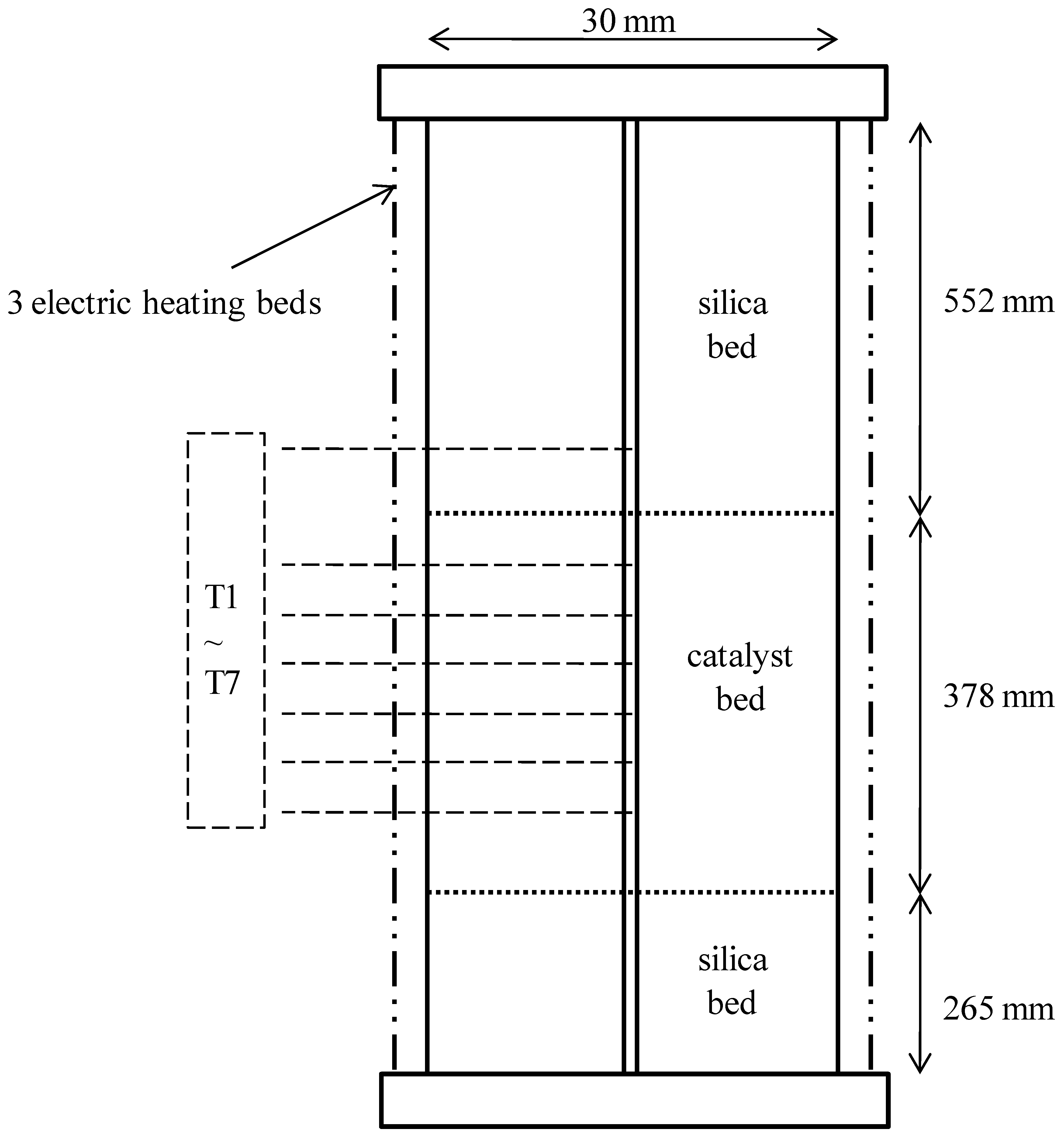
3.1. Experimental Setup
3.2. Operation and Troubleshooting
3.3. Analysis Methods
4. Development of the Reactor Model
4.1. Reaction Kinetics
4.2. Reactor Model
5. Conclusions
Conflicts of Interest
Nomenclature
| av | external particle surface area per unit reactor volume, m2/m3 |
| Biot number, m | |
| C | mole concentration, mol/m3 |
| Cpf | fluid heat capacity, J/kg K |
| Csj | mole concentration in catalysts, mol/m3 |
| Csj,s | mole concentration at the surface of the catalyst, mol/m3 |
| logarithmic mean diameter, m | |
| dp | diameter of a catalyst, m |
| dt | diameter of a reactor, m |
| De | effective diffusivity within a catalyst, m2/s |
| mean diffusivity coefficient, m2/s | |
| E | activation energy, J/mol |
| F | mass flow rate, g/s |
| Fin | mass flow rate at the inlet of the catalyst bed, g/s |
| hf | heat transfer coefficient between catalyst surface and fluid, W/m2 K |
| hi | tube inside heat transfer coefficient, W/m2 K |
| k | reaction rate constant, mol(1−n) m3(n−1) s−1 |
| kf | mass transfer coefficient between catalyst surface and fluid, m/s |
| transformed adsorption pre-exponential factor, m3/mol | |
| Lt | length of the catalyst bed, m |
| n | reaction order |
| Nusselt number for fluid-solid heat transfer | |
| P | pressure, Pa |
| Prandtl number for the fluid | |
| r | reaction rate, mol/kg-cat s |
| rp | catalyst radius, m |
| rsi | reaction rate in catalysts, mol/kg-cat s |
| rsi,s | reaction rate at the surface of the catalyst, mol/kg-cat s |
| R | ideal gas law constant, J/mol K |
| Reynolds number for packed bed | |
| S | mass selectivity, % |
| Schmidt number | |
| Sherwood number for packed bed | |
| T | temperature, K |
| Tin | temperature at the inlet of the catalyst bed, K |
| Ts | temperature in catalysts, K |
| Tss | temperature at the surface of catalysts, K |
| Tref | reference temperature, K |
| Tw | temperature of electric heaters around the reactors, K |
| us | superficial fluid velocity, m/s |
| U | overall heat transfer coefficient, W/m2 K |
| X | conversion, % |
| tube wall thickness, m | |
| z | axial reactor coordinate, m |
| Greek Letters | |
| effective bed-wall heat transfer coefficient, W/m2 K | |
| static term of the effective bed-wall heat transfer coefficient, W/m2 K | |
| static term of the effective bed-wall heat transfer coefficient, W/m2 K | |
| heat of reaction, J/mol | |
| catalyst porosity | |
| effectiveness factor | |
| wall thermal conductivity, W/m K | |
| catalyst heat conductivity, W/m K | |
| effective bed heat conductivity, W/m K | |
| static term of effective bed heat, W/m K | |
| dynamic term of effective bed heat conductivity, W/m K | |
| stoichiometric coefficient of species j in reaction i | |
| bulk density of catalyst bed, kg/m3 | |
| fluid density, kg/m3 | |
| catalyst density, kg/m3 | |
| catalyst tortuosity | |
| Subscripts | |
| i | reaction i |
| j | species j |
| react | reactant |
| rxn | reaction |
References
- Daniell, J.; Köpke, M.; Simpson, S.D. Commercial biomass syngas fermentation. Energies 2012, 5, 5372–5417. [Google Scholar] [CrossRef]
- Kopke, M.; Mihalcea, C.; Liew, F.; Tizard, J.H.; Ali, M.S.; Conolly, J.J.; Al-Sinawi, B.; Simpson, S.D. 2,3-Butanediol Production by Acetogenic Bacteria, an Alternative Route to Chemical Synthesis, Using Industrial Waste Gas. Appl. Environ. Microbiol. 2011, 77, 5467–5475. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Wales, M.D.; Heidlage, M.G.; Rezac, M.; Wang, H.; Bossmann, S.H.; Hohn, K.L. Conversion of 2,3-butanediol to butenes over bifunctional catalysts in a single reactor. J. Catal. 2015, 330, 222–237. [Google Scholar] [CrossRef]
- Bourns, A.N.; Nicholls, R.V.V. The Catalytic Action of Aluminium Silicates: I. The Dehydration of Butanediol-2,3 and Butanone-2 over Activated Morden Bentonite. Can. J. Res. 1947, 25b, 80–89. [Google Scholar] [CrossRef]
- Winfield, M.E. The catalytic dehydration of 2,3-butanediol to butadiene. Aust. J. Sci. Res. Ser. A 1945, 3, 290–305. [Google Scholar]
- Winfield, M.E. The Catalytic Dehydration of 2,3-Butanediol to Butadiene. II. Adsorption Equilibriaitle. Aust. J. Sci. Res. Ser. A 1950, 3, 290–305. [Google Scholar]
- Kannan, S.V.; Pillai, C.N. Dehydration of meso- and dl-hydrobenzoins and 2,3-butanediols over alumina. Indian J. Chem. 1969, 7, 1164–1166. [Google Scholar]
- Duan, H.; Sun, D.; Yamada, Y.; Sato, S. Dehydration of 2,3-butanediol into 3-buten-2-ol catalyzed by ZrO2. Catal. Commun. 2014, 48, 1–4. [Google Scholar] [CrossRef]
- Duan, H.; Yamada, Y.; Sato, S. Applied Catalysis A: General Efficient production of 1,3-butadiene in the catalytic dehydration of 2,3-butanediol. Appl. Catal. A Gen. 2015, 491, 163–169. [Google Scholar] [CrossRef]
- Bucsi, I.; Molnár, Á.; Bartók, M.; Olah, G.A. Transformation of 1,3-, 1,4- and 1,5-diols over perfluorinated resinsulfonic acids (Nafion-H). Tetrahedron 1995, 51, 3319–3326. [Google Scholar] [CrossRef]
- Molnár, Á.; Bucsi, I.; Bartók, M. Pinacol rearrangement on zeolites. Stud. Surf. Sci. Catal. 1988, 41, 203–210. [Google Scholar] [CrossRef]
- Lee, J.; Grutzner, J.B.; Walters, W.E.; Delgass, W.N. The conversion of 2,3-butanediol to methyl ethyl ketone over zeolites. Stud. Surf. Sci. Catal. 2000, 130, 2603–2608. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, D.; Ji, X.; Huang, H. Efficient dehydration of bio-based 2,3-butanediol to butanone over boric acid modified HZSM-5 zeolites. Green Chem. 2012, 14, 3441–3450. [Google Scholar] [CrossRef]
- Tsrsk, B.; Bucsi, I.; Beregsz, T.; Kapocsi, I.; Molnfir, A. Transformation of diols in the presence of heteropoly acids under homogeneous and heterogeneous conditions. J. Mol. Catal. A Chem. 1996, 107, 305–311. [Google Scholar]
- Hahn, H.-D.; Dämbkes, G.; Rupprich, N.; Bahl, H.; Frey, G.D. Butanols. Ullmanns Encycl. Ind. Chem. 2013, 1–13. [Google Scholar] [CrossRef]
- Kim, S.J.; Seo, S.O.; Park, Y.C.; Jin, Y.S.; Seo, J.H. Production of 2,3-butanediol from xylose by engineered Saccharomyces cerevisiae. J. Biotechnol. 2014, 192, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Nikitina, M.A.; Sushkevich, V.L.; Ivanova, I.I. Dehydration of 2,3-butanediol over zeolite catalysts. Pet. Chem. 2016, 56, 230–236. [Google Scholar] [CrossRef]
- Song, D. Kinetic Model Development for Dehydration of 2,3-Butanediol to 1,3-Butadiene and Methyl Ethyl Ketone over an Amorphous Calcium Phosphate Catalyst. Ind. Eng. Chem. Res. 2016, 55, 11664–11671. [Google Scholar] [CrossRef]
- Song, D. Development of a deactivation model for the dehydration of 2,3-butanediol to 1,3-butadiene and methyl ethyl ketone over an amorphous calcium phosphate catalyst. Ind. Eng. Chem. Res. 2017, 56, 11013–11020. [Google Scholar] [CrossRef]
- Tsukamoto, D.; Sakami, S.; Ito, M.; Yamada, K.; Ito, M.; Yonehara, T. Production of Bio-based 1,3-Butadiene by Highly Selective Dehydration of 2,3-Butanediol over SiO2-supported Cesium Dihydrogen Phosphate Catalyst. Chem. Lett. 2016, 45, 831–833. [Google Scholar] [CrossRef]
- Kim, T.Y.; Baek, J.; Song, C.K.; Yun, Y.S.; Park, D.S.; Kim, W.; Han, J.W.; Yi, J. Gas-phase dehydration of vicinal diols to epoxides: Dehydrative epoxidation over a Cs/SiO2 catalyst. J. Catal. 2015, 323, 85–99. [Google Scholar] [CrossRef]
- Kim, W.; Shin, W.; Lee, K.J.; Song, H.; Kim, H.S.; Seung, D.; Filimonov, I.N. Applied Catalysis A: General 2,3-Butanediol dehydration catalyzed by silica-supported sodium phosphates. Appl. Catal. A Gen. 2016, 511, 156–167. [Google Scholar] [CrossRef]
- Makshina, E.V.; Dusselier, M.; Janssens, W.; Degrève, J.; Jacobs, P.A.; Sels, B.F. Review of old chemistry and new catalytic advances in the on-purpose synthesis of butadiene. Chem. Soc. Rev. 2014, 43, 7917–7953. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Yamada, Y.; Sato, S. Future prospect of the production of 1,3-butadiene from butanediols. Chem. Lett. 2016, 45, 1036–1047. [Google Scholar] [CrossRef]
- Song, D.; Yoon, Y.-G.; Lee, C.-J. Conceptual design for the recovery of 1,3-Butadiene and methyl ethyl ketone via a 2,3-Butanediol-dehydration process. Chem. Eng. Res. Des. 2017, 123, 268–276. [Google Scholar] [CrossRef]
- Kelley, K.K. [Part] 13. High-Temperature Heat-Content, Heat-Capacity, and Entropy Data for the Elements and Inorganic Compounds; Bureau of Mines: Washington, DC, USA, 1960. [Google Scholar]
- Bhatia, S.K. Directional autocorrelation and the diffusional tortuosity of capillary porous media. J. Catal. 1985, 93, 192–196. [Google Scholar] [CrossRef]
- Dykhuizen, R.C.; Casey, W.H. An analysis of solute diffusion in rocks. Geochim. Cosmochim. Acta 1989, 53, 2797–2805. [Google Scholar] [CrossRef]
- Ergun, S. Fluid Flow through Packed Columns. Chem. Eng. Prog. 1952, 48, 89–94. [Google Scholar]
- Hougen, O. Engineering Aspects of Solid Catalysts. Ind. Eng. Chem. 1961, 53, 509–528. [Google Scholar] [CrossRef]
- Dixon, A.G. An improved equation for the overall heat transfer coefficient in packed beds. Chem. Eng. Process. Process Intensif. 1996, 35, 323–331. [Google Scholar] [CrossRef]
- Specchia, V.; Baldi, G.; Sicardi, S. Heat Transfer in Packed Bed Reactors with One Phase Flow. Chem. Eng. Commun. 1980, 4, 361–380. [Google Scholar] [CrossRef]
- Process Systems Enterprise Ltd. gPROMS Advanced User Guide; Process Systems Enterprise Ltd.: London, UK, 2004. [Google Scholar]
- McCabe, W.L.; Smith, J.; Harriott, P. Unit Operations of Chemical Engineering, 6th ed.; McGraw Hill: New York, NY, USA, 2001. [Google Scholar]
- Riggs, J.M. Introduction to Numerical Methods for Chemical Engineer, 2nd ed.; Texas Tech University Press: Lubbock, TX, USA, 1994. [Google Scholar]
- Poling, B.E.; Prausnitz, J.M.; O’s Connell, J.P. The Properties of Gases & Liquids; McGraw Hill: New York, NY, USA, 2001. [Google Scholar]
- Perry, R.H.; Green, D.W. Perry’s Chemical Engineering Hand-Book; McGraw Hill: New York, NY, USA, 1997. [Google Scholar]


| Property | Value | Unit | |
|---|---|---|---|
| Catalyst | Type | Amorphous Calcium Phosphate (Ca/P = 1.3) | |
| average diameter | 2.855 | mm | |
| weight | 80 | g | |
| density | 460.5 | kg/m3 | |
| heat capacity [26] | 995 | J/(kg K) | |
| conductivity | 0.251 | W/(m K) | |
| porosity | 0.121 | - | |
| tortuosity [27,28] | 1.73 | - | |
| tube | inner diameter | 30 | mm |
| tube length | 1195 | mm | |
| tube wall | thickness | 3.937 | mm |
| thermal conductivity | 16 | W/(m K) | |
| heat capacity | 2000 | J/(kg K) | |
| catalyst bed | length | 378 | mm |
| density | 299.4 | kg/m3 | |
| porosity | 0.35 | - |
| Operating Conditions | Test 1 | Test 2 | Unit |
|---|---|---|---|
| inlet temperature of the catalyst bed | 330 | 330 | °C |
| pressure | 1 | 1 | bar |
| N2 flow rate | 0 | 393 | g/h |
| 2,3-BDO flow rate | 80 | 39 | g/h |
| temperature of 3 electric heating beds | 330 | 330 | °C |
| Model Parameter | Unit | Value |
|---|---|---|
| E1 | J/mol | 2.33 × 105 |
| E2 | J/mol | 2.82 × 105 |
| E3 | J/mol | 1.93 × 105 |
| E4 | J/mol | 1.66 × 105 |
| kTref,1 | mol(1−n1) m3(n1−1) s−1 | 7.45 × 10−4 |
| kTref,2 | mol(1−n2) m3(n2−1) s−1 | 4.41 × 10−4 |
| kTref,3 | mol(1−n3) m3(n3−1) s−1 | 6.44 × 10−4 |
| kTref,4 | mol(1−n4) m3(n4−1) s−1 | 1.27 × 10−4 |
| n1, n3, n4 | - | 0.0187 |
| n2 | - | 0.146 |
| Parameter | Formula |
|---|---|
| mass and heat transfer coefficient between catalyst surface and fluid [30] | |
| overall heat transfer coefficient [34] | |
| tube inside heat transfer coefficient [31,32] | |
| effective diffusivity within a catalyst [35] | |
| effectiveness factor [35] |
| Property | Method |
|---|---|
| fluid density | Peng-Robinson [36] |
| fluid viscosity | Lucas [36] |
| fluid heat capacity | ideal gas [36] |
| fluid conductivity | Steil-Thodos [37] |
| binary diffusion coefficient, components i and j | Fuller-Schettler-Gidding (FSG) [37] |
| fluid compressibility factor | Peng-Robinson [36] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, D. Modeling of a Pilot-Scale Fixed-Bed Reactor for Dehydration of 2,3-Butanediol to 1,3-Butadiene and Methyl Ethyl Ketone. Catalysts 2018, 8, 72. https://doi.org/10.3390/catal8020072
Song D. Modeling of a Pilot-Scale Fixed-Bed Reactor for Dehydration of 2,3-Butanediol to 1,3-Butadiene and Methyl Ethyl Ketone. Catalysts. 2018; 8(2):72. https://doi.org/10.3390/catal8020072
Chicago/Turabian StyleSong, Daesung. 2018. "Modeling of a Pilot-Scale Fixed-Bed Reactor for Dehydration of 2,3-Butanediol to 1,3-Butadiene and Methyl Ethyl Ketone" Catalysts 8, no. 2: 72. https://doi.org/10.3390/catal8020072