Mn-Ce-V-WOx/TiO2 SCR Catalysts: Catalytic Activity, Stability and Interaction among Catalytic Oxides
Abstract
:1. Introduction
2. Results and Discussion
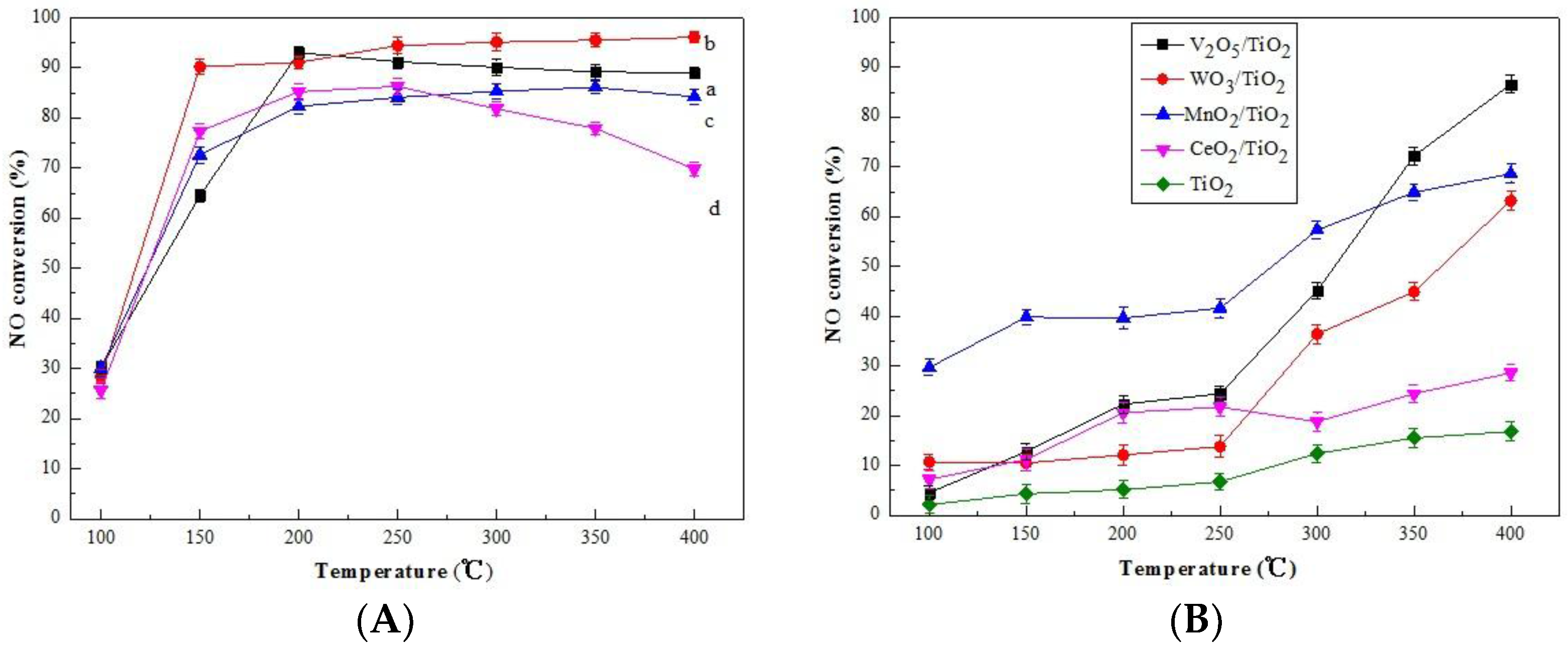
2.1. Catalytic Behavior
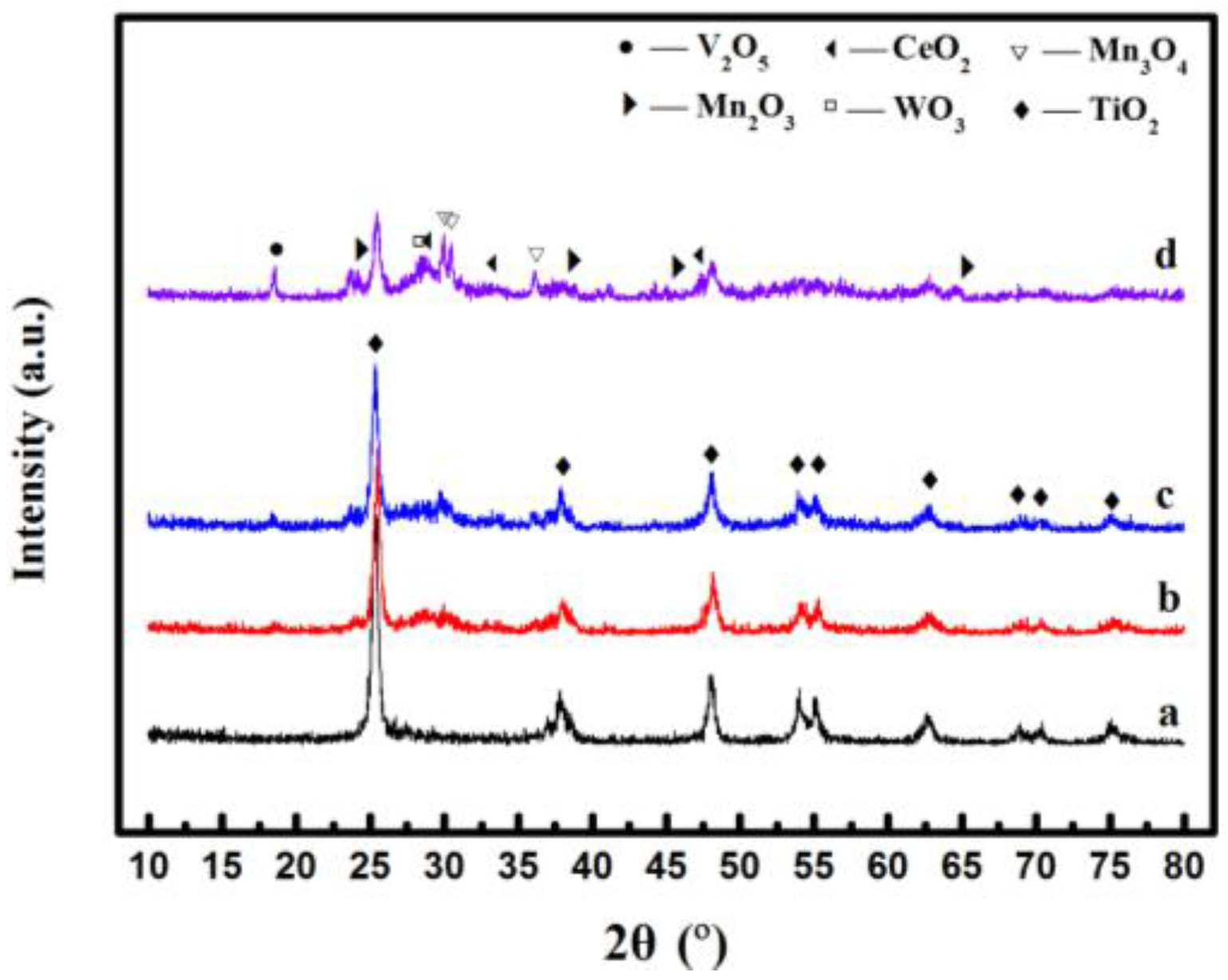
2.2. XRD Analysis
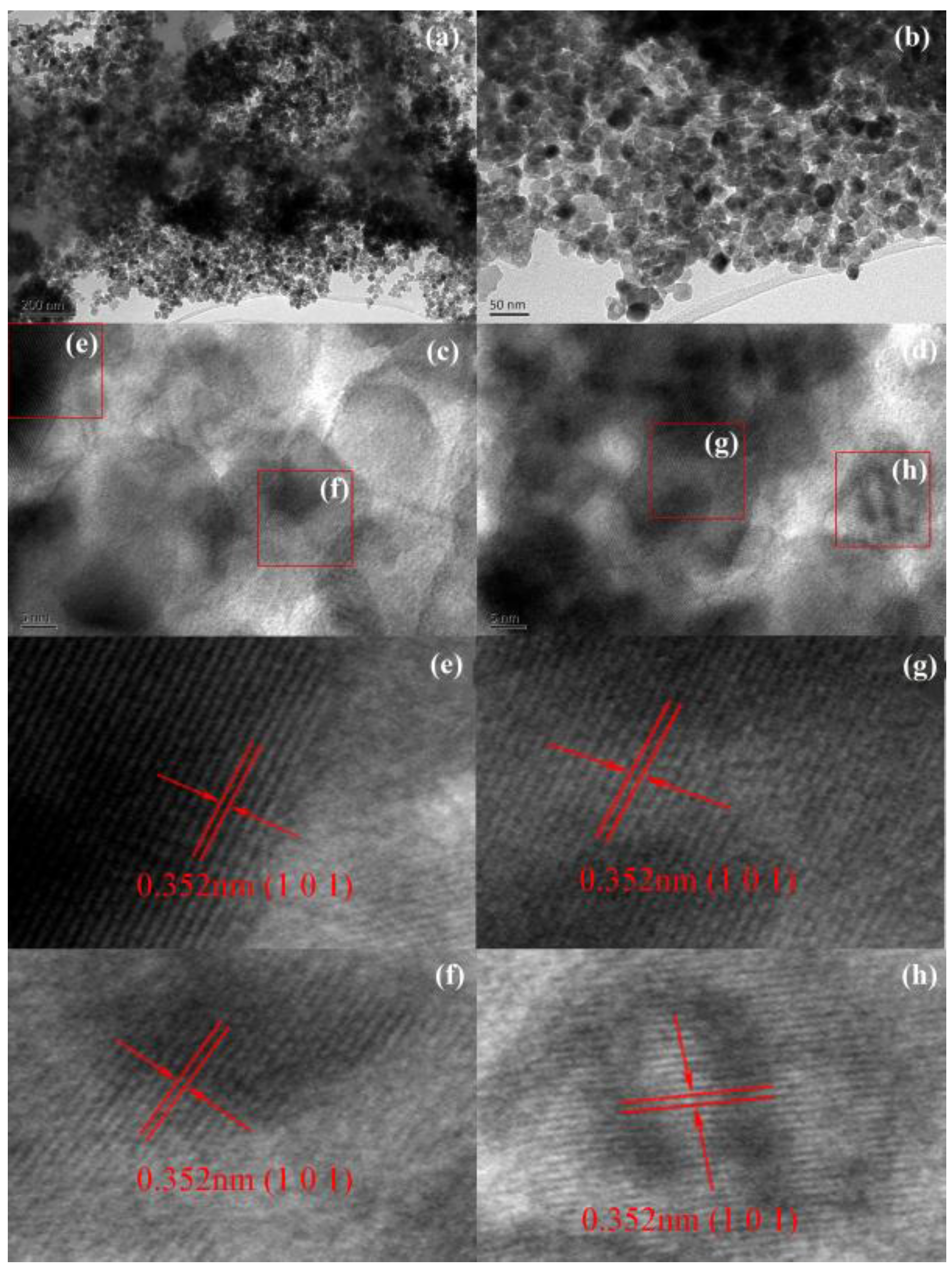
2.3. TEM and HRTEM Analysis
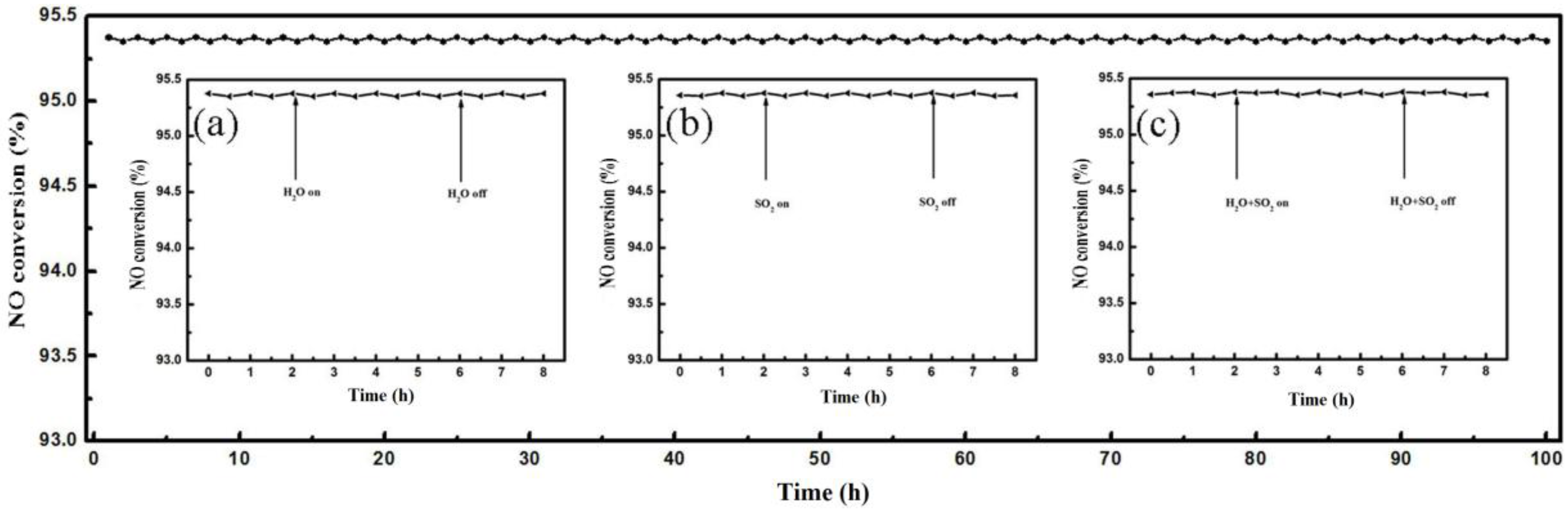
2.4. Catalyst Stability and H2O/SO2 Resistance
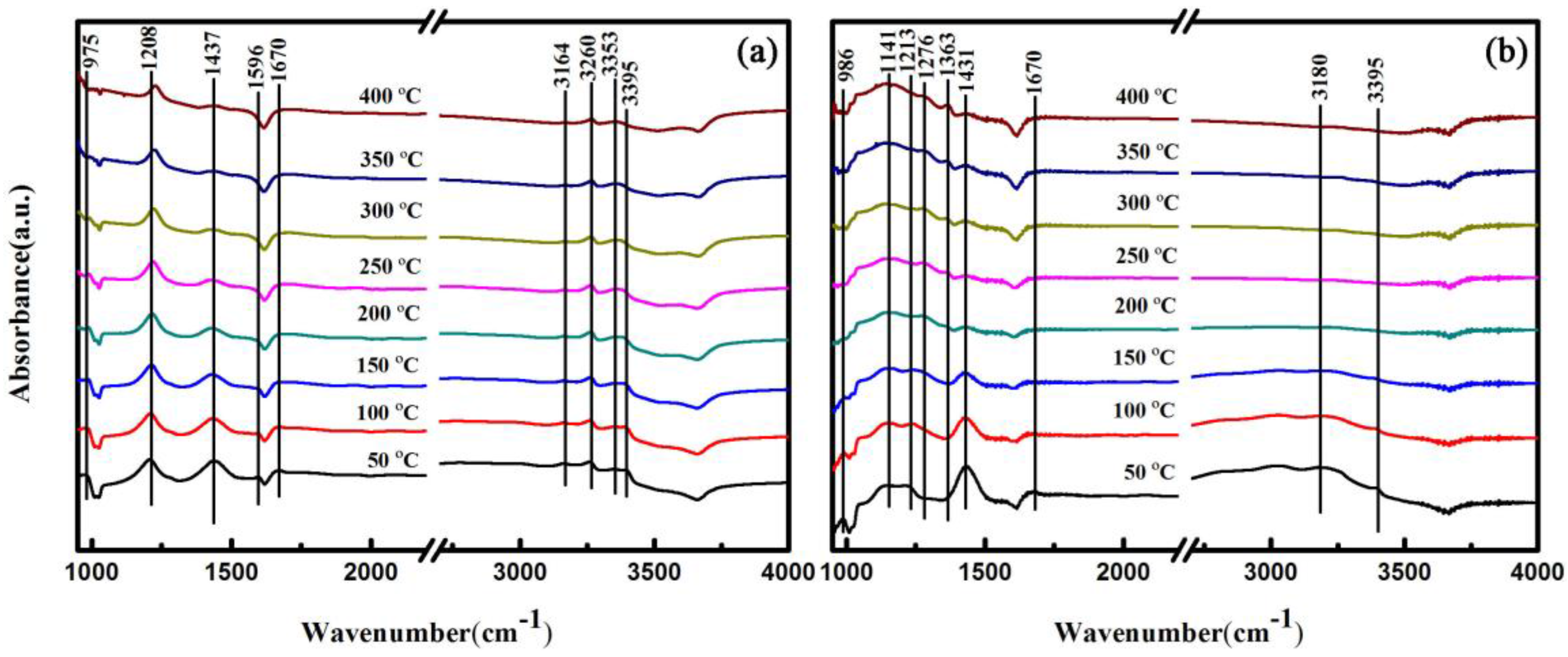
2.5. In Situ FTIR Analysis
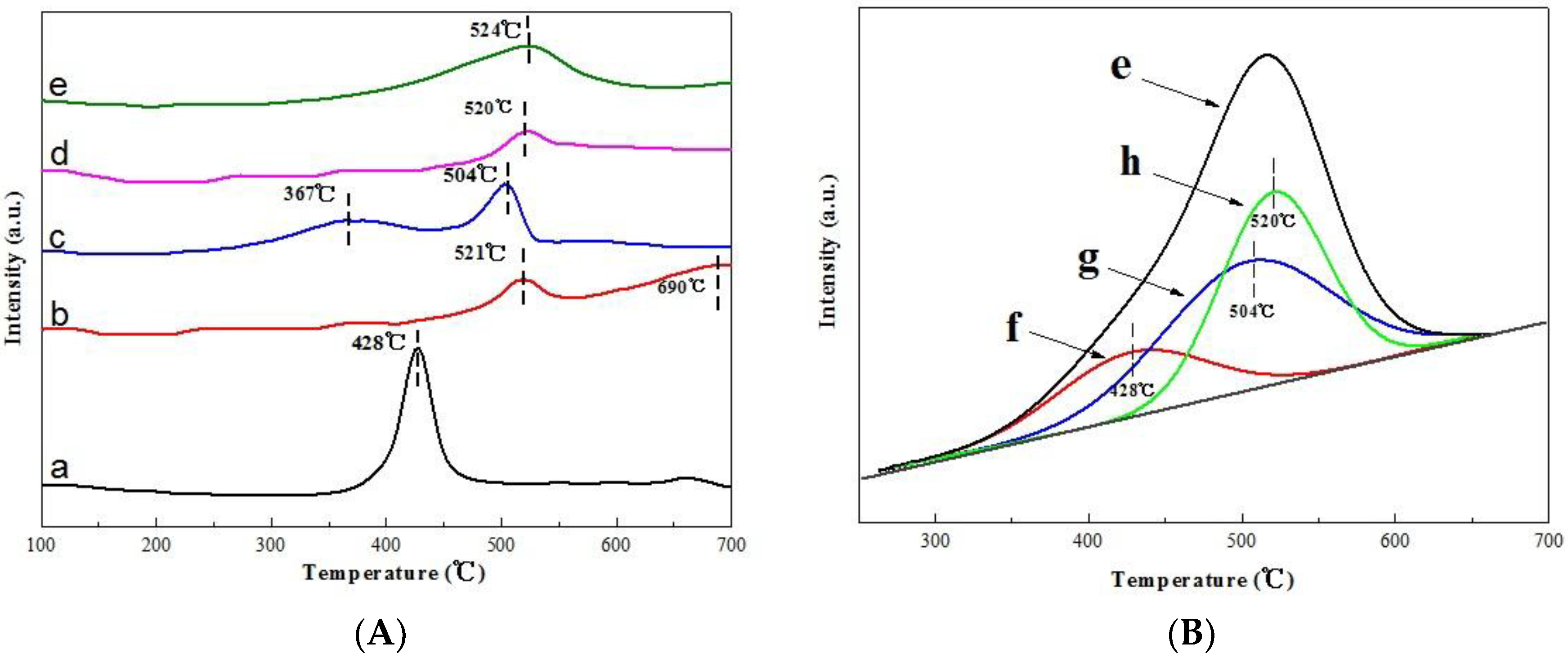
2.6. H2-TPR Analyses
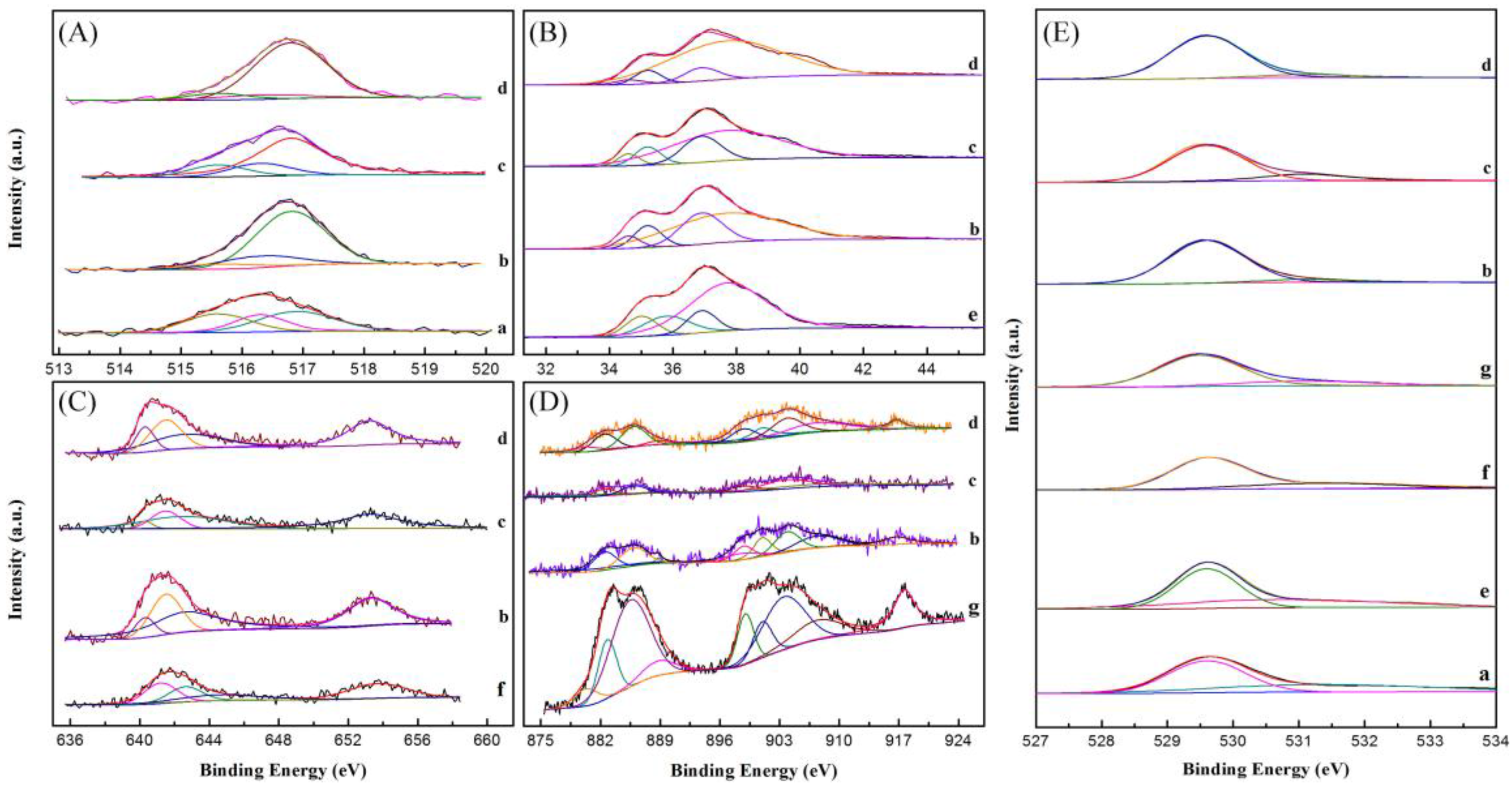
2.7. XPS Analysis
3. Materials and Methods
3.1. Catalysts Preparation
3.2. NH3-SCR Activity Test
3.3. Catalyst Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Park, E.; Kim, M.; Jung, H.; Chin, S.; Jurng, J. Effect of Sulfur on Mn/Ti Catalysts Prepared Using Chemical Vapor Condensation (CVC) for Low-Temperature NO Reduction. ACS Catal. 2013, 3, 1518–1525. [Google Scholar] [CrossRef]
- Boudali, L.K.; Ghorbel, A.; Grange, P. SCR of NO by NH3 over V2O5 supported sulfated Ti-pillared clay: Reactivity and reducibility of catalysts. Appl. Catal. A 2006, 305, 7–14. [Google Scholar] [CrossRef]
- Lónyi, F.; Solt, H.E.; Pászti, Z.; Valyon, J. Mechanism of NO-SCR by methane over Co,H-ZSM-5 and Co,H-mordenite catalysts. Appl. Catal. B 2014, 150–151, 218–229. [Google Scholar] [CrossRef] [Green Version]
- Nicosia, D.; Czekaj, I.; Kröcher, O. Chemical deactivation of V2O5/WO3-TiO2 SCR catalysts by additives and impurities from fuels, lubrication oils and urea solution. Appl. Catal. B 2008, 77, 228–236. [Google Scholar] [CrossRef]
- Si, Z.; Weng, D.; Wu, X.; Li, J.; Li, G. Structure, acidity and activity of CuOx/WOx-ZrO2 catalyst for selective catalytic reduction of NO by NH3. J. Catal. 2010, 271, 43–51. [Google Scholar] [CrossRef]
- Thirupathi, B.; Smirniotis, P.G. Nickel-doped Mn/TiO2 as an efficient catalyst for the low-temperature SCR of NO with NH3: Catalytic evaluation and characterizations. J. Catal. 2012, 288, 74–83. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Ge, M. Promotional Effect of Ce-doped V2O5-WO3/TiO2 with Low Vanadium Loadings for Selective Catalytic Reduction of NOx by NH3. J. Phys. Chem. C 2009, 113, 21177–21184. [Google Scholar] [CrossRef]
- Xie, G.; Liu, Z.; Zhu, Z.; Liu, Q.; Ge, J.; Huang, Z. Simultaneous removal of SO2 and NOx from flue gas using a CuO/Al2O3 catalyst sorbentII. Promotion of SCR activity by SO2 at high temperatures. J. Catal. 2014, 224, 42–49. [Google Scholar] [CrossRef]
- Pan, S.; Luo, H.; Li, L.; Wei, Z.; Huang, B. H2O and SO2 deactivation mechanism of MnOx/MWCNTs for low-temperature SCR of NOx with NH3. J. Mol. Catal. A 2013, 377, 154–161. [Google Scholar] [CrossRef]
- Tian, Q.; Liu, H.; Yao, W.; Wang, Y.; Liu, Y.; Wu, Z.; Wang, H.; Weng, X. SO2 Poisoning Behaviors of Ca-Mn/TiO2 Catalysts for Selective Catalytic Reduction of NO with NH3 at Low Temperature. J. Nanomater. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Xie, J.; Fang, D.; He, F.; Chen, J.; Fu, Z.; Chen, X. Performance and mechanism about MnOx species included in MnOx/TiO2 catalysts for SCR at low temperature. Catal. Commun. 2012, 28, 77–81. [Google Scholar] [CrossRef]
- Ettireddy, P.R.; Ettireddy, N.; Boningari, T.; Pardemann, R.; Smirniotis, P.G. Investigation of the selective catalytic reduction of nitric oxide with ammonia over Mn/TiO2 catalysts through transient isotopic labeling and in situ FT-IR studies. J. Catal. 2012, 292, 53–63. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Q.; Li, H.; Li, X.; Wang, L.; Tsang, S.C. Cr–MnOx mixed-oxide catalysts for selective catalytic reduction of NOx with NH3 at low temperature. J. Catal. 2010, 276, 56–65. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; Shi, L.; Fang, C.; Li, H.; Gao, R.; Huang, L.; Zhang, J. In situ supported MnO(x)-CeO(x) on carbon nanotubes for the low-temperature selective catalytic reduction of NO with NH3. Nanoscale 2013, 5, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, D.; Zhang, J.; Cai, S.; Fang, C.; Huang, L.; Li, H.; Gao, R.; Shi, L. Design of meso-TiO2@MnO(x)-CeO(x)/CNTs with a core-shell structure as DeNO(x) catalysts: Promotion of activity, stability and SO2-tolerance. Nanoscale 2013, 5, 9821–9829. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zhang, D.; Cai, S.; Zhang, L.; Huang, L.; Li, H.; Maitarad, P.; Shi, L.; Gao, R.; Zhang, J. Low-temperature selective catalytic reduction of NO with NH3 over nanoflaky MnOx on carbon nanotubes in situ prepared via a chemical bath deposition route. Nanoscale 2013, 5, 9199–9207. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Liu, Y.; Wang, Y.; Cen, W.; Wu, Z.; Wang, H.; Weng, X. The role of cerium in the improved SO2 tolerance for NO reduction with NH3 over Mn-Ce/TiO2 catalyst at low temperature. Appl. Catal. B 2014, 148–149, 582–588. [Google Scholar] [CrossRef]
- Yang, S.; Guo, Y.; Chang, H.; Ma, L.; Peng, Y.; Qu, Z.; Yan, N.; Wang, C.; Li, J. Novel effect of SO2 on the SCR reaction over CeO2: Mechanism and significance. Appl. Catal. B 2013, 136–137, 19–28. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; Fang, C.; Gao, R.; Qian, Y.; Shi, L.; Zhang, J. MnOx–CeOx/CNTs pyridine-thermally prepared via a novel in situ deposition strategy for selective catalytic reduction of NO with NH3. RSC Adv. 2013, 3, 8811–8819. [Google Scholar] [CrossRef]
- Shu, Y.; Aikebaier, T.; Quan, X.; Chen, S.; Yu, H. Selective catalytic reaction of NOx with NH3 over Ce-Fe/TiO2-loaded wire-mesh honeycomb: Resistance to SO2 poisoning. Appl. Catal. B 2014, 150–151, 630–635. [Google Scholar] [CrossRef]
- Thirupathi, B.; Smirniotis, P.G. Co-doping a metal (Cr, Fe, Co, Ni, Cu, Zn, Ce, and Zr) on Mn/TiO2 catalyst and its effect on the selective reduction of NO with NH3 at low-temperatures. Appl. Catal. B 2011, 110, 195–206. [Google Scholar] [CrossRef]
- Tang, F.; Zhuang, K.; Yang, F.; Yang, L.; Xu, B.; Qiu, J.; Fan, Y. Effect of Dispersion State and Surface Properties of Supported Vanadia on the Activity of V2O5/TiO2, Catalysts for the Selective Catalytic Reduction of NO by NH3. Chin. J. Catal. 2012, 33, 933–940. [Google Scholar] [CrossRef]
- Li, H.; Zhang, S.; Zhong, Q. Effect of nitrogen doping on oxygen vacancies of titanium dioxide supported vanadium pentoxide for ammonia-SCR reaction at low temperature. J. Colloid Interface Sci. 2013, 402, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Jiang, B.; Liu, Y.; Wang, H.; Jin, R. DRIFT Study of Manganese/Titania-Based Catalysts for Low-Temperature Selective Catalytic Reduction of NO with NH3. Environ. Sci. Technol. 2007, 41, 5812–5817. [Google Scholar] [CrossRef] [PubMed]
- Larrubia, M.A.; Ramis, G.; Busca, G. An FT-IR study of the adsorption of urea and ammonia over V2O5-MoO3-TiO2 SCR catalysts. Appl. Catal. B 2000, 27, L145–L151. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Ge, M. DRIFT Study on Cerium-Tungsten/Titiania Catalyst for Selective Catalytic Reduction of NOx with NH3. Environ. Sci. Technol. 2010, 44, 9590–9596. [Google Scholar] [CrossRef] [PubMed]
- Mihaylov, M.; Chakarova, K.; Hadjiivanov, K. Formation of carbonyl and nitrosyl complexes on titania-and zirconia-supported nickel: FTIR spectroscopy study. J. Catal. 2004, 228, 273–281. [Google Scholar] [CrossRef]
- Zhao, L.; Li, X.; Hao, C.; Raston, C.L. SO2 adsorption and transformation on calcined NiAl hydrotalcite-like compounds surfaces: An in situ FTIR and DFT study. Appl. Catal. B 2012, 117–118, 339–345. [Google Scholar] [CrossRef]
- Liu, F.; Asakura, K.; He, H.; Shan, W.; Shi, X.; Zhang, C. Influence of sulfation on iron titanate catalyst for the selective catalytic reduction of NOx with NH3. Appl. Catal. B 2011, 103, 369–377. [Google Scholar] [CrossRef]
- Vargas, M.A.L.; Casanova, M.; Trovarelli, A.; Busca, G. An IR study of thermally stable V2O5-WO3-TiO2 SCR catalysts modified with silica and rare-earths (Ce, Tb, Er). Appl. Catal. B 2007, 75, 303–311. [Google Scholar] [CrossRef]
- Yu, W.; Wu, X.; Si, Z.; Weng, D. Influences of impregnation procedure on the SCR activity and alkali resistance of V2O5-WO3/TiO2 catalyst. Appl. Surf. Sci. 2013, 283, 209–214. [Google Scholar] [CrossRef]
- Chang, H.; Li, J.; Yuan, J.; Chen, L.; Dai, Y.; Arandiyan, H.; Xu, J.; Hao, J. Ge, Mn-doped CeO2-WO3 catalysts for NH3-SCR of NOx: Effects of SO2 and H2 regeneration. Catal. Today 2013, 201, 139–144. [Google Scholar] [CrossRef]
- Li, Q.; Yang, H.; Nie, A.; Fan, X.; Zhang, X. Catalytic Reduction of NO with NH3 over V2O5-MnOX/TiO2-Carbon Nanotube Composites. Catal. Lett. 2011, 141, 1237–1242. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Song, W.; Chen, J.; Li, T.; Feng, Z. Synergetic Promotional Effects Between Cerium Oxides and Manganese Oxides for NH3-Selective Catalyst Reduction Over Ce-Mn/TiO2. Mater. Express 2011, 1, 167–175. [Google Scholar] [CrossRef]
- Cen, W.; Liu, Y.; Wu, Z.; Wang, H.; Weng, X. A theoretic insight into the catalytic activity promotion of CeO2 surfaces by Mn doping. Phys. Chem. Chem. Phys. 2012, 14, 5769–5777. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Zhao, W.; Tang, Y.; Li, L.; Wang, H.; Cui, Y.; Gu, J.; Li, Y.; Shi, J. Ni-Mn bi-metal oxide catalysts for the low temperature SCR removal of NO with NH3. Appl. Catal. B 2014, 148–149, 114–122. [Google Scholar] [CrossRef]
- Yang, S.; Wang, C.; Chen, J.; Peng, Y.; Ma, L.; Chang, H.; Chen, L.; Liu, C.; Xu, J.; Li, J.; et al. A novel magnetic Fe-Ti-V spinel catalyst for the selective catalytic reduction of NO with NH3 in a broad temperature range. Catal. Sci. Technol. 2012, 2, 915–917. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, D.; Liu, X.; Shi, L.; Maitarad, P.; Li, H.; Zhang, J.; Cao, W. Enhanced catalytic performance of V2O5-WO3/Fe2O3/TiO2 microspheres for selective catalytic reduction of NO by NH3. Catal. Sci. Technol. 2013, 3, 191–199. [Google Scholar] [CrossRef]
- Nefzi, H.; Sediri, F. Vanadium oxide nanotubes VOx-NTs: Hydrothermal synthesis, characterization, electrical study and dielectric properties. J. Solid State Chem. 2013, 201, 237–243. [Google Scholar] [CrossRef]
- Guo, X.; Bartholomew, C.; Hecker, W.; Baxter, L.L. Effects of sulfate species on V2O5/TiO2 SCR catalysts in coal and biomass-fired systems. Appl. Catal. B 2009, 92, 30–40. [Google Scholar] [CrossRef]
- Boningari, T.; Koirala, R.; Smirniotis, P.G. Low-temperature catalytic reduction of NO by NH3 over vanadia-based nanoparticles prepared by flame-assisted spray pyrolysis: Influence of various supports. Appl. Catal. B 2013, 140–141, 289–298. [Google Scholar] [CrossRef]
- Yang, S.; Wang, C.; Ma, L.; Peng, Y.; Qu, Z.; Yan, N.; Chen, J.; Chang, H.; Li, J. Substitution of WO3 in V2O5/WO3-TiO2 by Fe2O3 for selective catalytic reduction of NO with NH3. Catal. Sci. Technol. 2013, 3, 161–168. [Google Scholar] [CrossRef]
- Digregorio, F. Activation and isomerization of hydrocarbons over WO3/ZrO2 catalysts: I. Preparation, characterization, and X-ray photoelectron spectroscopy studies. J. Catal. 2004, 225, 45–55. [Google Scholar] [CrossRef]
- Nishiguchi, T.; Oka, K.; Matsumoto, T.; Kanai, H.; Utani, K.; Imamura, S. Durability of WO3/ZrO2-CuO/CeO2 catalysts for steam reforming of dimethyl ether. Appl. Catal. A 2006, 301, 66–74. [Google Scholar] [CrossRef]
- Wang, L.; Huang, B.; Su, Y.; Zhou, G.; Wang, K.; Luo, H.; Ye, D. Manganese oxides supported on multi-walled carbon nanotubes for selective catalytic reduction of NO with NH3: Catalytic activity and characterization. Chem. Eng. J. 2012, 192, 232–241. [Google Scholar] [CrossRef]
- Shan, W.; Liu, F.; He, H.; Shi, X.; Zhang, C. A superior Ce-W-Ti mixed oxide catalyst for the selective catalytic reduction of NOx with NH3. Appl. Catal. B 2012, 115–116, 100–106. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, Y.; Zhong, Y.; Luo, Z.; Cen, K. The activity and characterization of CeO2-TiO2 catalysts prepared by the sol-gel method for selective catalytic reduction of NO with NH3. J. Hazard. Mater. 2010, 174, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, A.; Duan, Y.; Wang, J.; Shen, M. Catalytic performance and hydrothermal durability of CeO2-V2O5-ZrO2/WO3-TiO2 based NH3-SCR catalysts. Catal. Sci. Technol. 2012, 2, 1386–1395. [Google Scholar] [CrossRef]
- Li, Q.; Hou, X.; Yang, H.; Ma, Z.; Zheng, J.; Liu, F.; Zhang, X.; Yuan, Z. Promotional effect of CeOX for NO reduction over V2O5/TiO2-carbon nanotube composites. J. Mol. Catal. A 2012, 356, 121–127. [Google Scholar] [CrossRef]
- Yang, S.; Qi, F.; Liao, Y.; Xiong, S.; Lan, Y.; Fu, Y.; Shan, W.; Li, J. Dual Effect of Sulfation on the Selective Catalytic Reduction of NO with NH3 over MnOx/TiO2: Key Factor of NH3 Distribution. Ind. Eng. Chem. Res. 2014, 53, 5810–5819. [Google Scholar] [CrossRef]
| Catalysts | ||||||||
|---|---|---|---|---|---|---|---|---|
| V2O5/TiO2 | WO3/TiO2 | MnO2/TiO2 | CeO2/TiO2 | Composite Catalyst | Peak of V2O5 | Peak of MnO2 | Peak of WO3 + CeO2 | |
| Hydrogen consumption (μmol/g) | 90.27 | 33.01 | 126.24 | 22.89 | 99.0 | 19.44 | 43.09 | 40.02 |
| Catalysts | Percent of Valence State, % | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V 2p | W 4f | Mn 2p | Ce 3d | O 1s | ||||||||
| V3+ | V4+ | V5+ | W5+ | W6+ | Mn2+ | Mn3+ | Mn4+ | Ce3+ | Ce4+ | Lattice Oxygen | Adsorbed Oxygen | |
| V2O5/TiO2 | 32.4 | 27.1 | 40.5 | - | - | - | - | - | - | - | 60.0 | 40.0 |
| V2O5/TiO2 ** | 100 | 0 | 0 | - | - | - | - | - | - | - | 53.5 | 46.5 |
| WO3/TiO2 | - | - | - | 20.7 | 79.3 | - | - | - | - | - | 58.8 | 41.2 |
| WO3/TiO2 ** | - | - | - | 46.8 | 53.2 | - | - | - | - | - | 58.4 | 41.6 |
| MnO2/TiO2 | - | - | - | - | - | 3.9 | 52.7 | 43.5 | - | - | 68.7 | 31.3 |
| MnO2/TiO2 ** | - | - | - | - | - | 22.3 | 61 | 16.7 | - | - | 28.1 | 71.9 |
| CeO2/TiO2 | - | - | - | - | - | - | - | - | 40.3 | 59.7 | 80.4 | 19.6 |
| CeO2/TiO2 ** | - | - | - | - | - | - | - | - | 46.1 | 53.9 | 63.5 | 36.5 |
| Composite catalyst * | 7.5 | 18.3 | 74.2 | 28.8 | 71.2 | 10.8 | 34.0 | 55.2 | 45.3 | 54.7 | 92.7 | 7.3 |
| Composite catalyst ** | 79.9 | 3.6 | 16.5 | 54 | 46 | 35.4 | 50.2 | 14.4 | 60.6 | 39.4 | 78.9 | 21.1 |
| Composite catalyst *** | 14.1 | 15.9 | 70.0 | 24.2 | 75.8 | 6.9 | 30.8 | 62.3 | 57.3 | 42.7 | 80.2 | 19.8 |
| Composite catalyst **** | 7.9 | 9.0 | 83.1 | 10.8 | 89.2 | 18.3 | 38.5 | 43.2 | 47.3 | 52.7 | 92.5 | 7.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Mao, L.; Dong, G. Mn-Ce-V-WOx/TiO2 SCR Catalysts: Catalytic Activity, Stability and Interaction among Catalytic Oxides. Catalysts 2018, 8, 76. https://doi.org/10.3390/catal8020076
Zhao X, Mao L, Dong G. Mn-Ce-V-WOx/TiO2 SCR Catalysts: Catalytic Activity, Stability and Interaction among Catalytic Oxides. Catalysts. 2018; 8(2):76. https://doi.org/10.3390/catal8020076
Chicago/Turabian StyleZhao, Xuteng, Lei Mao, and Guojun Dong. 2018. "Mn-Ce-V-WOx/TiO2 SCR Catalysts: Catalytic Activity, Stability and Interaction among Catalytic Oxides" Catalysts 8, no. 2: 76. https://doi.org/10.3390/catal8020076





