1. Introduction
Fisher–Tropsch synthesis (FTS) is a catalytic process that converts carbon sources like natural gas, coal and biomass into more valuable hydrocarbon fuels. Traditionally, the FTS industry uses supported cobalt and unsupported iron catalysts [
1,
2]. Iron catalysts are preferred over cobalt for FTS from coal or biomass because of their low cost, low methane selectivity and high water–gas shift (WGS) activity; WGS activity is needed for internal production of H
2 during FTS because of inherently low H
2/CO ratios of syngas produced from coal or biomass. Unsupported iron FT catalysts are limited by their weak mechanical strength making them a poor option for slurry-bubble column reactors, the most thermally efficient and economical FT reactors [
3]. Unfortunately, supported iron FT catalysts have historically had poor activities and selectivities making them commercially unavailable [
4,
5,
6]. Potassium and copper are structural promoters often used in industrial iron FT catalysts. It is widely accepted that potassium suppresses methane formation and increases the formation rate of heavy hydrocarbons [
7,
8,
9]. On the other hand, copper reduces the temperature required for the reduction of iron oxides through enhanced H
2 dissociation [
7,
8,
10].
The relatively poor performance of supported iron FTS catalysts is primarily attributed to the strong Fe oxide-support interactions [
6,
11]. The effect of inorganic oxide supports (Al
2O
3, SiO
2, TiO
2 and ZrO
2) have been tested for iron based FTS [
12,
13,
14]; it was shown that catalysts’ activity and selectivity are greatly affected by support-surface acidity and catalyst-support interaction. To decrease Fe oxide–support interactions a variety of carbon supports (for example carbon nanotubes, nanofibers, carbon spheres or activated carbon), which provide inert surface chemistry, have also been studied [
15,
16,
17,
18,
19,
20].
Of all the studies on supported Fe catalyst, there has been limited focus on the effects of support pretreatment methods, prior to adding the active phase and promoters [
12]. On the other hand, some studies have been undertaken on the effect of support type and support pore sizes [
11,
18,
21,
22]. Among the few studies on the effect of support pretreatment, Xu and Bartholomew prepared 10% Fe/silica catalyst via non-aqueous evaporative impregnation of a silica support, where the support was dehydroxylated at 600 °C before Fe impregnation [
6]. This was done using a non-aqueous solvent to decrease any further metal oxide–support interactions. Even with the careful catalyst preparation, interaction of metal oxide and the silica support was high which resulted in low catalyst activity. It could be expected that the supports with high interaction with metals could provide higher stability by preventing the agglomeration of metal particles [
21]. On the other hand, strong metal oxide–support interaction could lead to inactive species which are very difficult to reduce or to carbidize [
11]. Weak interactive supports such as activated carbon have been also modified with functional surface groups to increase interaction between support and iron species [
23].
Calcination of supports at high temperatures is a pathway to make reducible iron catalysts. However, the calcination temperature is usually limited to 500–800 °C due to the limited hydrothermal stability of typical supports, such as alumina and silica. Qu et al. showed higher dispersion of Ag on silica and, subsequently, higher CO oxidation activity as the support was pretreated at 550–700 °C [
24]. Higher calcination temperatures (900–950 °C) of the support resulted in an agglomeration of Ag particles due to a drastic decrease in surface area and pore volume of the support with calcination temperature. However, our group has recently reported a hydrothermally stable γ-alumina doped with 5 wt % silica. Silica enters the tetrahedral vacancies in the defect spinel structure of alumina and forms Si–Al spinel phase, which significantly postpones the alumina phase transition from γ to α even at temperatures as high as 1200 °C [
25,
26]. This unique feature enables pretreatment of the AlSi support at much higher temperatures than conventionally practiced for catalyst preparation and makes it a unique support for FT iron catalyst [
27].
In this paper, we explore the effect of support pretreatment temperature on the catalytic performance of iron catalysts supported on AlSi. The hydrothermal stability of the AlSi support enabled us, for the first time, to pretreat the support at high temperatures (1100–1200 °C) while still maintaining the γ-alumina phase with high surface area and large pore volume. High-temperature dehydroxylation of the AlSi facilitates the formation of ε′-Fe2.2C, which resulted in a very active supported iron catalyst.
3. Discussion
This work demonstrates that the support pretreatment temperature, before precursor loading, has a significant impact on the final catalyst morphology and FTS catalytic performance. The effect of pretreatment temperature of the support was studied, for the first time, over a wider range of temperatures, 700–1200 °C; higher than typically practiced by previous authors. This was made possible by incorporation of a unique silica-stabilized alumina support of high thermal stability [
25,
26]. The catalyst preparation and activation procedures used in this study led to the significant formation of ε′-Fe
2.2C, which had a great correlation with steady-state activity of the catalyst.
3.1. Effects of Support Pretreatment Temperature on Catalyst Physical Properties
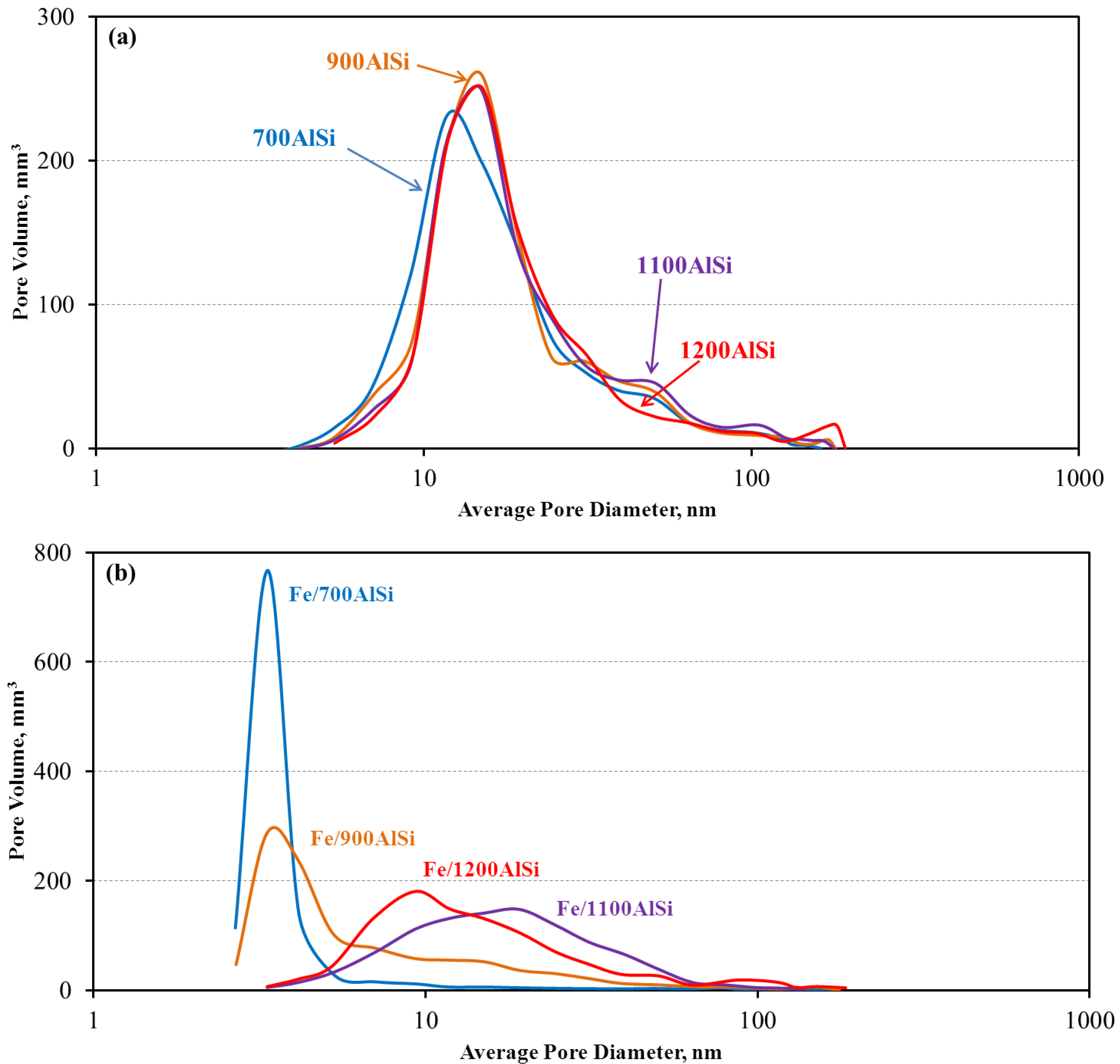
Support pretreatment temperature affects both the support and final catalyst pore properties, i.e., surface area, pore volume and pore size distribution. It is well known that γ-alumina is a metastable transition phase. When calcined at elevated temperatures, γ-alumina loses surface area and pore volume due to sintering and finally undergoes phase transition into α-phase with a lower surface area and pore volume. This trend is also observed in the present study, i.e., 700AlSi and 900AlSi have large surface areas (more than 250 m
2/g) and pore volumes (more than 1.5 cm
3/g), while 1100AlSi and 1200AlSi show relatively low surface areas (<170 m
2/g) and pore volumes (<1 cm
3/g). However, it should be mentioned that even at high-temperature pretreatment of 1200 °C, the AlSi support is still in γ-alumina phase with desirable surface area and pore volume. From the pore property point of view, 700AlSi and 900AlSi should be able to accommodate more Fe and be more suitable as catalyst supports. However, a drastic reduction in surface area, pore volume and pore size is observed in the final catalysts of Fe/700AlSi and Fe/900AlSi when Fe is loaded, as reported in
Table 1. Nucleation of FeO crystallites is favored on hydroxylated alumina surfaces [
34]. Therefore, much lower pore volume (0.16–0.26 cm
3/g) and pore diameter (5.6–10.7 nm) of Fe/700AlSi and Fe/900AlSi with higher hydroxyl group concentrations (
Table 5) suggest pore blockage of the support due to the presence of large Fe and Fe
3O
4 particles (>30 nm calculated from XRD).
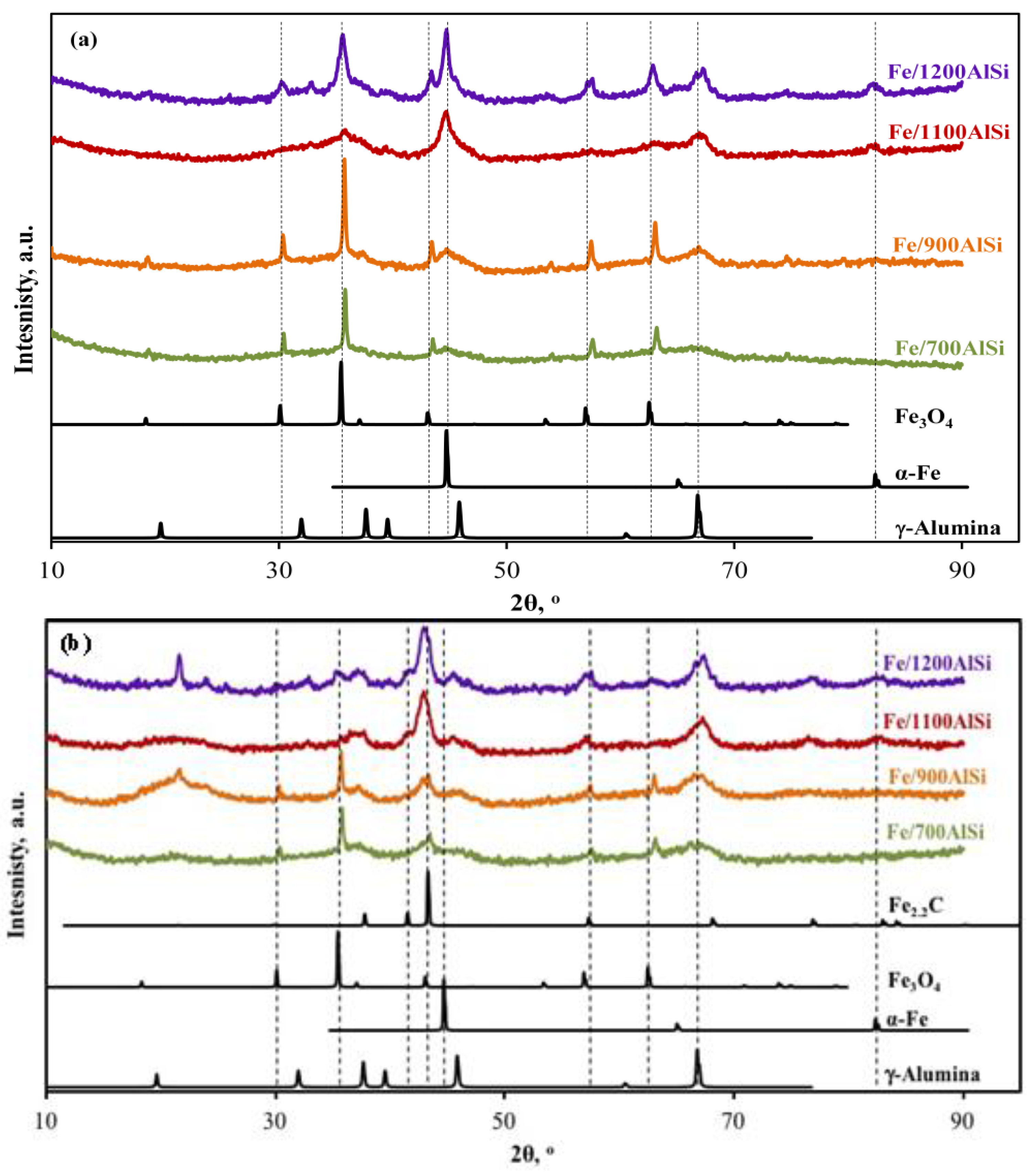
High-temperature dehydroxylation of Fe/1100AlSi and Fe/1200AlSi results in relatively uniform distribution of Fe
2O
3 and Fe
3O
4 crystallites inside the pores and, consequently, higher dispersion as evidenced by narrower iron carbide peaks observed in XRD. Although small by comparison, the pore volume and pore size of 1100AlSi and 1200AlSi are still remarkably large, especially when treated at such high temperatures to remove most of the hydroxyl groups. It should also be noted that the support pretreatment temperature up to 1100 °C improves the dispersion of iron particles as evidenced by decreasing iron particle sizes from ~40 nm to 4 nm (700–900 °C vs. 1100 °C support pretreatment temperature). However, higher support pretreatment temperatures (>1100 °C) further decreases the surface area and pore volume of the support, which consequently results in slightly lower dispersion and larger particle sizes (~8 nm). These results are in agreement with Qu et al. who found higher dispersion of Ag on silica as the calcination temperature increased to 700 °C, while lower dispersion resulted at higher calcination temperatures [
24].
3.2. Effects of Support Pretreatment Temperature on Catalyst Chemical Properties
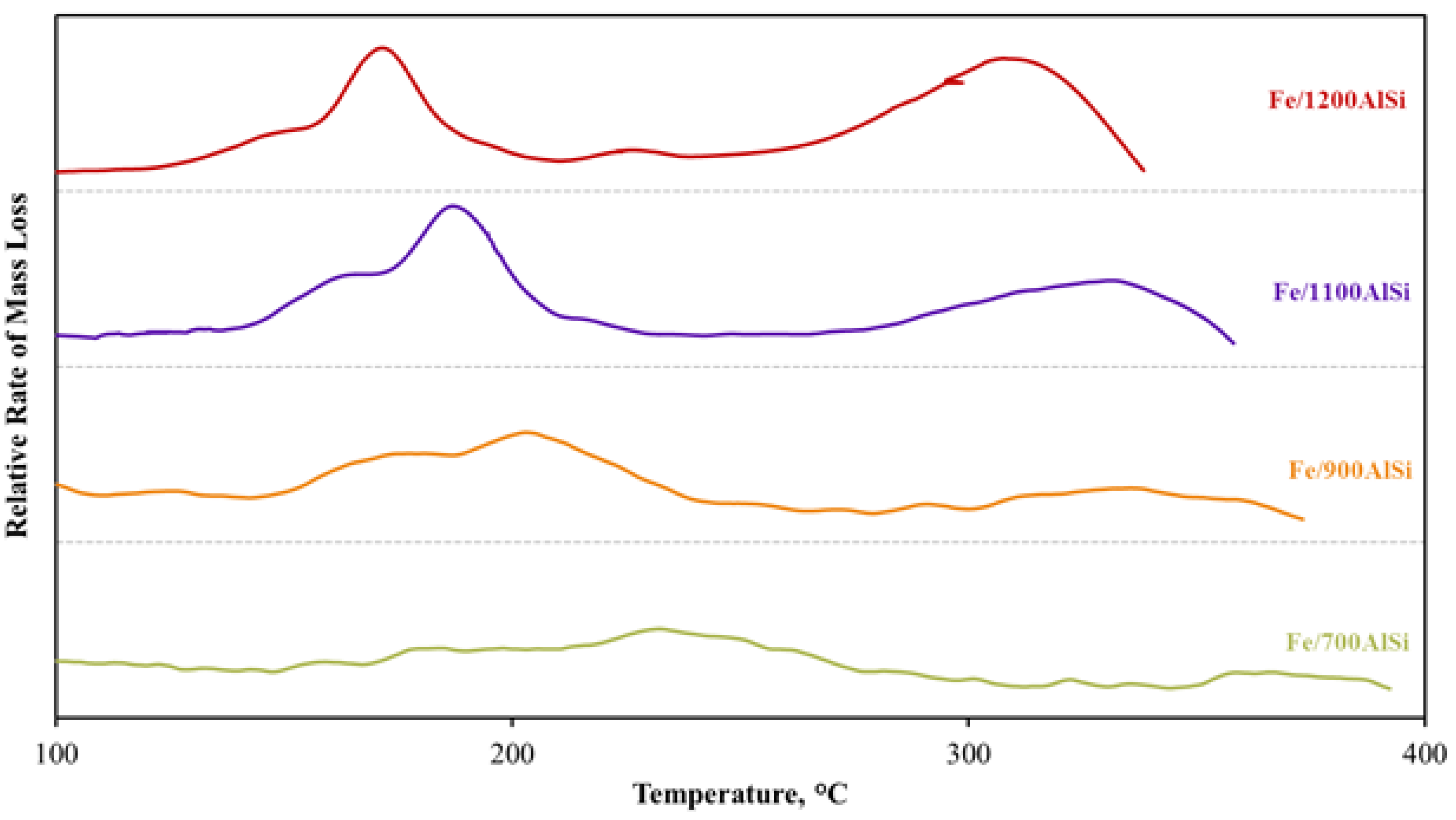
The results in this work provide correlations between support pretreatment temperature and the catalyst reducibility and extent of carbiding.
Figure 8 shows the correlations between the support pretreatment temperature and hydroxyl groups (
Table 5), and the extent of reduction from TPR (
Table 3) and ε′-Fe
2.2C from Mossbauer spectroscopy (
Table 6). Higher dehydroxylation temperature of the support for Fe/1100AlSi and Fe/1200AlSi leads to highly reducible Fe
2O
3 and Fe
3O
4, and subsequently a higher extent of reduction due to weaker FeO-support interactions. This observation is confirmed by H
2-TPR and CO-TPR data shown in
Figure 3 and
Figure 4. Lower temperature and higher area of the reduction and carbiding peaks clearly support higher reducibility and carbiding extent as the calcination temperature of the support increases. This observation is further evidenced by a much lower Fe
2+/Fe
3+ percentage from iron aluminate spinel in Fe/1100AlSi and Fe/1200AlSi than that of Fe/700AlSi and Fe/900AlSi measured by Mossbauer spectroscopy. Brenner et al. reported that oxidation of Fe
0 to Fe
3+ occurs on the hydroxyl groups of alumina supports and a high density of FeO crystallites, which are difficult to reduce or carbide, are produced [
35]. Therefore, highly reducible Fe
2O
3 and Fe
3O
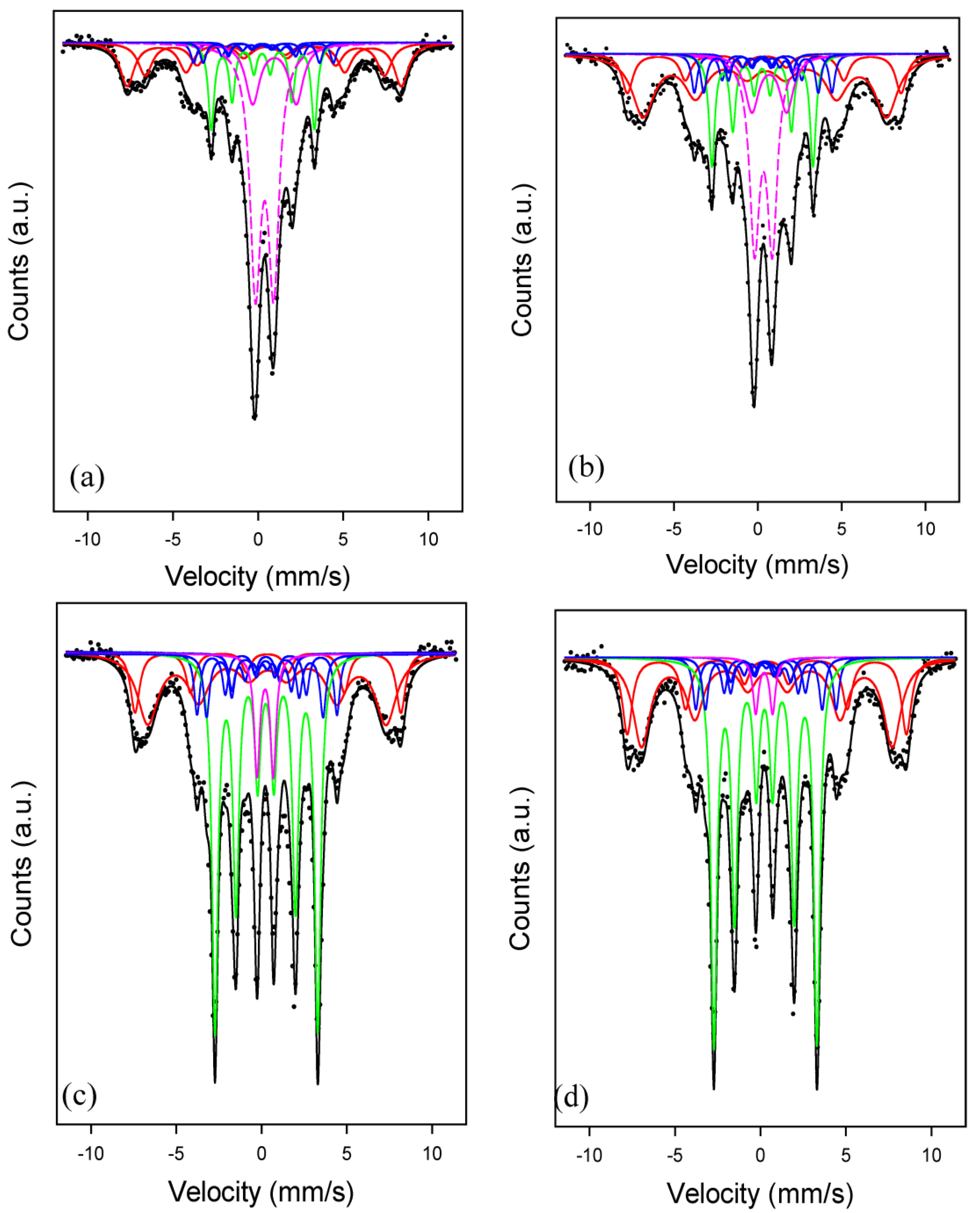
4 clusters are formed in the near absence of surface OH groups. That likely explains why Fe/700AlSi and Fe/900AlSi with higher concentrations of surface hydroxyl groups have lower extents of reduction and a lower iron carbide phase.
Degree of carbidization can also be affected by iron particle size. Generally, larger iron oxide crystallites are easier to carbidize than smaller iron oxide counterparts [
21]. However, this is only true if the surface chemistry of the support in supported iron catalyst would be relatively the same. For example, large iron particle sizes on Fe/700AlSi and Fe/900AlSi have the lowest degree of carbidization because of high metal oxide–support interaction at lower support dehydroxylation temperatures.
3.3. Effects of Support Pretreatment Temperature on Catalyst Performance
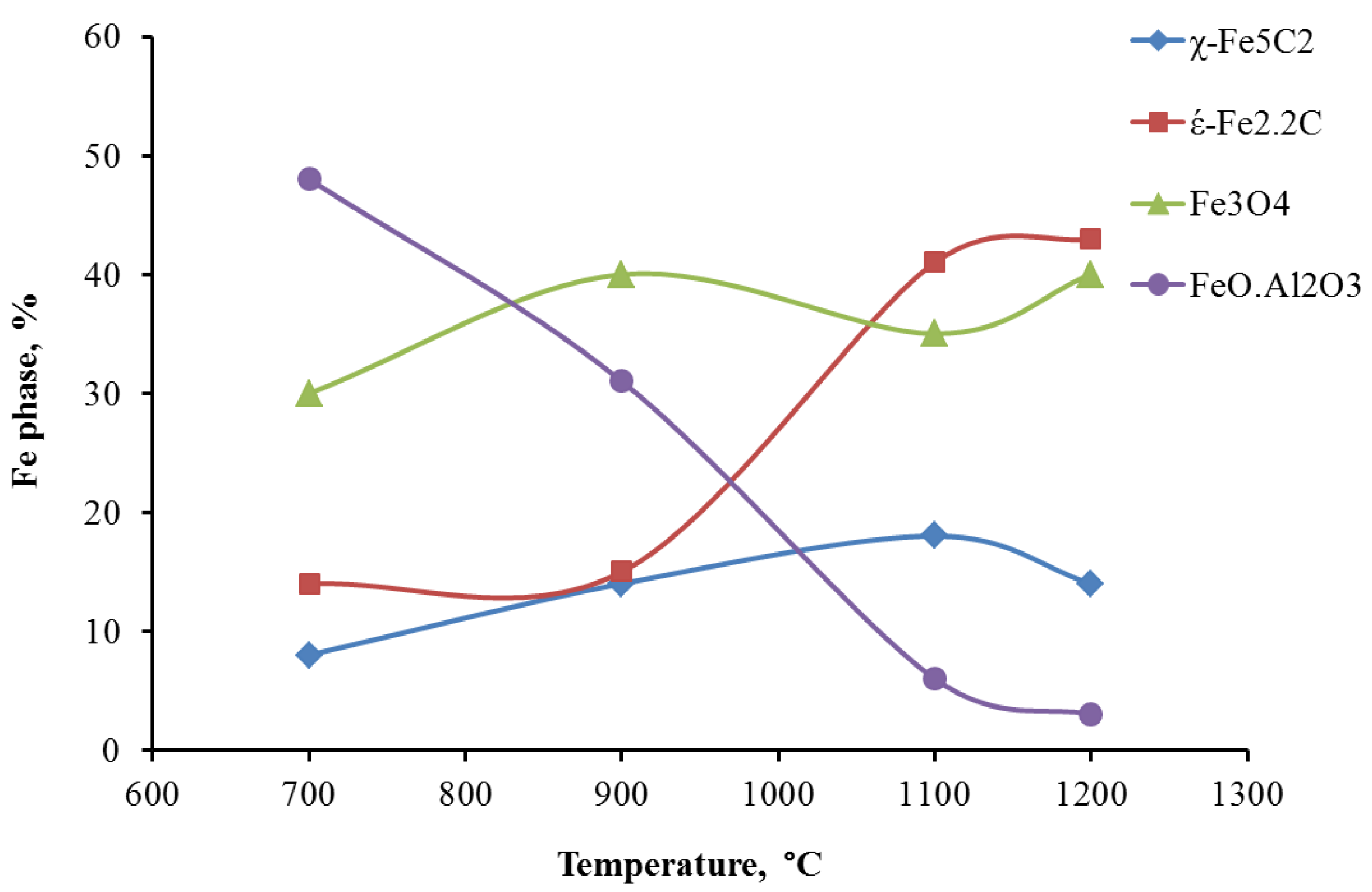
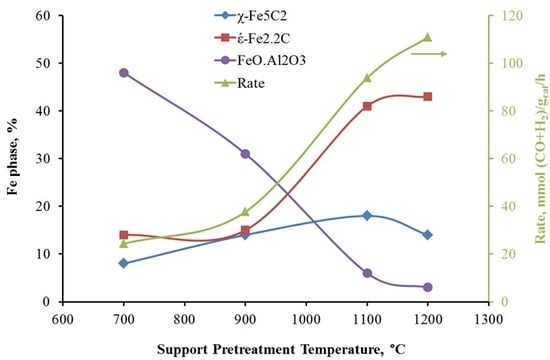
A positive correlation between the extent of reduction to Fe metal (from TPR data) following reduction in H
2 and Fe carbide content is evident (
Figure 8). This means that highly reducible Fe
2O
3 and Fe
3O
4 clusters result in a higher concentration of carbides, which are believed to be the active phase for FTS. The concentration of carbides increases significantly as the support pretreatment temperature increases, but it stays constant for pretreatment temperatures of 1100 °C and 1200 °C. This observation is consistent with syngas-TPR results, which show the carbiding of Fe/1100AlSi and Fe/1200AlSi is almost complete. In the present study, these iron carbide phases are identified as both ε′-Fe
2.2C and χ-Fe
2.5C by Mossbauer spectroscopy. The χ-Fe
2.5C is only increased from 8% to 14–18% by higher pretreatment temperature of the support while the formation of ε′-Fe
2.2C is significantly enhanced. In addition, the catalyst activity is also increased five-fold as the support pretreatment temperature increased from 700 °C to 1200 °C. The positive correlation between catalyst activity and ε′-Fe
2.2C content is shown in
Figure 9.
It has been reported that the formation of χ-Fe
2.5C is favored over unsupported Fe catalysts under typical FT conditions and identified as an active iron carbide phase [
36,
37]. Nevertheless, the catalysts used in most of those studies are unsupported, whereas the catalyst in the present study is supported with pretreated silica-stabilized alumina. In the study by de Smit et al. using chemical potentials calculated by statistical thermodynamics, ε′-Fe
2.2C formation is more favored for small particle sizes with less diffusion resistance and in the presence of a support material [
38]. One can expect higher dispersion of active sites using supports, especially highly dehydroxylated supports used in this study which lower the metal oxide-support interaction and produce highly reducible and well dispersed iron oxides. On the other hand, the formation of ε′-Fe
2.2C is more favored at low temperatures followed by its conversion to χ-Fe
2.5C at higher temperatures (>250 °C). In this study, the calcined Fe/AlSi catalysts were activated under syngas at 20 atm by increasing the temperature from 180 °C and holding every 10 °C up to 280 °C, while keeping conversion less than 60% in each step. The highly dispersed ε′-Fe
2.2C particles formed at low temperatures might have been stabilized on the support and their conversion to χ-Fe
2.5C was not completed at higher temperatures of the activation process. This finding is in accordance with Niemantsverdriet et al. who reported the formation of ε′-Fe
2.2C over iron supported on TiO
2/CaO after FT reaction at 240 °C [
39]. Raupp et al. also observed ε′-Fe
2.2C on Fe/SiO
2 exposed to FTS at 250 °C for 6 h [
40].
The activity of Fe/AlSi, in this study, is among the highest in the literature for both unsupported and supported iron catalysts, and the catalyst is stable over 800 h time on stream. In addition, the significant correlation of the catalyst activity with the amount of ε′-Fe2.2C clearly show that ε′-Fe2.2C on AlSi support can be as active as χ-Fe2.5C on unsupported Fe catalysts. Thus, historically ε′-Fe2.2C was not considered the active phase due to a lack of evidence on unsupported iron catalysts. This is an important finding because the supported iron catalysts reported in the literature prior to this study were usually much less active than unsupported iron catalysts due to their high metal oxide–support interaction, and thus careful characterization of supported catalysts is scarce in the literature.
4. Materials and Methods
4.1. Catalyst Preparation
A series of iron catalysts supported on BYU alumina supports (Brigham Young University, Provo, UT, USA) pretreated at different temperatures were investigated in this study. The alumina support was doped with 5% SiO
2 to increase the thermal stability, as described previously [
25]. The supports were sieved to 250–595 µm (30–60 mesh size) and calcined in air at 700 °C, 900 °C, 1100 °C or 1200 °C for 4 h prior to impregnation. The supports were named starting with their calcination temperature, e.g., the support calcined at 700 °C was named 700AlSi. All four catalysts were prepared by incipient wetness using an aqueous solution containing desired amounts of ferric nitrate, copper nitrate and potassium bicarbonate in four steps. In each step, 10 wt % Fe with the desired amount of Cu and K were dissolved in sufficient water for incipient wetness and mixed into the catalyst support. The catalysts were sat at room temperature for 4 h, dried in an oven at 80 °C for 16 h, and finally calcined for 16 h at 300 °C in air. Nominal compositions (on a relative mass basis) of reduced catalysts were 100Fe/7.5Cu/4K/150Al
2O
3 for an iron loading of 40%. The final catalysts were named Fe/xAlSi, where “x” was the calcination temperature. For example, Fe/700AlSi was the Fe/Cu/K catalyst supported on the support calcined at 700 °C.
The samples were reduced at 280–320 °C in 10% H2/He for 10 h followed by 100% H2 for 6 h for characterization of reduced catalysts. Reduced catalysts were carefully passivated by first exposing each to flowing air in He (<1%) at room temperature followed by gradually increasing concentrations of air in helium while monitoring the temperature of the catalyst bed. The passivated reduced catalysts were carbided at 280 °C, 20 atm, and H2/CO = 1 for 24 h for characterization of carbided samples.
4.2. Catalyst Characterization
4.2.1. Nitrogen Adsorption/Desorption
Surface area, pore volume, and pore size distribution were calculated by nitrogen adsorption/desorption isotherms measured using a Micromeritics TriStar 3000 instrument (Norcross, GA, USA). The samples (0.3 g) were degassed at 120 °C for 12 h before measurement. Surface area was calculated by the BET model using P/P0 ranging from 0.05 to 0.2. Pore volume was measured by the single point method using P/P0 = 0.990. Pore size distribution was calculated using the desorption branch of the isotherm according to a newly developed slit pore-geometry model [
41].
4.2.2. XRD
To determine the presence of different iron phases, X-ray diffraction patterns were collected for all the catalysts using a PANalytical X’Pert Pro diffractometer (Almelo, The Netherlands) with a Cu source and a Ge monochromator tuned to the Cu-K1 wavelength ( = 1.54 Å). Samples (reduced and passivated) were scanned from 10–90° using a step size of 0.016° and a step time of 350 s. Diffraction patterns were compared to standard patterns in the database.
4.2.3. TPR
TPR experiments were performed in a Mettler Toledo TGA/DSC 1 equipped with an automated GC 200 gas controller (Columbus, OH, USA) to understand the reduction/carbiding behavior of the catalysts. 10–20 mg of calcined samples were exposed to a reducing gas mixture of 10% H2/He (H2-TPR) or 10% syngas (H2:CO = 1) in He (syngas-TPR), while the temperature was increased at 3 °C/min from ambient to 700 °C.
4.2.4. Hydroxyl Group Content Measurement
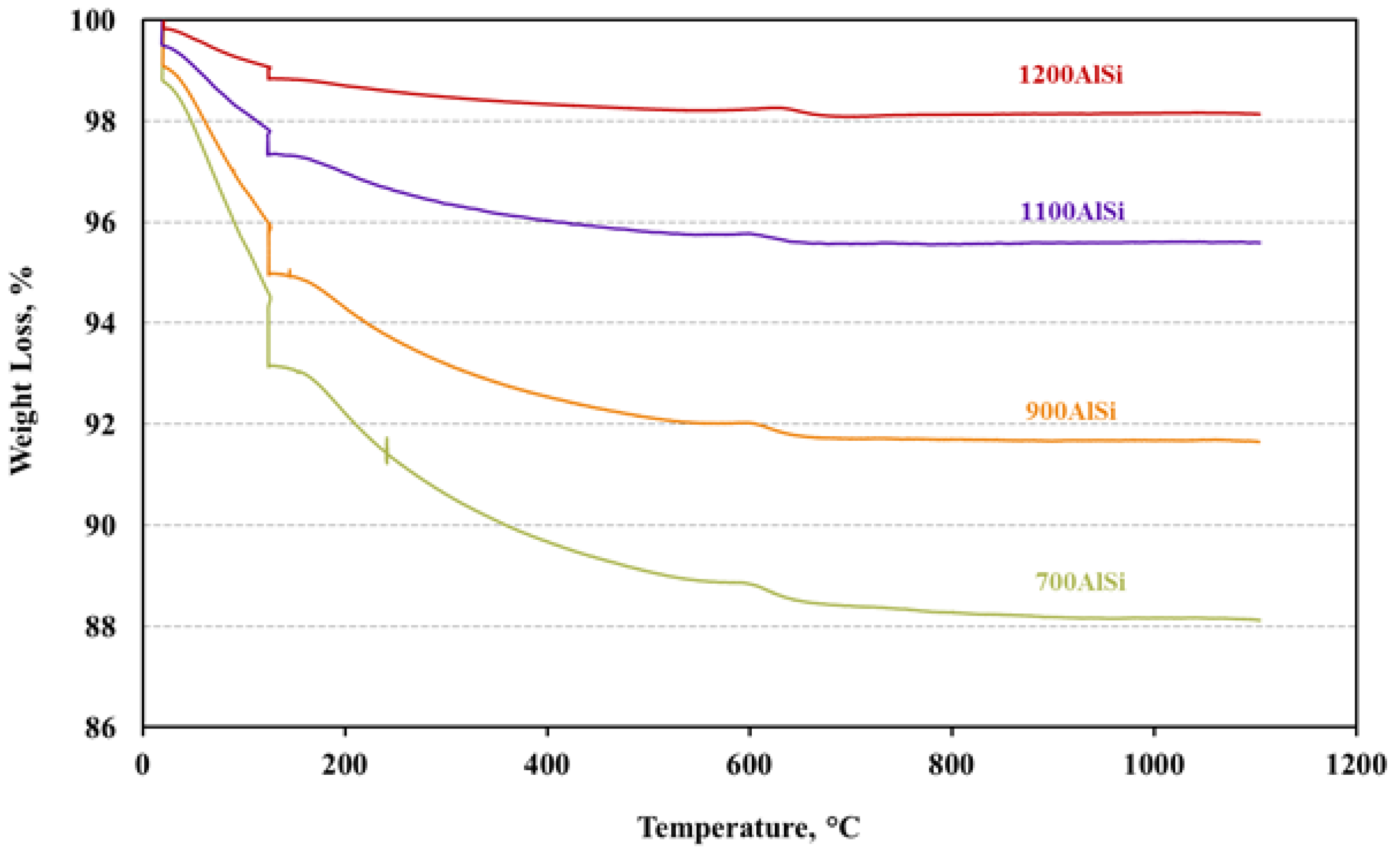
TGA was performed for alumina samples in the same TGA equipment (Columbus, OH, USA) described in
Section 2.2.3 to determine hydroxyl group content. Pre-calcined alumina samples were heated at a rate of 5 °C/min from room temperature to 1100 °C in He flow of 80 mL/min and held for 2 h. The weight loss between 130 °C and 1100 °C was used to determine the hydroxyl group content.
4.2.5. Mossbauer Spectroscopy
Mossbauer measurements were taken in transmission mode, with a 57Co source in Rh matrix mounted in a standard constant acceleration drive unit. Samples were placed inside a closed-cycle refrigerator for low-temperature measurements. All measurements were calibrated with respect to alpha-Fe. Analysis of measured spectra was carried out by using defined functions within the PeakFit program (version 4.12) using the least-squares-fitting routine. The parameters for each sub-spectrum in the fit consisted of the position, width and height of the first peak, the hyperfine magnetic field and the quadrupole electric field. Initially, the hyperfine magnetic fields of known FexC (2.2 ≤ x ≤ 3) were constrained but all other parameters could vary freely. Subsequently, the carbides’ hyperfine magnetic fields for the intense-distinct peaks could vary to obtain the best fit to the experimental data. The percentages of Fe in the different phases were determined from the areas under the peaks of the different sub-spectra. Calculated carbide sub-spectra of Fe percentages less than the experimental error of about ± 3% were then removed from the fitting procedure and data was fitted for the final results.
4.3. FTS Performance Measurement
FTS was conducted in a fixed-bed reactor (stainless steel, 3/8 inch OD) described previously [
34]. Each sample (0.25 g, 250–590 μm) was diluted with 1 g silicon carbide to improve heat distribution in the catalytic zone.
Before FTS, the samples were reduced in situ at 280–320 °C in 10% H2/He for 10 h followed by 100% H2 for 6 h. After cooling to 180 °C, the system was then pressurized to 20 atm in syngas (H2:CO = 1), and the catalysts were activated by ramping and holding every 10 °C up to 280 °C, while keeping conversion less than 60% in each step. Activity and stability data were then obtained over the next 200–700 h as reaction temperatures were varied from 220 °C to 260 °C.
After leaving the reactor, the exit gas and liquid effluent passed through a hot trap (90 °C) and a cold trap (0 °C) to collect heavy hydrocarbons and liquid products. The effluent gaseous product was analyzed using an HP5890 gas chromatograph (Santa Clara, CA, USA) equipped with a thermal conductivity detector and 60/80 carboxene-1000 column. CO conversion and selectivities were determined with the use of an internal standard (Ar).
5. Conclusions
In summary, the results of this work demonstrate that support pretreatment temperature has a significant impact on the properties (physical and chemical) and catalytic performance of silica-stabilized alumina (AlSi) supported iron FTS catalysts. The high thermal stability of the AlSi support prepared in this study compared with the traditional alumina supports enabled us to study the effect of support pretreatment temperature at much higher temperatures than traditional practice (500–800 °C). Pretreating AlSi material at higher temperatures (i.e., 1100–1200 °C) before catalyst loading significantly removes support surface hydroxyl groups and reduces the iron oxide–support interaction. Therefore, the Fe/AlSi catalyst where the support calcined at 1100–1200 °C is (1) more reduced and more easily reduced, (2) more effectively carbided, and (3) significantly more active and productive. In addition, the results of this work show an excellent correlation of the catalyst activity and ε′-Fe2.2C content, which provide new insights into the active phase for supported FTS catalysts.