Enhanced Low Temperature NO Reduction Performance via MnOx-Fe2O3/Vermiculite Monolithic Honeycomb Catalysts
Abstract
:1. Introduction
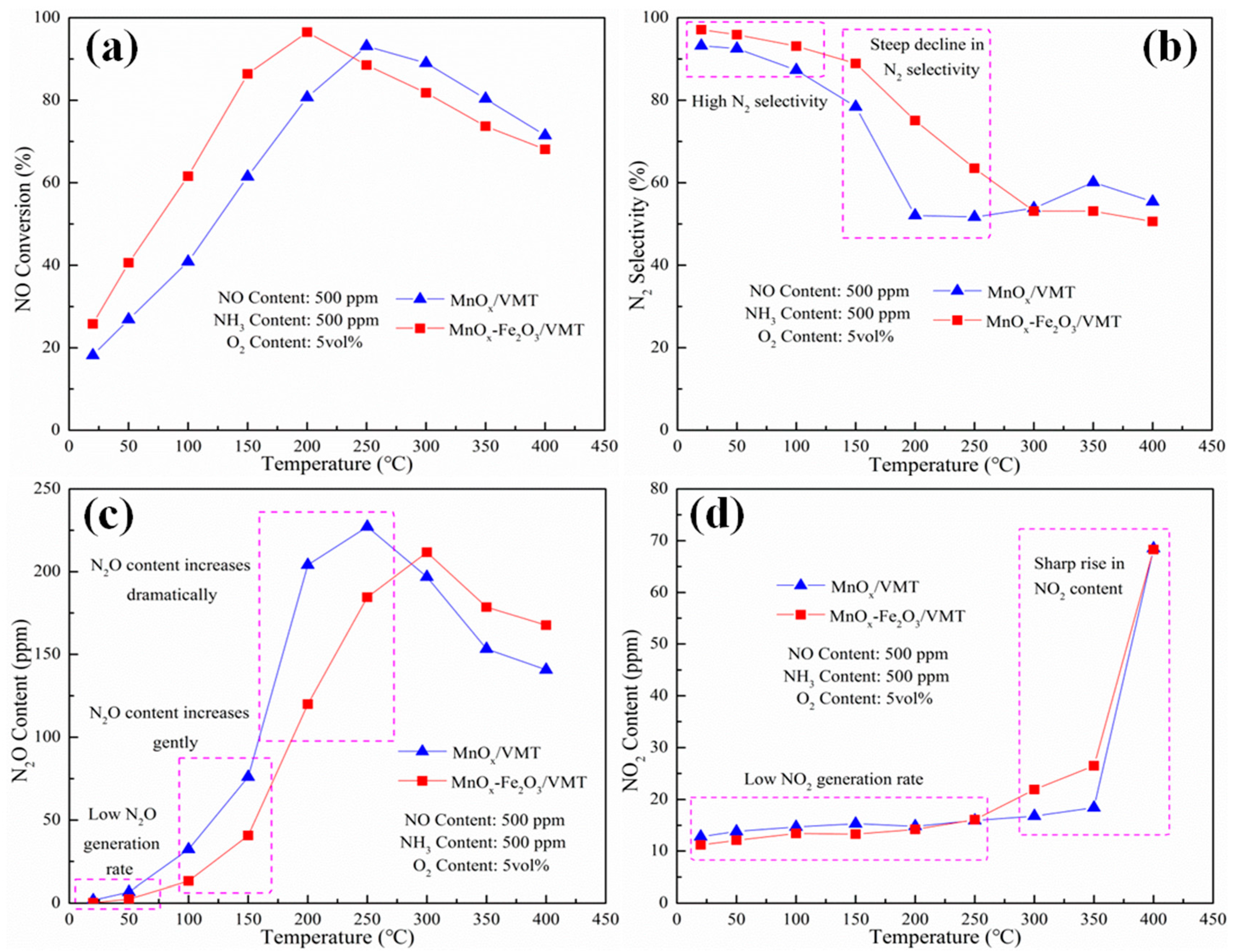
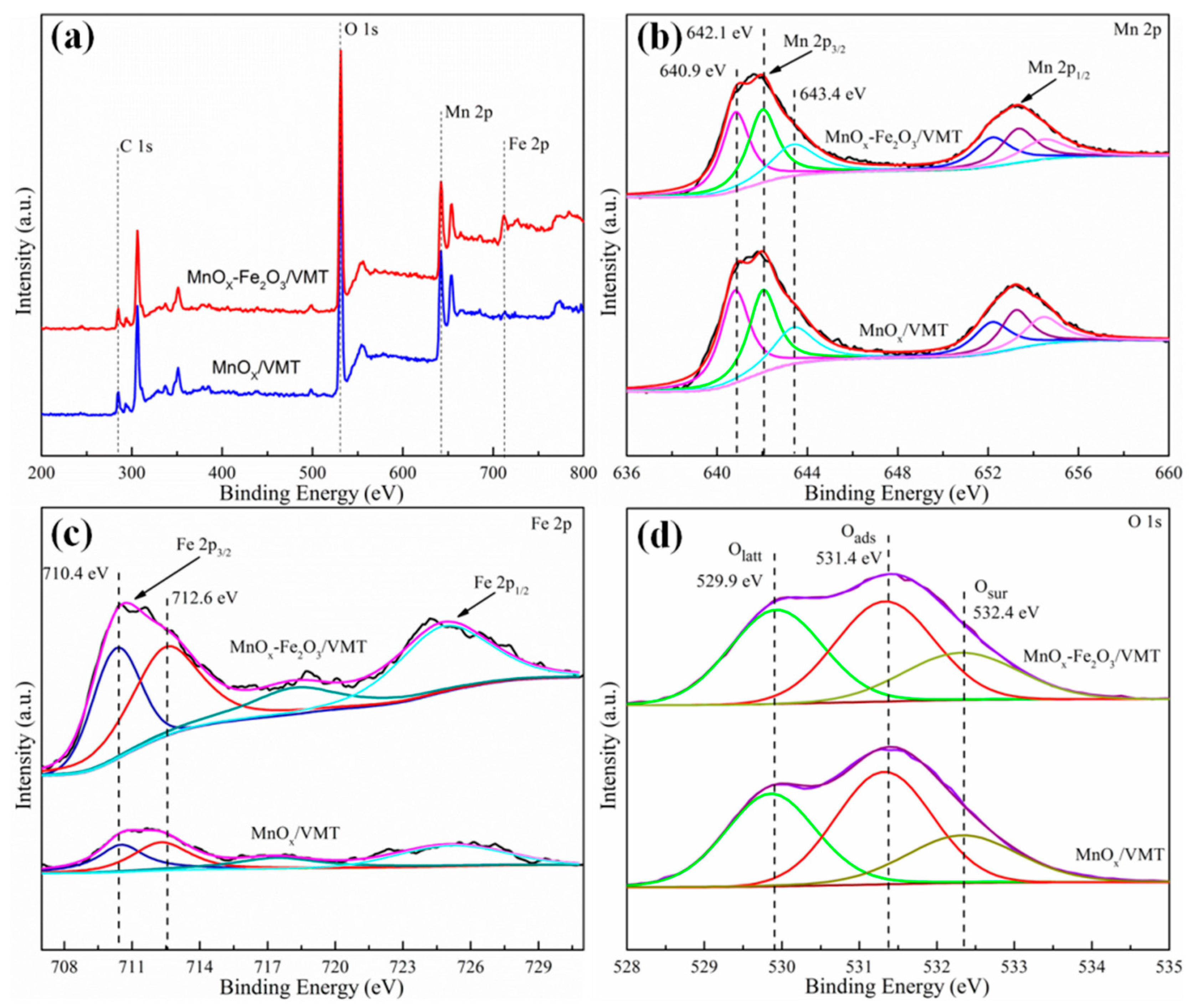
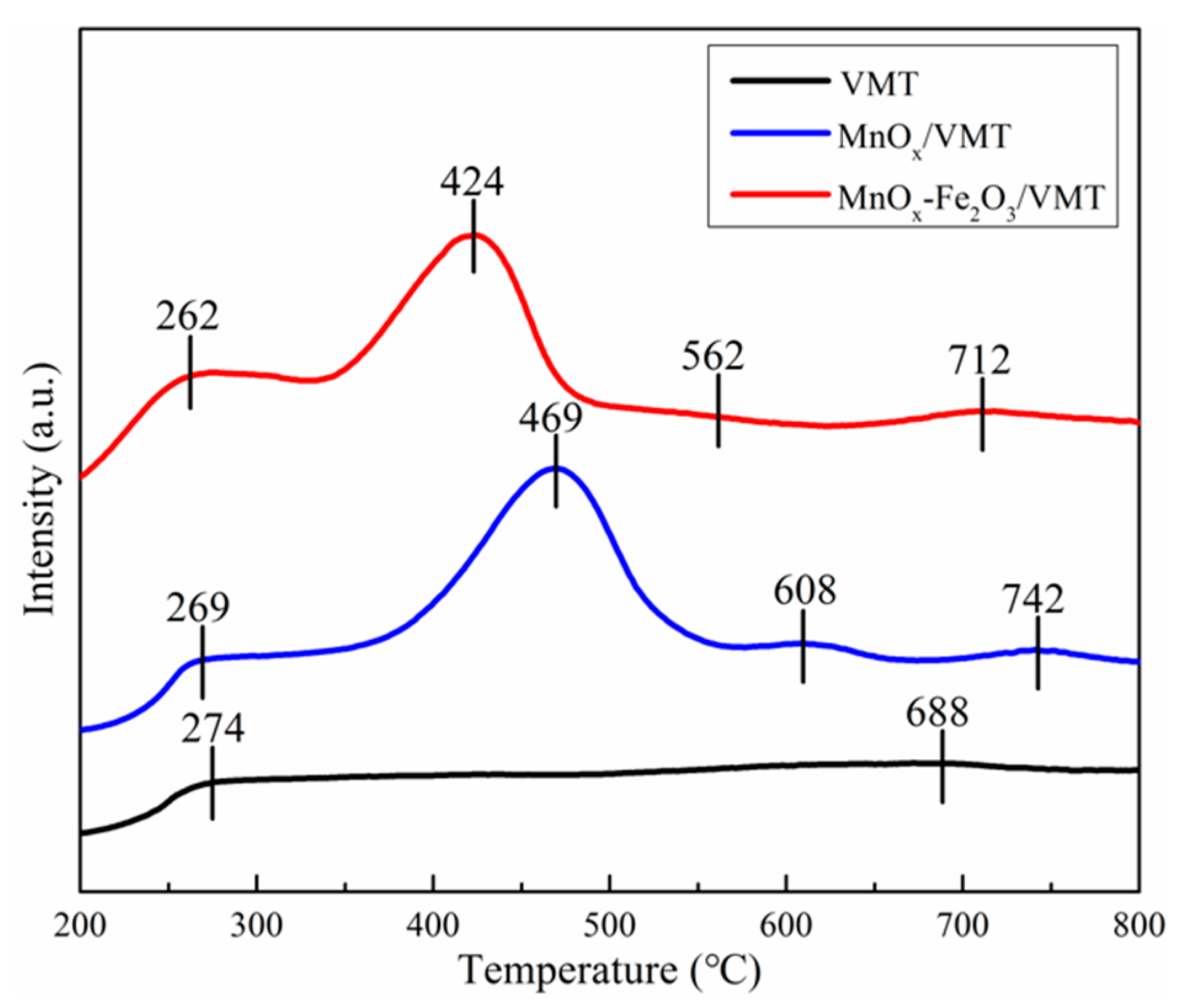
2. Results and Discussion
3. Materials and Methods
3.1. Catalysts Preparation
3.1.1. Preparation of MnOx/VMT and MnOx-Fe2O3/VMT Catalysts
3.1.2. Preparation of MnOx-Fe2O3/VMT Monolithic Honeycomb Catalysts
3.2. Catalyst Characterization
3.3. Activity Measurement
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cai, S.; Liu, J.; Zha, K.; Li, H.; Shi, L.; Zhang, D. A general strategy for the in situ decoration of porous Mn-Co bi-metal oxides on metal mesh/foam for high performance de-NOx monolith catalysts. Nanoscale 2017, 9, 5648–5657. [Google Scholar] [CrossRef] [PubMed]
- Boningari, T.; Smirniotis, P.G. Impact of nitrogen oxides on the environment and human health: Mn-based materials for the NOx abatement. Curr. Opin. Chem. Eng. 2016, 13, 133–141. [Google Scholar] [CrossRef]
- Li, J.H.; Chang, H.Z.; Ma, L.; Hao, J.M.; Yang, R.T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts—A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, B.; Zhao, R.; Zhao, Q.; Gies, H.; Xiao, F.; Vos, D.E.D.; Yokoi, T.; Bao, X.; Kolb, U.; et al. Fe-doped Beta Zeolite from Organotemplate-free Synthesis for NH3-SCR of NOx. Catal. Sci. Technol. 2016, 6, 6581–6592. [Google Scholar] [CrossRef]
- Liu, C.; Shi, J.W.; Niu, C. Manganese oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3: A review. Appl. Catal. A Gen. 2016, 522, 54–69. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T.; Chang, R. Low temperature selective catalytic reduction (SCR) of NO with NH3 over Fe-Mn based catalysts. Chem. Commun. 2012, 5, 452–453. [Google Scholar]
- Boningari, T.; Pappas, D.K.; Ettireddy, P.R.; Kotrba, A.; Smirniotis, P.G. Influence of SiO2 on M/TiO2 M = Cu, Mn, and Ce) formulations for low-temperature selective catalytic reduction of NOx with NH3: Surface properties and key components in relation to the activity of NOx reduction. Ind. Eng. Chem. Res. 2015, 54, 2261–2273. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, F.; Li, H.; Yang, Q.; Wang, L.F.; Li, X.H. Low-Temperature Selective Catalytic Reduction of NOx with NH3 over Fe–Mn Mixed-Oxide Catalysts Containing Fe3Mn3O8 Phase. Ind. Eng. Chem. Res. 2012, 51, 202–212. [Google Scholar] [CrossRef]
- Li, R.; Li, Z.B.; Chen, L.Q.; Dong, Y.L.; Ma, S.B.; Yuan, F.L.; Zhu, Y.J. Synthesis of MnNi–SAPO-34 by a one-pot hydrothermal method and its excellent performance for the selective catalytic reduction of NO by NH3. Catal. Sci. Technol. 2017, 7, 4989–4995. [Google Scholar] [CrossRef]
- Lou, X.; Liu, P.; Li, J.; Li, Z.; He, K. Effects of calcination temperature on Mn species and catalytic activities of Mn/ZSM-5 catalyst for selective catalytic reduction of NO with ammonia. Appl. Sur. Sci. 2014, 307, 382–387. [Google Scholar] [CrossRef]
- Yu, C.; Dong, L.; Feng, C.; Liu, X.; Huang, B. Low-temperature SCR of NOx by NH3 over MnOx/SAPO-34 prepared by two different methods: A comparative study. Environ. Technol. 2017, 38, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, K.; Si, M.; Lian, J.H.; Liu, L.S.; Gou, X. Experimental Research on Catalysts of V2O5/AC and V2O5/CNTs for Low Temperature SCR Denitrification. Appl. Mech. Mater. 2014, 694, 478–483. [Google Scholar] [CrossRef]
- Lu, X.; Zheng, Y.; Zhang, Y.; Qiu, H. Low-temperature selective catalytic reduction of NO over carbon nanotubes supported MnO2 fabricated by co-precipitation method. Micro Nano Lett. 2015, 10, 666–669. [Google Scholar] [CrossRef]
- Lin, F.; Wu, X.; Liu, S.; Weng, D.; Huang, Y. Preparation of MnOx–CeO2–Al2O3 mixed oxides for NOx-assisted soot oxidation: Activity, structure and thermal stability. Chem. Eng. J. 2013, 226, 105–112. [Google Scholar] [CrossRef]
- Jin, R.; Liu, Y.; Wu, Z.; Wang, H.; Gu, T. Low-temperature selective catalytic reduction of NO with NH3 over MnCe oxides supported on TiO2 and Al2O3: A comparative study. Chemosphere 2010, 78, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Pappas, D.K.; Boningari, T.; Boolchand, P.; Smirniotis, P.G. Novel manganese oxide confined interweaved titania nanotubes for the low-temperature selective catalytic reduction (SCR) of NOx by NH3. J. Catal. 2016, 334, 1–13. [Google Scholar] [CrossRef]
- Ettireddy, P.R.; Ettireddy, N.; Boningari, T.; Pardemann, R.; Smirniotis, P.G. Investigation of the selective catalytic reduction of nitric oxide with ammonia over Mn/TiO2 catalysts through transient isotopic labeling and in situ FT-IR studies. J. Catal. 2012, 292, 53–63. [Google Scholar] [CrossRef]
- Smirniotis, P.G.; Peña, D.A.; Uphade, B.S. Low-Temperature Selective Catalytic Reduction (SCR) of NO with NH3 by Using Mn, Cr, and Cu Oxides Supported on Hombikat TiO2. Angew. Chem. Int. Ed. Eng. 2001, 40, 2479–2482. [Google Scholar] [CrossRef]
- Singoredjo, L.; Korver, R.; Kapteijn, F.; Moulijn, J. Alumina supported manganese oxides for the low-temperature selective catalytic reduction of nitric oxide with ammonia. Appl. Catal. B 1992, 1, 297–316. [Google Scholar] [CrossRef]
- Ma, K.; Zou, W.X.; Zhang, L.; Li, L.L.; Yu, S.H.; Tang, C.J.; Gao, F.; Dong, L. Construction of hybrid multi-shell hollow structured CeO2-MnOx materials for selective catalytic reduction of NO with NH3. RSC Adv. 2017, 7, 5989–5999. [Google Scholar] [CrossRef]
- Liu, C.; Gao, G.; Shi, J.W.; He, C.; Li, G.D.; Bai, N.; Niu, C. MnOx-CeO2 shell-in-shell microspheres for NH3-SCR de-NOx at low temperature. Catal. Commun. 2016, 86, 36–40. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T.; Chang, R. MnOx-CeO2 mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B Environ. 2004, 51, 93–106. [Google Scholar] [CrossRef]
- Hu, H.; Zha, K.; Li, H.; Shi, L.; Zhang, D. In situ DRIFTs investigation of the reaction mechanism over MnOx-MOy/Ce0.75Zr0.25O2 (M = Fe, Co, Ni, Cu) for the selective catalytic reduction of NOx with NH3. Appl. Sur. Sci. 2016, 387, 921–928. [Google Scholar] [CrossRef]
- Xiao, F.; Gu, Y.; Tang, Z.; Han, F.; Shao, J.; Xu, Q.; Zhu, H. ZrO2 Modified MnOx/Attapulgite Catalysts for NH3-SCR of NO at Low Temperature. J. Chem. Eng. Jpn. 2015, 48, 481–487. [Google Scholar] [CrossRef]
- Xie, A.; Zhou, X.; Huang, X.; Ji, L.; Zhou, W.; Luo, S.; Yao, C. Cerium-loaded MnOx/attapulgite catalyst for the low-temperature NH3-selective catalytic reduction. J. Ind. Eng. Chem. 2017, 49, 230–241. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kuśtrowski, P.; Dziembaj, R.; Cool, P.; Vansant, E.F. Selective catalytic reduction of NO with ammonia over porous clay heterostructures modified with copper and iron species. Catal. Today 2007, 119, 181–186. [Google Scholar] [CrossRef]
- Hou, X.X.; Schmieg, S.J.; Wei, L.; Epling, W.S. NH3 pulsing adsorption and SCR reactions over a Cu-CHA SCR catalyst. Catal. Today 2012, 197, 9–17. [Google Scholar] [CrossRef]
- Schmieg, S.J.; Oh, S.H.; Kim, C.H.; Brown, D.B.; Lee, J.H.; Pedenc, C.H.F.; Kim, D.H. Thermal durability of Cu-CHA NH3-SCR catalysts for diesel NOx reduction. Catal. Today 2012, 184, 252–261. [Google Scholar] [CrossRef]
- Brookshear, D.W.; Nam, J.G.; Ke, N.; Toops, T.J.; Binder, A. Impact of sulfation and desulfation on NOx reduction using Cu-chabazite SCR catalysts. Catal. Today 2015, 258, 359–366. [Google Scholar] [CrossRef]
- Luo, S.; Zhou, W.; Xie, A.; Wu, F.; Yao, C.; Li, X.; Zuo, S.; Liu, T. Effect of MnO2 polymorphs structure on the selective catalytic reduction of NOx with NH3 over TiO2–Palygorskite. Chem. Eng. J. 2016, 286, 291–299. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kuśtrowski, P.; Piwowarska, Z.; Dudek, B.; Gil, B.; Michalik, M. Montmorillonite, vermiculite and saponite based porous clay heterostructures modified with transition metals as catalysts for the DeNOx process. Appl. Catal. B Environ. 2009, 88, 331–340. [Google Scholar] [CrossRef]
- Samojeden, B.; Możdżeń, M. The influence of amount of copper of modified vermiculites on catalytic properties in SCR-NH3. Energy Fuels 2017, 14, 02020. [Google Scholar] [CrossRef]
- Ying, S. Preparation and Properties of Vermiculite Supported TiO2 Photocatalyst. Chin. J. Inorg. Chem. 2011, 27, 40–46. [Google Scholar]
- Li, P.; Zhu, M.; Dan, J.; Kang, L.; Lai, L.; Cai, X.; Zhang, J.; Yu, F.; Tian, Z.; Dai, B. Two-dimensional porous SiO2 nanomesh supported high dispersed Ni nanoparticles for CO methanation. Chem. Eng. J. 2017, 326, 774–780. [Google Scholar] [CrossRef]
- Li, P.; Wen, B.; Yu, F.; Zhu, M.; Guo, X.; Han, Y.; Kang, L.; Huang, X.; Dan, J.; Ouyang, F.; et al. High efficient nickel/vermiculite catalyst prepared via microwave irradiation-assisted synthesis for carbon monoxide methanation. Fuel 2016, 171, 263–269. [Google Scholar] [CrossRef]
- Huang, X.; Yu, F.; Zhu, M.; Ouyang, F.; Dan, J. Hydrochlorination of acetylene using expanded multilayered vermiculite (EML-VMT)-supported catalysts. Chin. Chem. Lett. 2015, 26, 1101–1104. [Google Scholar] [CrossRef]
- Shen, B.; Liu, T.; Zhao, N.; Yang, X.; Deng, L. Iron-doped Mn-Ce/TiO2 catalyst for low temperature selective catalytic reduction of NO with NH3. J. Environ. Sci. Chin. 2010, 22, 1447–1454. [Google Scholar] [CrossRef]
- Lin, Q.; Li, J.; Ma, L.; Hao, J. Selective catalytic reduction of NO with NH3 over Mn–Fe/USY under lean burn conditions. Catal. Today 2010, 151, 251–256. [Google Scholar] [CrossRef]
- Thirupathi, B.; Smirniotis, P.G. Co-doping a metal (Cr, Fe, Co, Ni, Cu, Zn, Ce, and Zr) on Mn/TiO2 catalyst and its effect on the selective reduction of NO with NH3 at low-temperatures. Appl. Catal. B Environ. 2011, 110, 195–206. [Google Scholar] [CrossRef]
- Fan, Z.; Shi, J.W.; Gao, C.; Gao, G.; Wang, B.; Niu, C. Rationally Designed Porous MnOx-FeOx Nanoneedles for Low-Temperature Selective Catalytic Reduction of NOx by NH3. ACS Appl. Mater. Interfaces 2017, 9, 16117. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, B.; Smirniotis, P.G. Nickel-doped Mn/TiO2 as an efficient catalyst for the low-temperature SCR of NO with NH3: Catalytic evaluation and characterizations. J. Catal. 2012, 288, 74–83. [Google Scholar] [CrossRef]
- Kapteijn, F.; Singoredjo, L.; Andreini, A.; Moulijn, J.A. Activity and selectivity of pure manganese oxides in the selective catalytic reduction of nitric oxides with ammonia. Appl. Catal. B Environ. 1994, 3, 173–189. [Google Scholar] [CrossRef]
- Reddy, G.K.; He, J.; Thiel, S.W.; Pinto, N.G.; Smirniotis, P.G. Sulfur-tolerant Mn-Ce-Ti sorbents for elemental mercury removal from flue gas: Mechanistic investigation by XPS. J. Phys. Chem. C 2015, 119, 8634–8644. [Google Scholar] [CrossRef]
- Gao, G.; Shi, J.W.; Liu, C.; Gao, C.; Fan, Z.; Niu, C. Mn/CeO2 catalysts for SCR of NOx with NH3: Comparative study on the effect of supports on low-temperature catalytic activity. Appl. Sur. Sci. 2017, 411, 338–346. [Google Scholar] [CrossRef]
- Li, Y.; Wan, Y.; Li, Y.; Zhan, S.; Guan, Q.; Tian, Y. Low-Temperature Selective Catalytic Reduction of NO with NH3 over Mn2O3-doped Fe2O3 Hexagonal Microsheets. ACS Appl. Mater. Interfaces 2016, 8, 5224–5233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qu, H. Low-temperature selective catalytic reduction of NOx with NH3 over Fe–Cu mixed oxide/ZSM-5 catalysts containing Fe2CuO4 phase. Res. Chem. Interfaces 2015, 41, 4961–4975. [Google Scholar] [CrossRef]
- Lin, Z.; Lei, Z.; Qu, H.; Qin, Z. A study on chemisorbed oxygen and reaction process of Fe-CuOx/ZSM-5 via ultrasonic impregnation method for low-temperature NH3-SCR. J. Mol. Catal. A Chem. 2015, 409, 207–215. [Google Scholar]
- Haidi, X.U.; Fang, Z.; Cao, Y.; Kong, S.; Lin, T.; Gong, M.; Chen, Y. Influence of Mn/(Mn + Ce) Ratio of MnOx-CeO2/WO3-ZrO2 Monolith Catalyst on Selective Catalytic Reduction of NOx with Ammonia. Chin. J. Catal. 2012, 33, 1927–1937. [Google Scholar]
- Fang, C.; Zhang, D.; Cai, S.; Zhang, L.; Huang, L.; Li, H.; Maitarad, P.; Shi, L.; Gao, R.; Zhang, J. Low-temperature selective catalytic reduction of NO with NH3; over nanoflaky MnOx on carbon nanotubes in situ prepared via a chemical bath deposition route. Nanoscale 2013, 5, 9199–9207. [Google Scholar] [CrossRef] [PubMed]
- Boningari, T.; Ettireddy, P.R.; Somogyvari, A.; Liu, Y.; Vorontsov, A.; Mcdonald, C.A.; Smirniotis, P.G. Influence of elevated surface texture hydrated titania on Ce-doped Mn/TiO2 catalysts for the low-temperature SCR of NOx under oxygen-rich conditions. J. Catal. 2015, 325, 145–155. [Google Scholar] [CrossRef]
- Khan, A.; Smirniotis, P.G. Relationship between temperature-programmed reduction profile and activity of modified ferrite-based catalysts for WGS reaction. J. Mol. Catal. A Chem. 2008, 280, 43–51. [Google Scholar] [CrossRef]
- Cai, S.; Hu, H.; Li, H.; Shi, L.; Zhang, D. Design of multi-shell Fe2O3@MnOx@CNTs for the selective catalytic reduction of NO with NH3: Improvement of catalytic activity and SO2 tolerance. Nanoscale 2016, 8, 3588–3598. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhu, J.; Li, J.; Ma, L.; Woo, S.I. Novel Mn-Ce-Ti mixed-oxide catalyst for the selective catalytic reduction of NOx with NH3. ACS Appl. Mater. Interfaces 2014, 6, 14500–14508. [Google Scholar] [CrossRef] [PubMed]
- France, L.J.; Yang, Q.; Li, W.; Chen, Z.; Guang, J.; Guo, D.; Wang, L.F.; Li, X.H. Ceria modified FeMnOx-Enhanced performance and sulphur resistance for low-temperature SCR of NOx. Appl. Catal. B Environ. 2017, 206, 203–215. [Google Scholar] [CrossRef]
- Kobayashi, M.; Miyoshi, K. WO3–TiO2 monolithic catalysts for high temperature SCR of NO by NH3: Influence of preparation method on structural and physico-chemical properties, activity and durability. Appl. Catal. B Environ. 2007, 72, 253–261. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, B.; Du, J.; Tang, Q.; Liu, Z.; Liu, R.; Tao, C. The monolithic cordierite supported V2O5–MoO3/TiO2 catalyst for NH3-SCR. Chem. Eng. J. 2016, 294, 264–272. [Google Scholar] [CrossRef]
- Hwang, J.; Ha, H.J.; Ryu, J.; Choi, J.J.; Ahn, C.W.; Kim, J.W.; Hahn, B.D.; Yoon, W.H.; Lee, H.; Choi, J.H. Enhancement of washcoat adhesion for SCR catalysts to convert nitrogen oxide using powder spray coating of TiO2 on metallic honeycomb substrate. Catal. Commun. 2017, 94, 1–4. [Google Scholar] [CrossRef]
- Gan, L.; Lei, S.; Yu, J.; Ma, H.; Yamamoto, Y.; Suzuki, Y.; Xu, G.; Zhang, Z. Development of highly active coated monolith SCR catalyst with strong abrasion resistance for low-temperature application. Front. Environ. Sci. Eng. 2015, 9, 979–987. [Google Scholar] [CrossRef]
- Huang, H.F.; Jin, L.L.; Lu, H.F.; Yu, H.; Chen, Y.J. Monolithic Cr–V/TiO2/cordierite catalysts prepared by in-situ precipitation and impregnation for low-temperature NH3-SCR reactions. Catal. Commun. 2013, 34, 1–4. [Google Scholar] [CrossRef]
- Gao, C.; Shi, J.W.; Fan, Z.; Niu, C. Sulfur and Water Resistance of Mn-Based Catalysts for Low-Temperature Selective Catalytic Reduction of NOx: A Review. Catalysts 2018, 8, 11. [Google Scholar] [CrossRef]
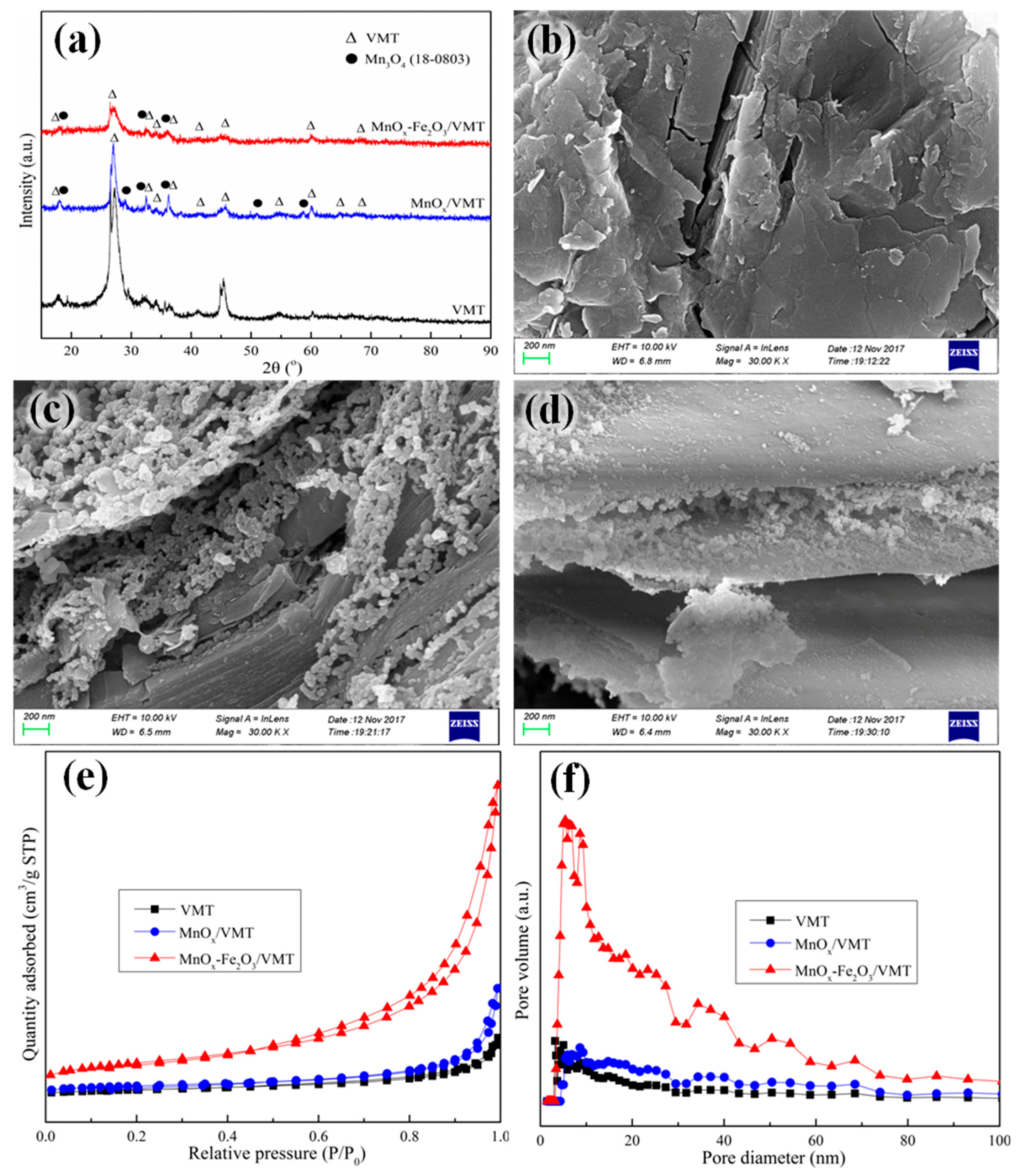


| Samples | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| VMT support | 2.9 | 0.02 | 26.2 |
| MnOx/VMT | 6.7 | 0.03 | 18.4 |
| MnOx-Fe2O3/VMT | 21.9 | 0.09 | 16.2 |
| MnOx-Fe2O3/VMT MHCs | 15.4 | 0.07 | 17.9 |
| Samples | Mn4+/Mntotal (%) | Oads/Ototal (%) | Fe3+/Fetotal (%) |
|---|---|---|---|
| MnOx/VMT | 26.3 | 38.5 | 51.5 |
| MnOx-Fe2O3/VMT | 35.9 | 46.0 | 59.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Yu, F.; Zhu, M.; Dan, J.; Wang, X.; Zhang, J.; Dai, B. Enhanced Low Temperature NO Reduction Performance via MnOx-Fe2O3/Vermiculite Monolithic Honeycomb Catalysts. Catalysts 2018, 8, 100. https://doi.org/10.3390/catal8030100
Zhang K, Yu F, Zhu M, Dan J, Wang X, Zhang J, Dai B. Enhanced Low Temperature NO Reduction Performance via MnOx-Fe2O3/Vermiculite Monolithic Honeycomb Catalysts. Catalysts. 2018; 8(3):100. https://doi.org/10.3390/catal8030100
Chicago/Turabian StyleZhang, Ke, Feng Yu, Mingyuan Zhu, Jianming Dan, Xugen Wang, Jinli Zhang, and Bin Dai. 2018. "Enhanced Low Temperature NO Reduction Performance via MnOx-Fe2O3/Vermiculite Monolithic Honeycomb Catalysts" Catalysts 8, no. 3: 100. https://doi.org/10.3390/catal8030100