Solvent-Free Mizoroki-Heck Reaction Applied to the Synthesis of Abscisic Acid and Some Derivatives
Abstract
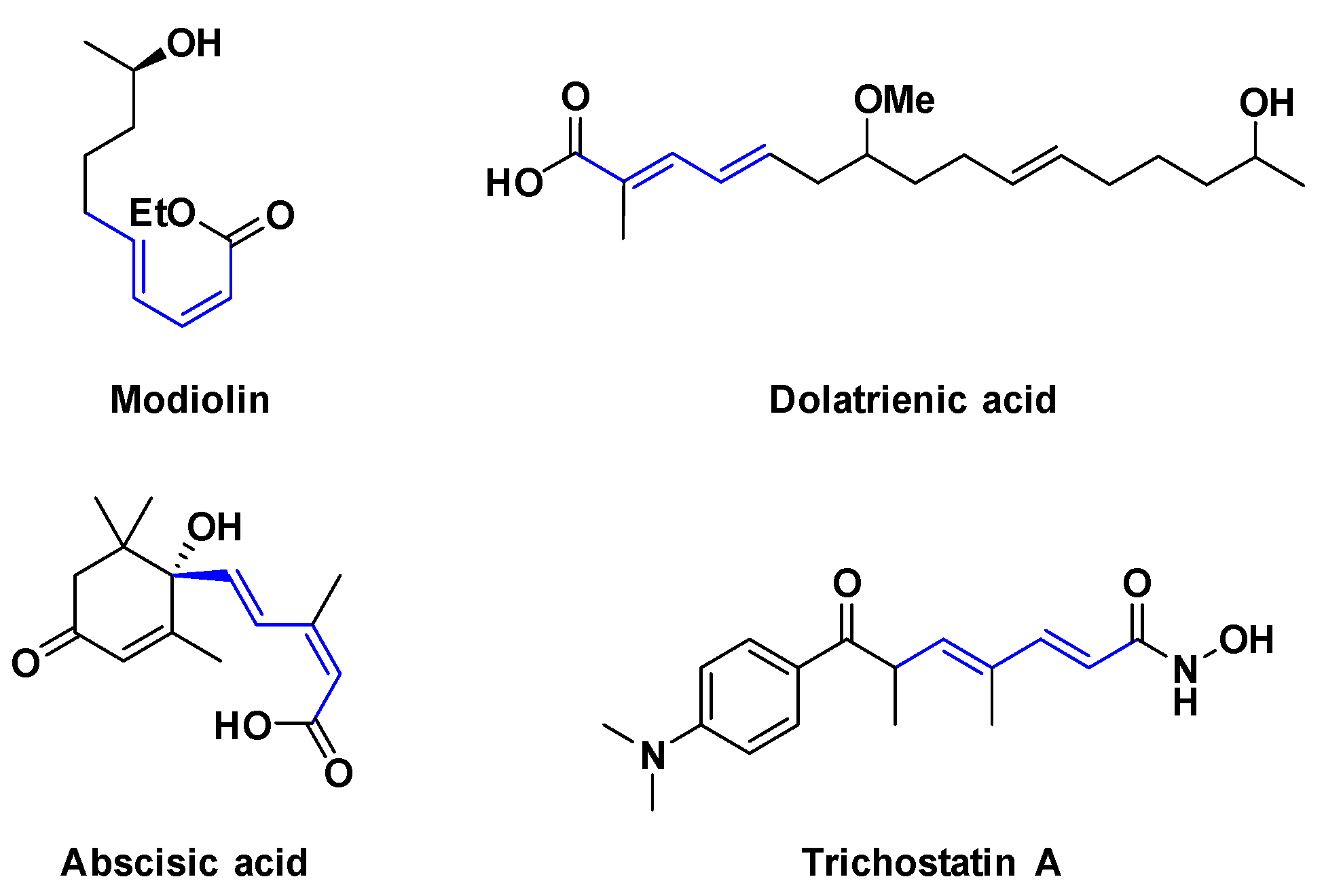
:1. Introduction
2. Results and Discussion
2.1. Optimization of the Mizoroki-Heck Reaction
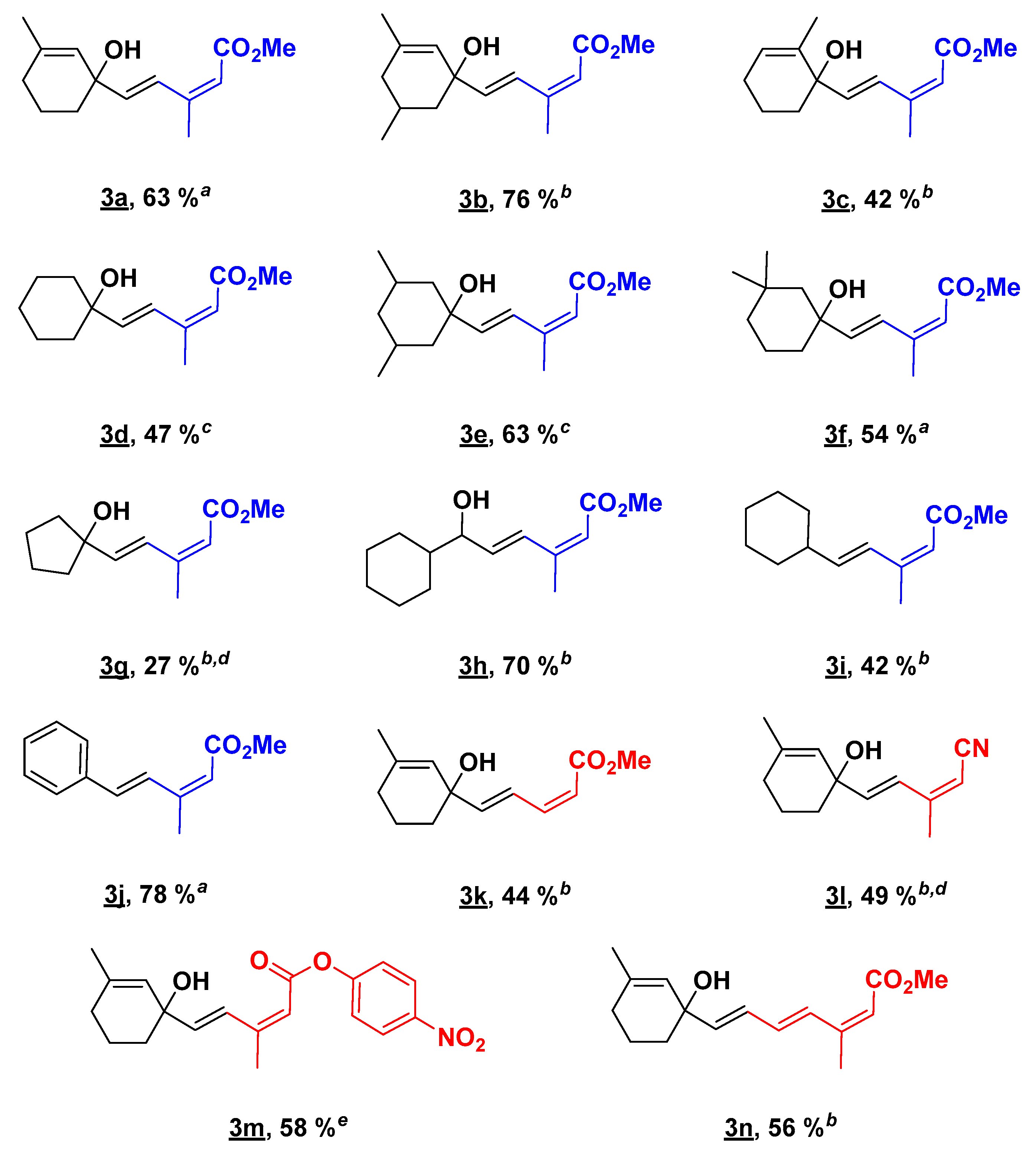
2.2. Substrate Scope
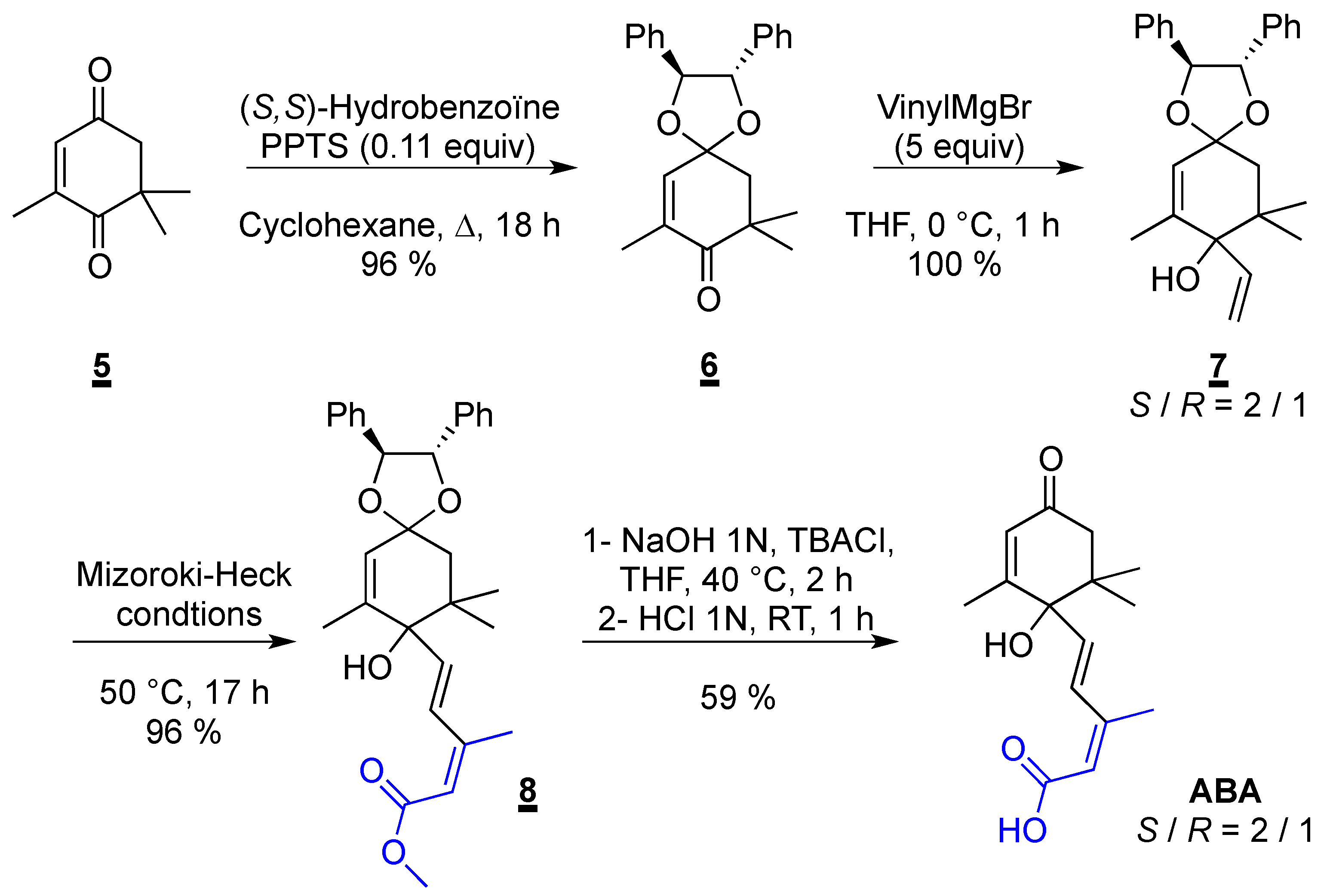
2.3. Synthesis of ABA
3. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hopf, H.; Maas, G. Synthesis and Transformation of Radialenes. In The Chemistry of Dienes and Polyenes, Vol. 1; Rappoport, Z., Ed.; Wiley: Chichester, UK, 1997; ISBN 978-0-471-96512-1. [Google Scholar]
- De Paolis, M.; Chataigner, I.; Maddaluno, J. Recent advances in stereoselective synthesis of 1,3-dienes. Top. Curr. Chem. 2012, 327, 87–146. [Google Scholar] [CrossRef]
- Takeda, T. (Ed.) Modern Carbonyl Olefination: Methods and Applications; Wiley-VCH: Weinheim, Germany, 2004; ISBN 978-3-527-60538-5. [Google Scholar]
- Grubbs, R.H. (Ed.) Handbook of Metathesis, Vol. 1; Wiley-VCH: Weinheim, Germany, 2003; ISBN 978-3-527-30616-9. [Google Scholar]
- Van Horn, D.E.; Negishi, E. Selective carbon-carbon bond formation via transition metal catalysts. 8. Controlled carbometalation. Reaction of acetylenes with organoalane-zirconocene dichloride complexes as a route to stereo- and regio-defined trisubstituted olefins. J. Am. Chem. Soc. 1978, 100, 2252–2254. [Google Scholar] [CrossRef]
- Negishi, E. Bimetallic catalytic systems containing Ti, Zr, Ni, and Pd. Their applications to selective organic syntheses. Pure Appl. Chem. 1981, 53, 2333–2356. [Google Scholar] [CrossRef]
- Negishi, E.; Van Horn, D.E.; Yoshida, T. Controlled carbometalation. 20. Carbometalation reaction of alkynes with organoalene-zirconocene derivatives as a route to stereo-and regiodefined trisubstituted alkenes. J. Am. Chem. Soc. 1985, 107, 6639–6647. [Google Scholar] [CrossRef]
- Robinson, C.Y.; Brouilllette, W.J.; Muccio, D.D. Reactions of vinylogous phosphonate carbanions and Reformatskii reagents with a sterically hindered ketone and enone. J. Org. Chem. 1989, 54, 1992–1997. [Google Scholar] [CrossRef]
- Horie, H.; Kurahashi, T.; Matsubara, S. Selective synthesis of trienes and dienes via nickel-catalyzed intermolecular cotrimerization of acrylates and alkynes. Chem. Commun. 2010, 46, 7229–7231. [Google Scholar] [CrossRef] [PubMed]
- Negishi, E.; Okukado, N.; King, A.O.; Van Horn, D.E.; Spiegel, B.I. Selective carbon-carbon bond formation via transition metal catalysts. 9. Double metal catalysis in the cross-coupling reaction and its application to the stereo- and regioselective synthesis of trisubstituted olefins. J. Am. Chem. Soc. 1978, 100, 2254–2256. [Google Scholar] [CrossRef]
- Negishi, E.; Hu, Q.; Wang, Z.; Yin, N. The Chemistry of Organozinc Compounds; Rappoport, Z., Marek, I., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2006; Chapter 11; p. 453. ISBN 978-0-470-09339-9. [Google Scholar]
- Stille, J.K.; Milstein, D. A general, selective, and facile method for ketone synthesis from acid chlorides and organotin compounds catalyzed by palladium. J. Am. Chem. Soc. 1978, 100, 3636–6638. [Google Scholar] [CrossRef]
- Stille, J.K. The Palladium-Catalyzed Cross-Coupling Reactions of Organotin Reagents with Organic Electrophiles. Angew. Chem. Int. Ed. Engl. 1986, 25, 508–524. [Google Scholar] [CrossRef]
- Farina, V. New perspectives in the cross-coupling reactions of organostannanes. Pure Appl. Chem. 1996, 68, 73–78. [Google Scholar] [CrossRef]
- Farina, V.; Krishnamurthy, V.; Scott, W.J. The Stille Reaction. Org. React. 1997, 50. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Stanforth, S.P. Catalytic cross-coupling reactions in biaryl synthesis. Tetrahedron 1998, 54, 263–303. [Google Scholar] [CrossRef]
- Suzuki, A. Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles 1995–1998. J. Organomet. Chem. 1999, 576, 147–168. [Google Scholar] [CrossRef]
- Kotovshchikov, Y.N.; Latyshev, G.V.; Lukashev, N.V.; Beletskaya, I.P. Alkynylation of steroids via Pd-free Sonogashira coupling. Org. Biomol. Chem. 2015, 13, 5542–5555. [Google Scholar] [CrossRef] [PubMed]
- Murahashi, S.-I. Palladium-catalyzed cross-coupling reaction of organic halides with Grignard reagents, organolithium compounds and heteroatom nucleophiles. J. Organomet. Chem. 2002, 653, 27–33. [Google Scholar] [CrossRef]
- Dounay, A.B.; Overman, L.E. The Asymmetric Intramolecular Heck Reaction in Natural Product Total Synthesis. Chem. Rev. 2003, 103, 2945–2964. [Google Scholar] [CrossRef] [PubMed]
- Bräse, S.; De Meijeren, F. Metal-Catalyzed Cross-Coupling Reactions; De Meijere, A., Diederich, F., Eds.; Wiley-VCH: New York, NY, USA, 2004; Chapter 5; ISBN 978-3-527-61953-5. [Google Scholar]
- Fayol, A.; Fang, Y.-Q.; Lautens, M. Synthesis of 2-Vinylic Indoles and Derivatives via a Pd-Catalyzed Tandem Coupling Reaction. Org. Lett. 2006, 8, 4203–4206. [Google Scholar] [CrossRef] [PubMed]
- Battace, A.; Zair, T.; Doucet, H.; Santelli, M. Heck Vinylations Using Vinyl Sulfide, Vinyl Sulfoxide, Vinyl Sulfone, or Vinyl Sulfonate Derivatives and Aryl Bromides Catalyzed by a Palladium Complex Derived from a Tetraphosphine. Synthesis 2006, 20, 3495–3505. [Google Scholar] [CrossRef]
- McConville, M.; Saidi, O.; Blacker, J.; Xiao, J. Regioselective Heck Vinylation of Electron-Rich Olefins with Vinyl Halides: Is the Neutral Pathway in Operation? J. Org. Chem. 2009, 74, 2692–2698. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, J.F. Handbook of Organopalladium Chemistry for Organic Synthesis; Negishi, E., Ed.; Wiley: Chichester, UK, 2002; ISBN 978-0-47-121246-1. [Google Scholar]
- Beletskaya, I.P.; Cheprakov, A.V. Comprehensive Coordination Chemistry II; McCleverty, J.A., Meyer, T.J., Eds.; Wiley-VCH: Weinheim, UK, 2004; ISBN 978-0-080-91316-2. [Google Scholar]
- Wang, K.; Chen, S.; Zhang, H.; Xu, S.; Ye, F.; Zhanga, Y.; Wang, J. Pd(0)-catalyzed cross-coupling of allyl halides with α-diazocarbonyl compounds or N-mesylhydrazones: Synthesis of 1,3-diene compounds. Org. Biomol. Chem. 2016, 14, 3809–3820. [Google Scholar] [CrossRef] [PubMed]
- O’Nei, G.W.; Phillips, A.J. Total Synthesis of (−)-Dictyostatin. J. Am. Chem. Soc. 2006, 128, 5340–5341. [Google Scholar] [CrossRef] [PubMed]
- Negishi, E.; Wang, G.; Rao, H.; Xu, Z. Alkyne Elementometalation-Pd-Catalyzed Cross-Coupling. Toward Synthesis of All Conceivable Types of Acyclic Alkenes in High Yields, Efficiently, Selectively, Economically, and Safely: “Green” Way. J. Org. Chem. 2010, 75, 3151–3182. [Google Scholar] [CrossRef] [PubMed]
- Eto, K.; Yoshino, M.; Takahashi, K.; Ishihara, J.; Hatakeyama, S. Total Synthesis of Oxazolomycin A. Org. Lett. 2011, 13, 5398–5401. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Kunz, O. One-Flask Tethered Ring Closing Metathesis–Electrocyclic Ring Opening for the Highly Stereoselective Synthesis of Conjugated Z/E-Dienes. Eur. J. Org. Chem. 2012, 5, 1008–1018. [Google Scholar] [CrossRef]
- Souris, C.; Frébault, F.; Patel, A.; Audisio, D.; Houk, K.N.; Maulide, N. Stereoselective Synthesis of Dienyl-Carboxylate Building Blocks: Formal Synthesis of Inthomycin C. Org. Lett. 2013, 15, 3242–3245. [Google Scholar] [CrossRef] [PubMed]
- Hiebel, M.-A.; Fall, Y.; Scherrmann, M.-C.; Berteina-Raboin, S. Straightforward Synthesis of Various 2,3-Diarylimidazo[1,2-a]pyridines in PEG400 Medium through One-Pot Condensation and C–H Arylation. Eur. J. Org. Chem. 2014, 21, 4643–4650. [Google Scholar] [CrossRef]
- Hiebel, M.-A.; Berteina-Raboin, S. Iodine-catalyzed regioselective sulfenylation of imidazoheterocycles in PEG400. Green Chem. 2015, 17, 937–944. [Google Scholar] [CrossRef]
- Tber, Z.; Hiebel, M.-A.; Akssira, M.; Guillaumet, G.; Berteina-Raboin, S. Use of Ligand-Free Iron/Copper Cocatalyst for Nitrogen and Sulfur Cross-Coupling Reaction with 6-Iodoimidazo[1,2-a]pyridine. Synthesis 2015, 47, 1780–1790. [Google Scholar] [CrossRef]
- Addicott, F.T.; Carns, H.R. Abscisic Acids; Addicott, F.T., Ed.; Praeger: New York, NY, USA, 1983; Chapter 1. [Google Scholar]
- Finkelstein, R.F.; Tenbarge, K.M.; Shumway, J.E.; Crunch, M.L. Role of ABA in Maturation of Rapeseed Embryos. Plant Physiol. 1985, 78, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Léon-Kloosterziel, K.M.; Van de Bunt, G.A.; Zeevaart, J.A.D.; Koornneef, M. Arabidopsis Mutants with a Reduced Seed Dormancy. Plant Physiol. 1996, 110, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Molecular responses to dehydration and low temperature: Differences and cross-talk between two stress signaling pathways. Curr. Opin. Plant. Biol. 2000, 3, 217–223. [Google Scholar] [CrossRef]
- Himmelbach, A.; Yang, Y.; Grill, E. Relay and control of abscisic acid signaling. Curr. Opin. Plant. Biol. 2003, 6, 470–479. [Google Scholar] [CrossRef]
- Nambara, E.; Marion-Poll, A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant. Biol. 2005, 56, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Todoroki, Y.; Narita, K.; Muramatsu, T.; Shimamura, H.; Ohnishi, T.; Mizutani, M.; Ueno, K.; Hirai, N. Synthesis and biological activity of amino acid conjugates of abscisic acid. Bioorg. Med. Chem. 2011, 19, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Guri, A.J.; Hontecillas, R.; Si, H.; Liu, D.; Bassaganya-Riera, J. Dietary abscisic acid ameliorates glucose tolerance and obesity-related inflammation in db/db mice fed high-fat diets. Clin. Nutr. 2007, 26, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Bellotti, M.; Salis, A.; Grozio, A.; Damonte, G.; Vigliarolo, T.; Galatini, A.; Zocchi, E.; Benatti, U.; Millo, E. Synthesis, structural characterization and effect on human granulocyte intracellular cAMP levels of abscisic acid analogs. Bioorg. Med. Chem. 2015, 23, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Brandt, D.; Bellosta, V.; Cossy, J. Stereoselective Synthesis of Conjugated Trienols from Allylic Alcohols and 1-Iodo-1,3-dienes. Org. Lett. 2012, 14, 5594–5597. [Google Scholar] [CrossRef] [PubMed]
- McCubbin, J.A.; Voth, S.; Krokhin, O.V. Mild and Tunable Benzoic Acid Catalysts for Rearrangement Reactions of Allylic Alcohols. J. Org. Chem. 2011, 76, 8537–8542. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-F.; Wang, H.-L.; Qu, J. 1,n-Rearrangement of Allylic Alcohols Promoted by Hot Water: Application to the Synthesis of Navenone B, a Polyene Natural Product. J. Org. Chem. 2014, 79, 3955–3962. [Google Scholar] [CrossRef] [PubMed]
- Knowles, J.P.; O’Connor, V.E.; Whiting, A. Studies towards the synthesis of the northern polyene of viridenomycin and synthesis of Z-double bond analogues. Org. Biomol. Chem. 2011, 9, 1876–1886. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Ortiz, A.; Prieto, P.; Vazquez, E. Heck Reactions under Microwave Irradiation in Solvent-Free Conditions. Synlett 1997, 3, 269–270. [Google Scholar] [CrossRef]
- Leadbeater, N.E.; Williams, V.A.; Barnard, T.M.; Collins, M.J., Jr. Solvent-Free, Open-Vessel Microwave-Promoted Heck Couplings: From the mmol to the mol Scale. Synlett 2006, 18, 2953–2958. [Google Scholar] [CrossRef]
- Du, L.-H.; Wang, Y.-G. Microwave-Promoted Heck Reaction Using Pd(OAc)2 as Catalyst under Ligand-Free and Solvent-Free Conditions. Synth. Commun. 2007, 37, 217–222. [Google Scholar] [CrossRef]
- Liu, G.; Hou, M.; Song, J.; Jiang, T.; Fan, H.; Zhang, Z.; Han, B. Immobilization of Pd nanoparticles with functional ionic liquid grafted onto cross-linked polymer for solvent-free Heck reaction. Green Chem. 2010, 12, 65–69. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Iranpoor, N.; Kazemi, F.; Gholinejad, M. Palladium nano-particles supported on agarose as efficient catalyst and bioorganic ligand for CC bond formation via solventless Mizoroki–Heck reaction and Sonogashira–Hagihara reaction in polyethylene glycol (PEG 400). J. Mol. Catal. A Chem. 2012, 357, 154–161. [Google Scholar] [CrossRef]
- Khazaei, A.; Rahmati, S.; Hekmatian, Z.; Saeednia, S. A green approach for the synthesis of palladium nanoparticles supported on pectin: Application as a catalyst for solvent-free Mizoroki–Heck reaction. J. Mol. Catal. A Chem. 2013, 372, 160–166. [Google Scholar] [CrossRef]
- Zolfigol, M.A.; Azadbakht, T.; Khakyzadeh, V.; Nejatyami, R.; Perrin, D.M. C(sp2)-C(sp2) cross coupling reactions catalyzed by an active and highly stable magnetically separable Pd-nanocatalyst in aqueous media. RSC Adv. 2014, 4, 40036–40042. [Google Scholar] [CrossRef]
- Khazaei, A.; Khazaei, M.; Rahmati, S. A green method for the synthesis of gelatin/pectin stabilized palladium nano-particles as efficient heterogeneous catalyst for solvent-free Mizoroki–Heck reaction. J. Mol. Catal. A Chem. 2015, 398, 241–247. [Google Scholar] [CrossRef]
- Trost, B.M.; Conway, W.P.; Strege, P.E.; Dietsche, T.J. New Synthetic reactions. Alkylative elimination. J. Am. Chem. Soc. 1974, 96, 7165–7167. [Google Scholar] [CrossRef]
- Roberts, D.L.; Heckmann, R.A.; Hege, B.P.; Bellin, S.A. Synthesis of (RS)-abscisic acid. J. Org. Chem. 1968, 33, 3566–3569. [Google Scholar] [CrossRef]
- Constantino, M.G.; Losco, P. A novel synthesis of (±)-abscisic acid. J. Org. Chem. 1989, 54, 681–683. [Google Scholar] [CrossRef]
- Cornforth, J.; Hawes, J.E.; Mallaby, R. A Stereospecific Synthesis of (±)-Abscisic Acid. Aust. J. Chem. 1992, 45, 179–185. [Google Scholar] [CrossRef]
- Mayer, H.J.; Rigassi, N.; Schwieter, U.; Weedon, B.C.L. Synthesis of Abscisic Acid. Helv. Chim. Acta 1976, 59, 1424–1427. [Google Scholar] [CrossRef]
- Kienzle, F.; Mayer, H.; Minder, R.E.; Thommen, H. Synthese von optisch aktiven, natürlichen Carotinoiden und strukturell verwandten Verbindungen. III. Synthese von (+)-Abscisinsäure, (−)-Xanthoxin, (−)-Loliolid, (−)-Actinidiolid und (−)-Dihydroactinidiolid. Helv. Chim. Acta 1978, 61, 2616–2627. [Google Scholar] [CrossRef]
- Constantino, M.G.; Donate, P.M.; Petragnani, N. An efficient synthesis of (±)-abscisic acid. J. Org. Chem. 1986, 51, 253–254. [Google Scholar] [CrossRef]
- Rose, P.A.; Abrams, S.R.; Shaw, A.C. Synthesis of chiral acetylenic analogs of the plant hormone abscisic acid. Tetrahedron Asymmetry 1992, 3, 443–450. [Google Scholar] [CrossRef]
- Hanson, J.R.; Uyanik, C. An efficient synthesis of the plant hormone abscisic acid. J. Chem. Res. 2003, 7, 426–427. [Google Scholar] [CrossRef]
- Smith, T.R.; Clark, A.J.; Clarkson, G.J.; Taylor, P.C.; Marsh, A. Concise enantioselective synthesis of abscisic acid and a new analogue. Org. Biomol. Chem. 2006, 4, 4186–4192. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, E.; Alibés, R.; Busqué, F.; Figueredo, M.; Font, J.; De March, P. Enantiodivergent Synthesis of Cyclohexenyl Nucleosides. J. Org. Chem. 2009, 74, 2425–2432. [Google Scholar] [CrossRef] [PubMed]
- Mash, E.A.; Torok, D.S. Homochiral ketals in organic synthesis. Diastereoselective cyclopropanation of alpha beta-unsaturated ketals derived from (S,S)-(−)-hydrobenzoin. J. Org. Chem. 1989, 54, 250–253. [Google Scholar] [CrossRef]


| Entry | 1 (equiv.) | 2 (equiv.) | Additive (equiv.) | Solvent | Time (h) | Yield 3:4 (%) |
|---|---|---|---|---|---|---|
| 1 | 1.2 | 1 | AgOAc (1.1) | DMF | 17 | 0:0 a |
| 2 | 1.2 | 1 | AgOAc (1.1) | DMF | 17 | 5:4 b |
| 3 | 1.2 | 1 | AgOAc (1.1) | MeCN | 17 | 9:0 b |
| 4 | 1.2 | 1 | AgOAc (1.1) P(oTol)3 (0.1) | MeCN | 17 | 9:20 b |
| 5 | 1.2 | 1 | Ag2CO3 (1.5) | MeCN | 17 | 63:0 b,c |
| 6 | 1.2 | 1 | Ag2CO3 (2) | MeCN | 17 | 63:0 b,c |
| 7 | 1.2 | 1 | Ag2CO3 (1.1) | MeCN | 17 | 54:0 b,c |
| 8 | 1.2 | 1 | Ag2CO3 (1.5) | MeCN | 17 | 10:0 b,c,d |
| 9 | 1.2 | 1 | Ag2CO3 (1.5) | none | 1 | 63:0 b,c |
| 10 | 1 | 2 | Ag2CO3 (1.5) | none | 1 | 60:0 b,c |
| 11 | 1 | 1 + 1 | Ag2CO3 (1.5) | none | 1 | 50:0 b,c |
| 12 | 1.2 | 1 | Ag2CO3 (1.5) | none | 1 | 62:0 b,d |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumonteil, G.; Hiebel, M.-A.; Berteina-Raboin, S. Solvent-Free Mizoroki-Heck Reaction Applied to the Synthesis of Abscisic Acid and Some Derivatives. Catalysts 2018, 8, 115. https://doi.org/10.3390/catal8030115
Dumonteil G, Hiebel M-A, Berteina-Raboin S. Solvent-Free Mizoroki-Heck Reaction Applied to the Synthesis of Abscisic Acid and Some Derivatives. Catalysts. 2018; 8(3):115. https://doi.org/10.3390/catal8030115
Chicago/Turabian StyleDumonteil, Geoffrey, Marie-Aude Hiebel, and Sabine Berteina-Raboin. 2018. "Solvent-Free Mizoroki-Heck Reaction Applied to the Synthesis of Abscisic Acid and Some Derivatives" Catalysts 8, no. 3: 115. https://doi.org/10.3390/catal8030115







