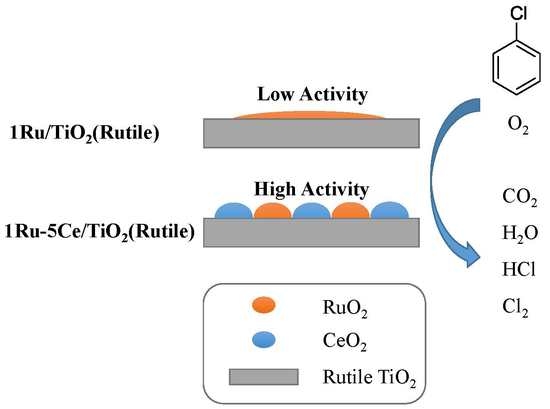
Catalytic Oxidation of Chlorobenzene over Ruthenium-Ceria Bimetallic Catalysts
Abstract
:1. Introduction
2. Results and Discussion
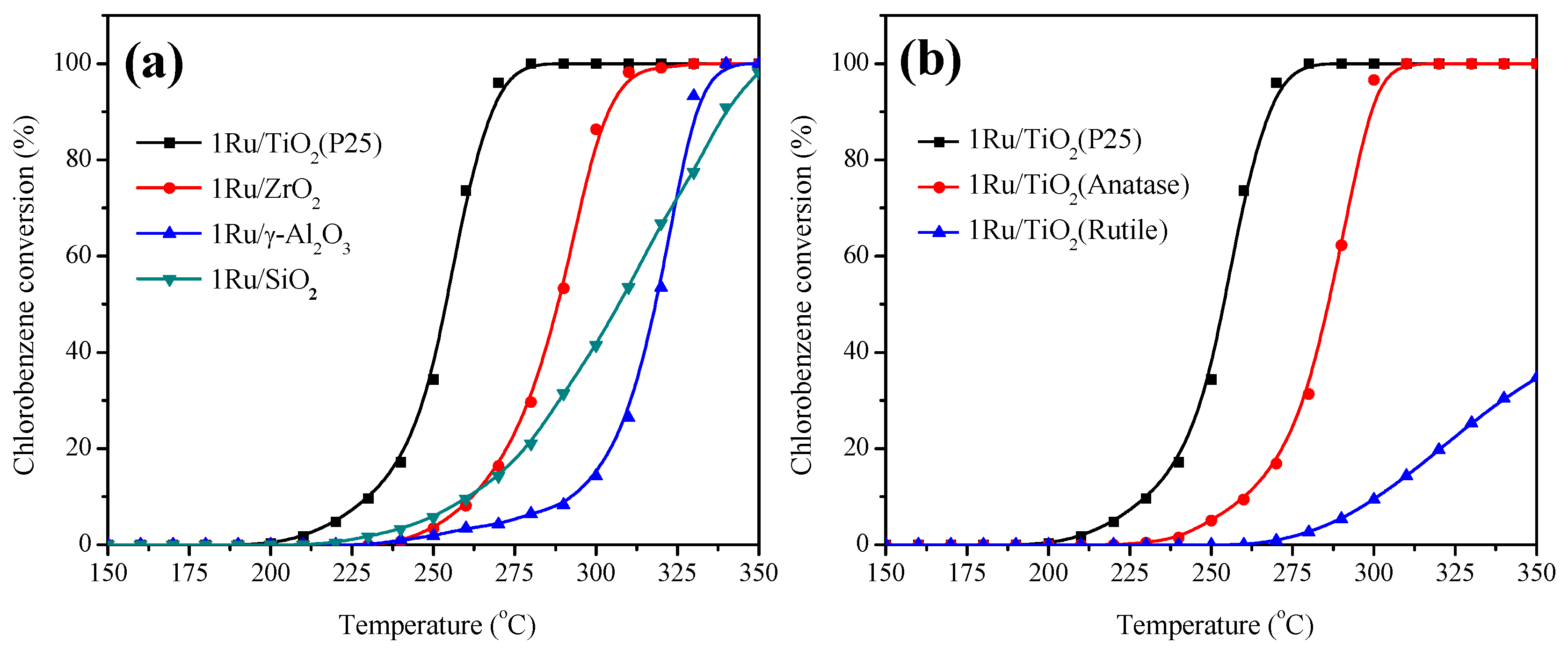
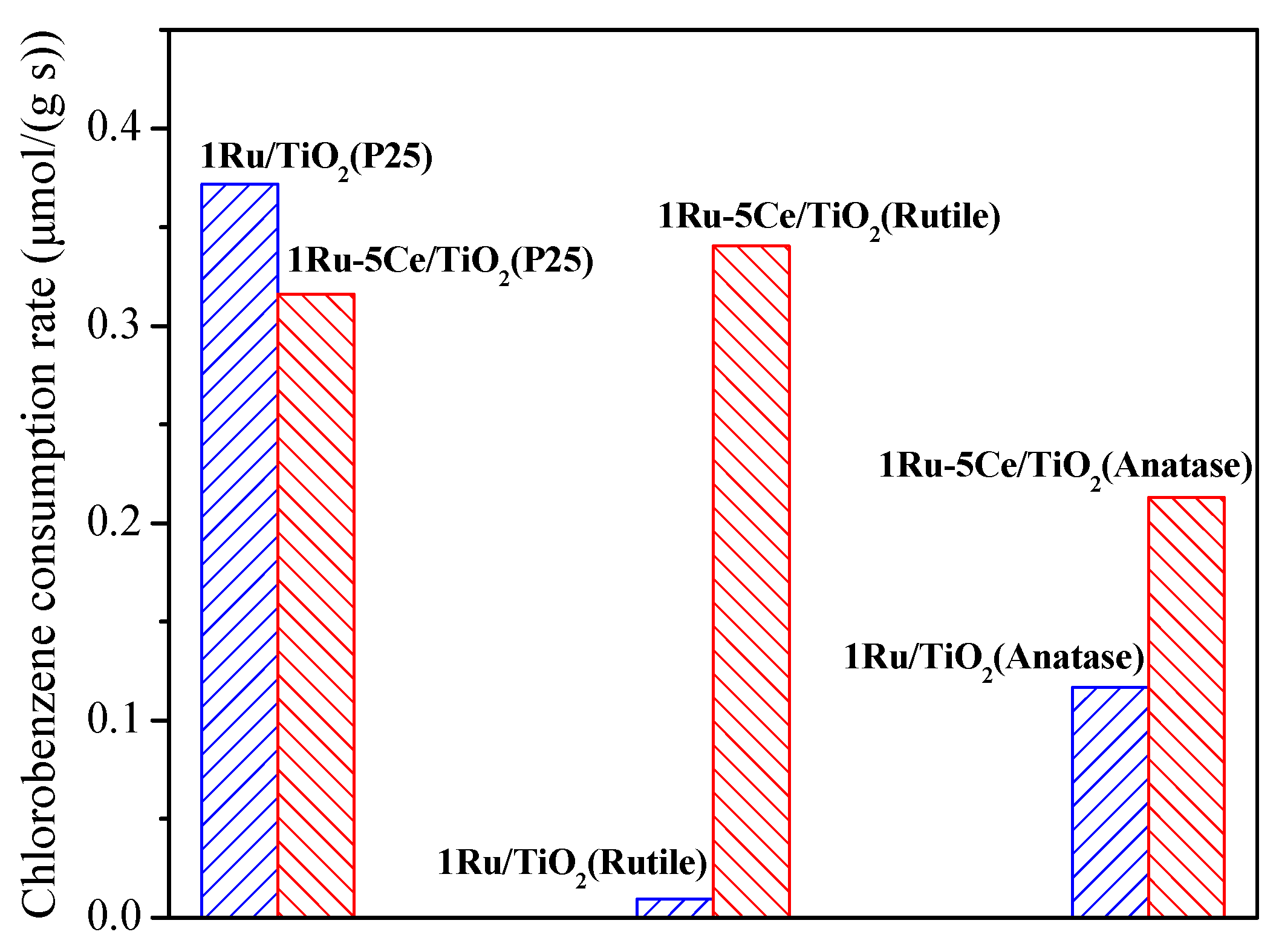
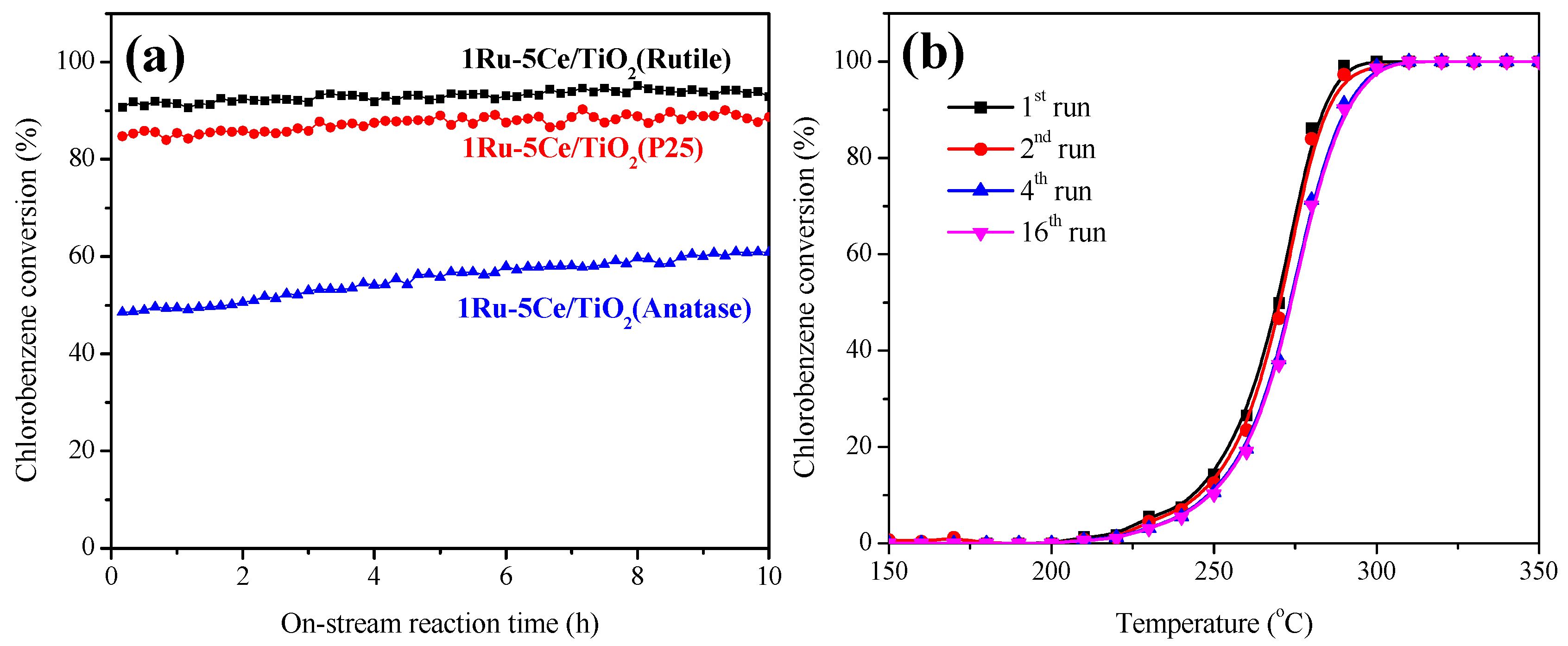
2.1. Catalytic Oxidation of Chlorobenzene
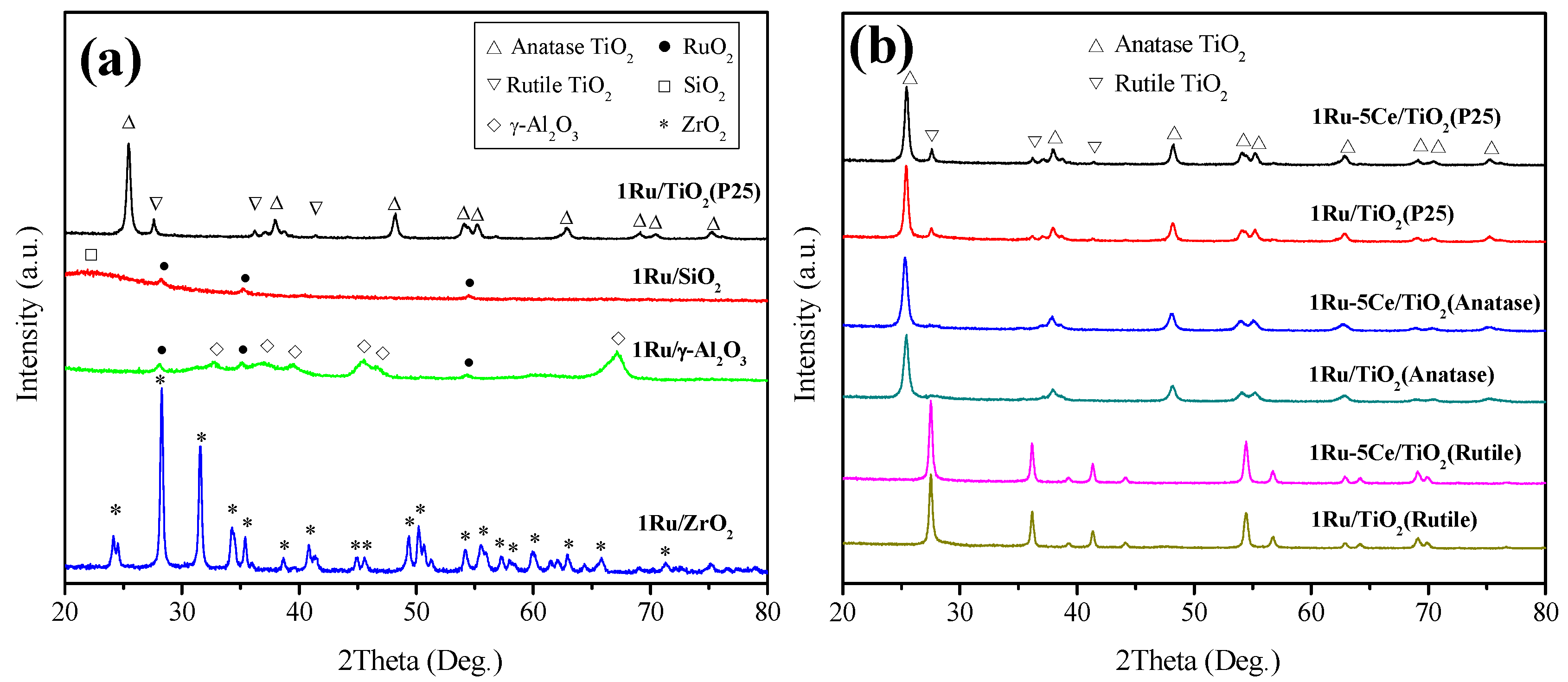
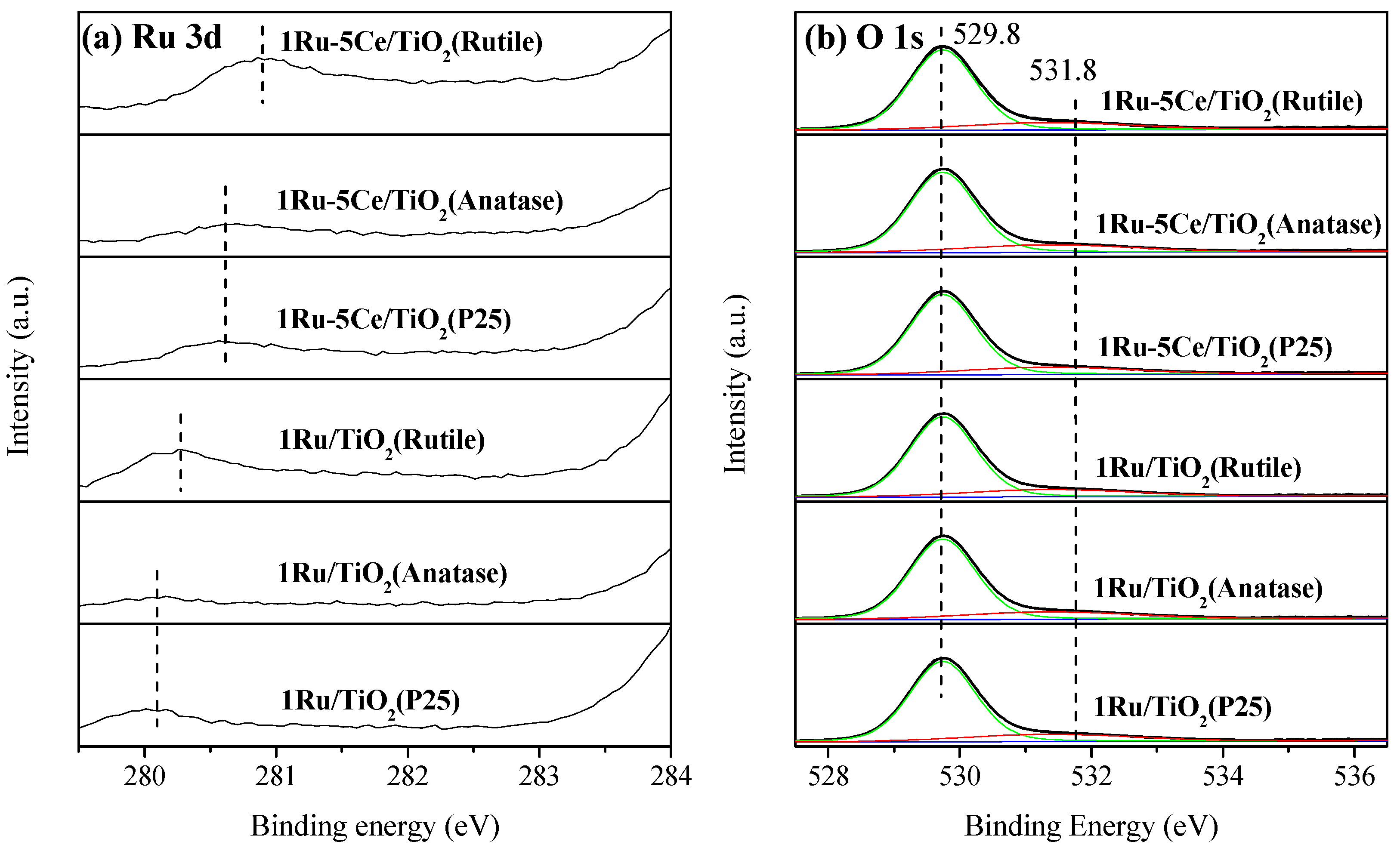
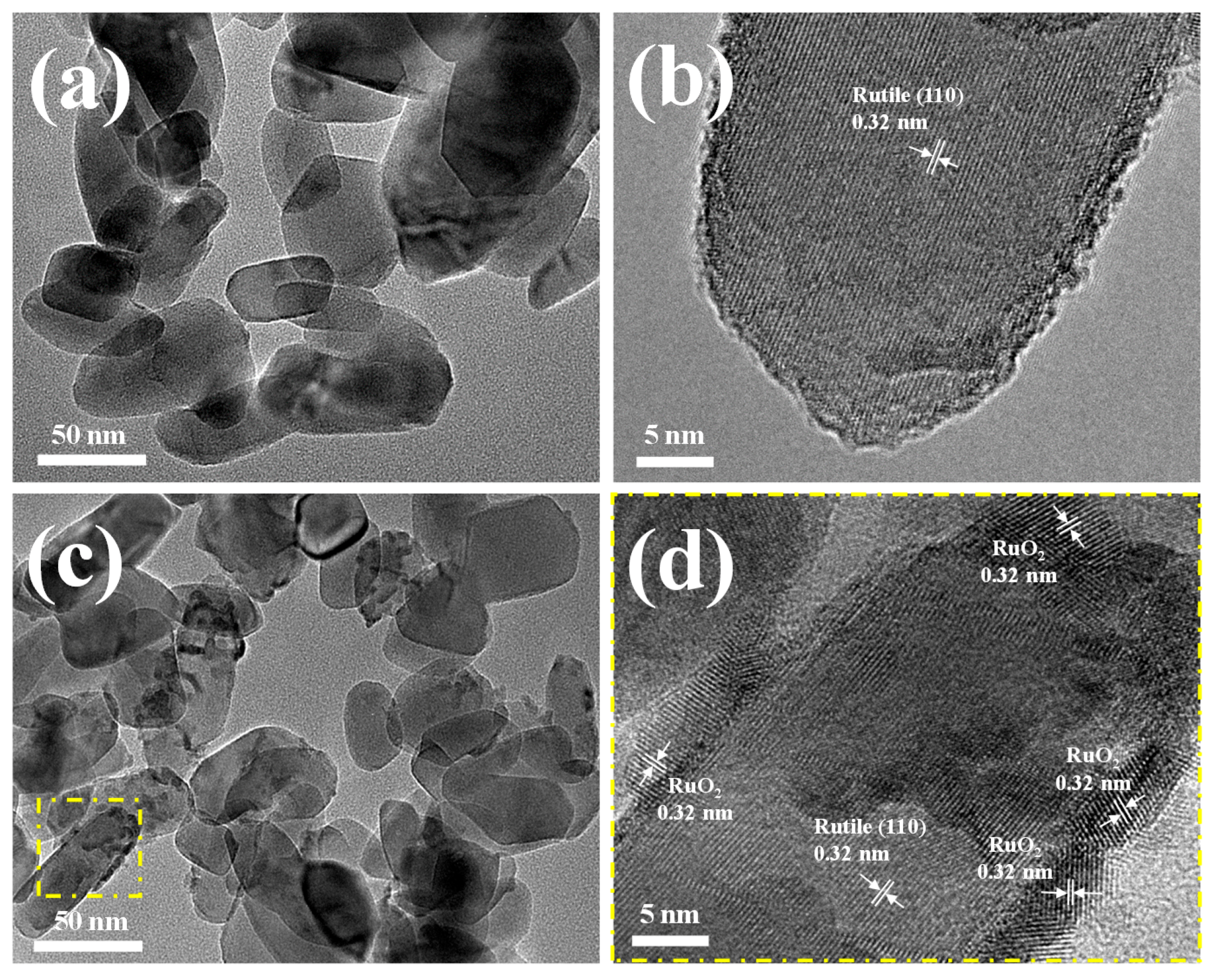
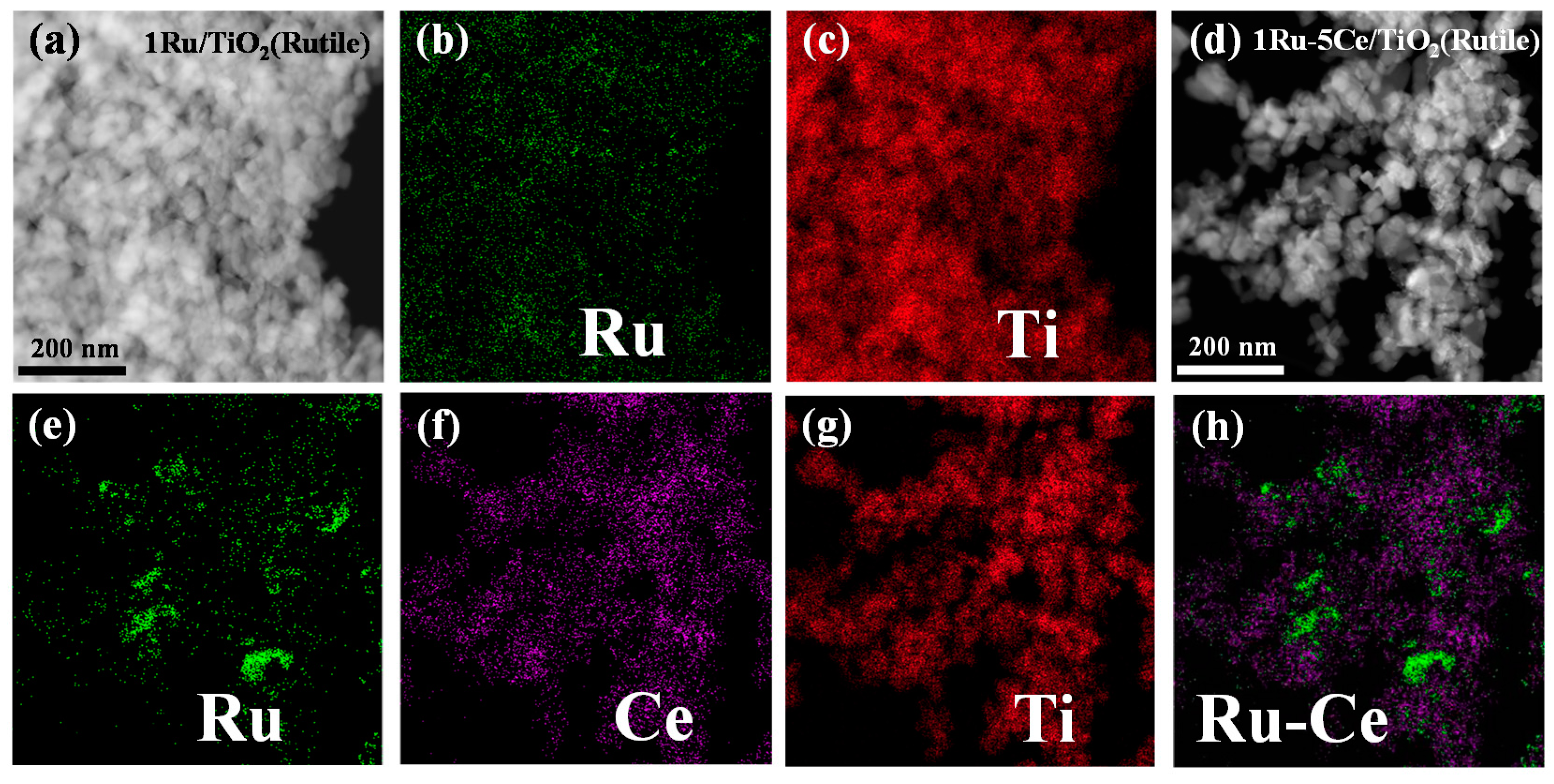
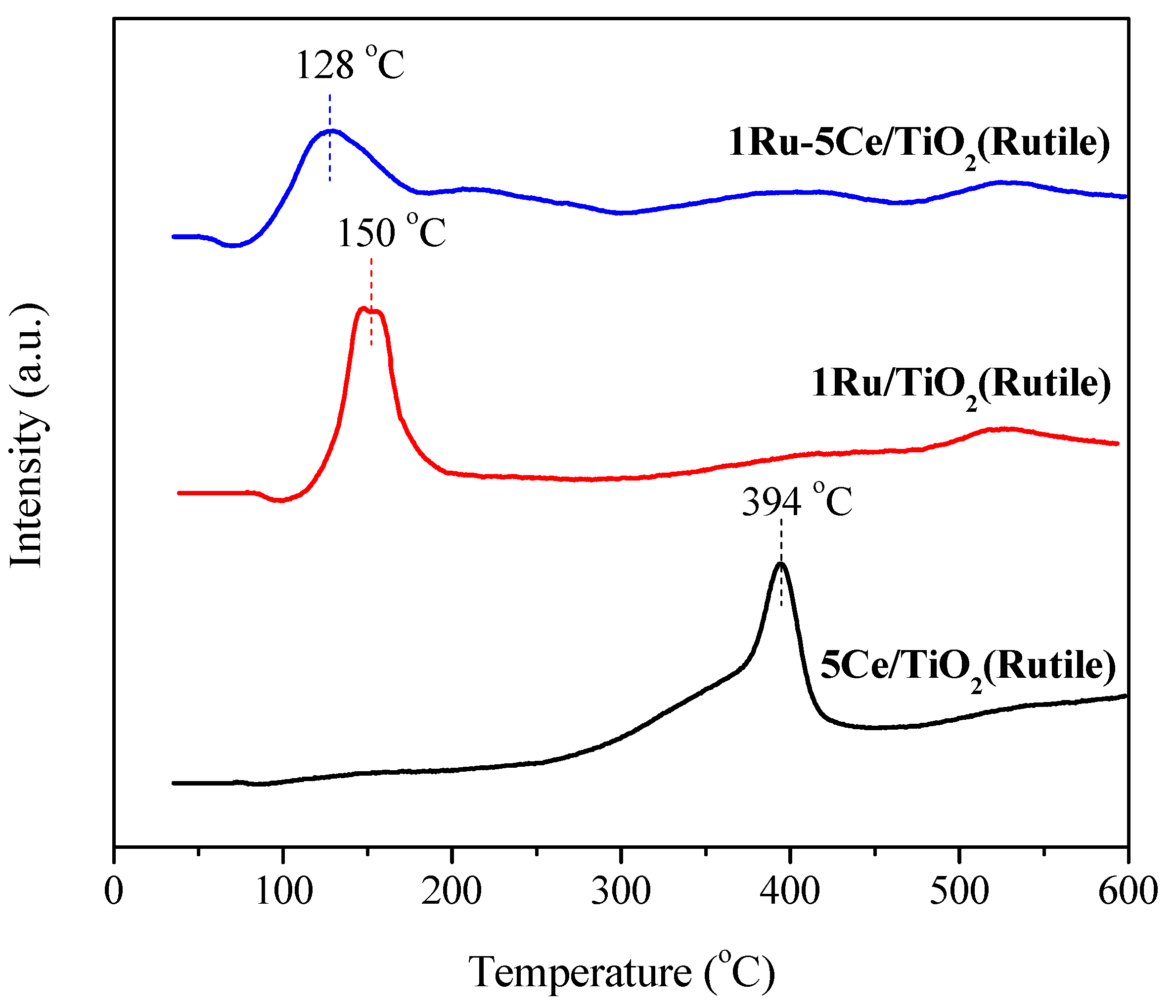
2.2. Catalyst Characterization
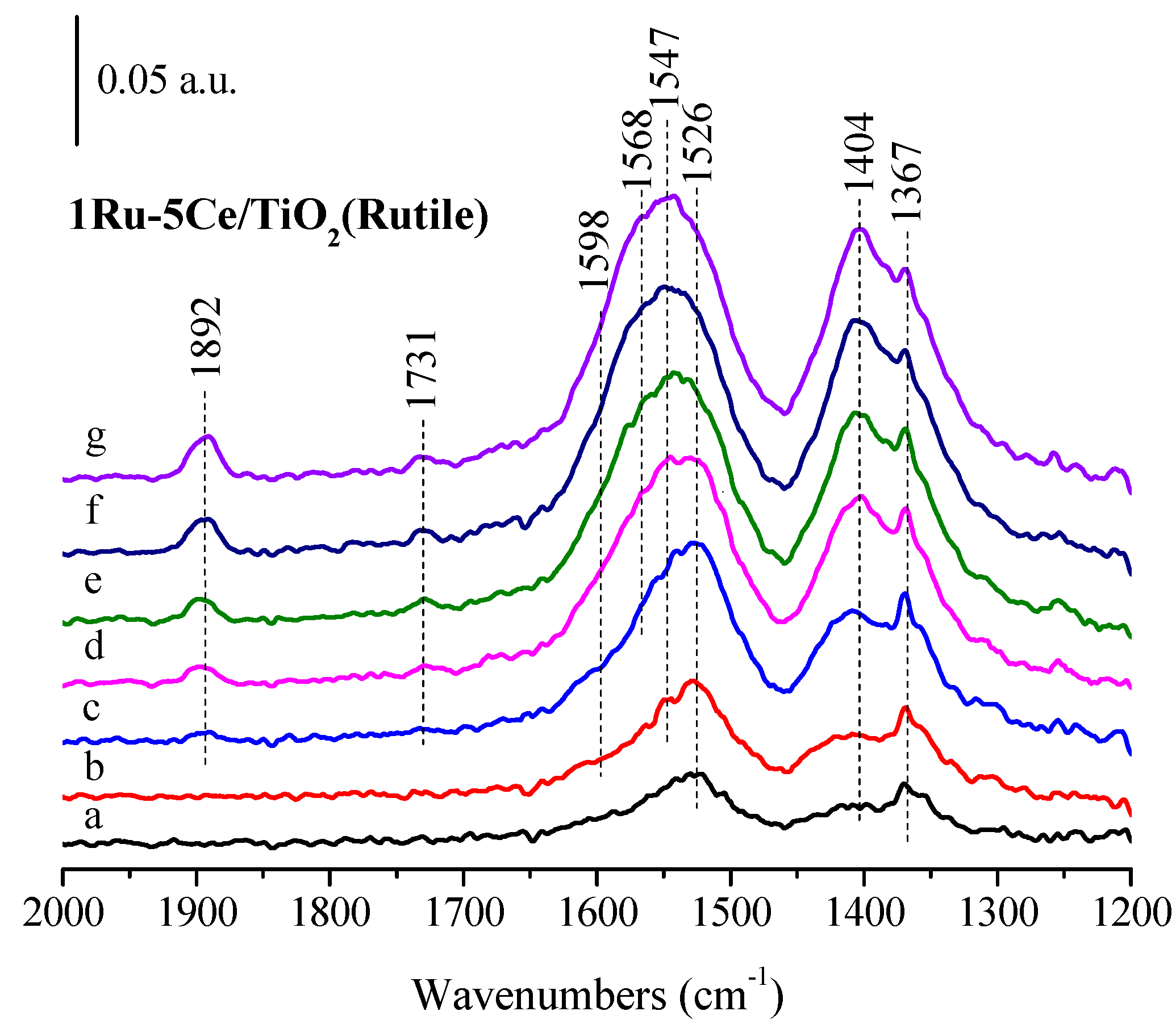
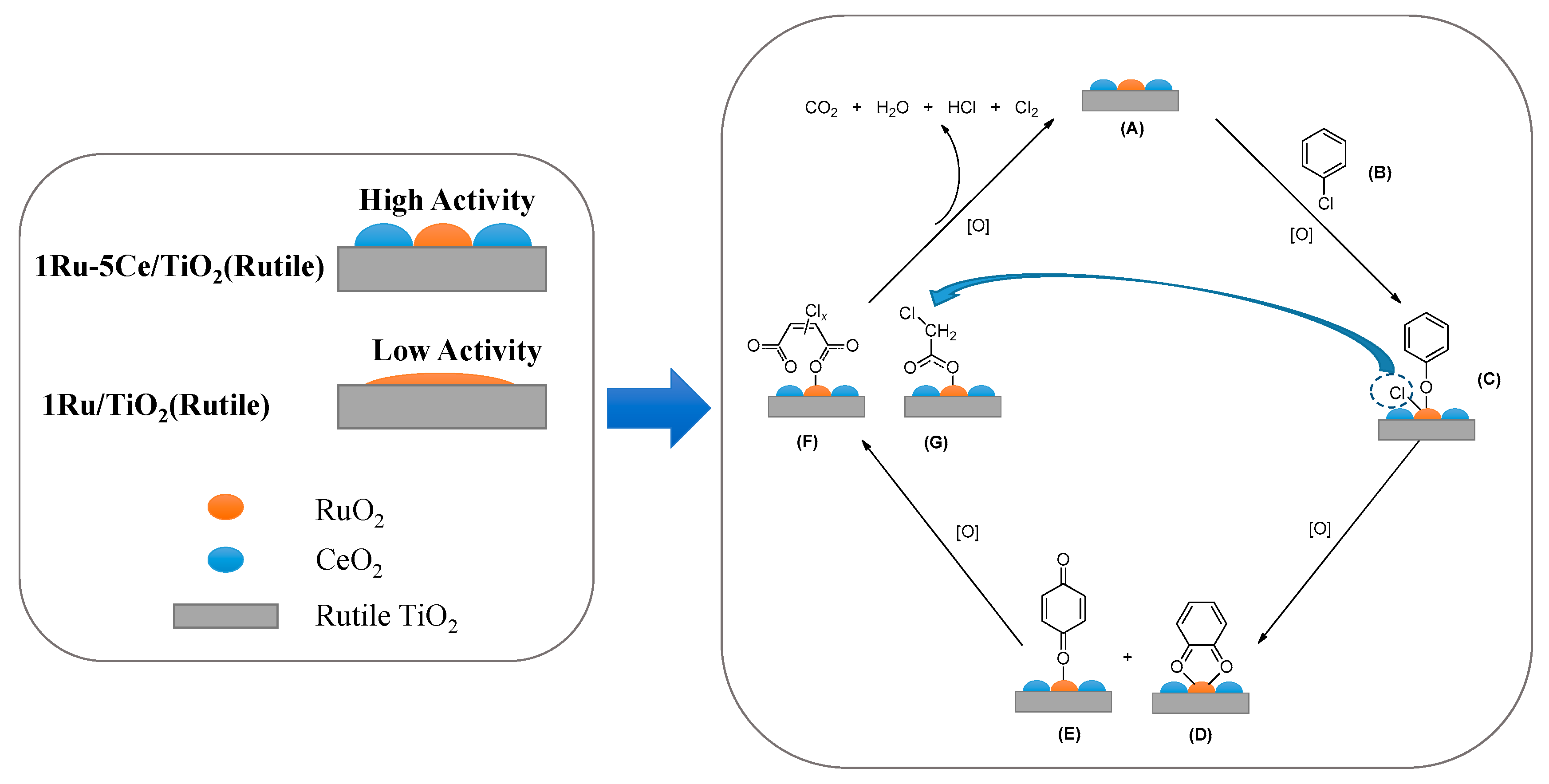
2.3. In Situ FTIR Studies and Reaction Mechanism
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Evaluation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jiang, L.; Nie, G.; Zhu, R.; Wang, J.; Chen, J.; Mao, Y.; Cheng, Z.; Anderson, W.A. Efficient degradation of chlorobenzene in a non-thermal plasma catalytic reactor supported on CeO2/HZSM-5 catalysts. J. Environ. Sci. 2017, 55, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xue, Z.; Hong, L.; Li, Y.; Yuan, Y.; Yi, W.; Duan, J.; Lei, L.; Chai, F.; Cheng, M. Ambient volatile organic compounds pollution in China. J. Environ. Sci. 2017, 55, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, X.; Zhao, Q.; Liu, J.; Liu, S.; Wang, S.; Tade, M.O. Insight into the Mechanism of Photocatalytic Degradation of Gaseous o-dichlorobenzene over Flower-Type V2O5 Hollow Spheres. J. Mater. Chem. A 2015, 3, 15163–15170. [Google Scholar] [CrossRef]
- Huang, B.; Lei, C.; Wei, C.; Zeng, G. Chlorinated volatile organic compounds (Cl-VOCs) in environment—Sources, potential human health impacts, and current remediation technologies. Environ. Int. 2014, 71, 118–138. [Google Scholar] [CrossRef] [PubMed]
- Delaigle, R.; Debecker, D.P.; Bertinchamps, F.; Gaigneaux, E.M. Revisiting the behaviour of vanadia-based catalysts in the abatement of (chloro)-aromatic pollutants: Towards an integrated understanding. Top. Catal. 2009, 52, 501. [Google Scholar] [CrossRef]
- Gan, J.; Megonnell, N.E.; Yates, S.R. Adsorption and catalytic decomposition of methyl bromide and methyl iodide on activated carbons. Atmos. Environ. 2001, 35, 941–947. [Google Scholar] [CrossRef]
- Everaert, K.; Baeyens, J. Catalytic combustion of volatile organic compounds. J. Hazard. Mater. 2004, 109, 113–139. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, R.; Dou, B.; Ma, C.; Hu, Q.; Liang, Y.; Wu, F.; Qiao, S.; Hao, Z. Porous graphitized carbon for adsorptive removal of benzene and the electrothermal regeneration. Environ. Sci. Technol. 2012, 46, 12648–12654. [Google Scholar] [CrossRef] [PubMed]
- Fiorenza, R.; Bellardita, M.; Palmisano, L.; Scirè, S. A comparison between photocatalytic and catalytic oxidation of 2-Propanol over Au/TiO2-CeO2 catalysts. J. Mol. Catal. A Chem. 2016, 415, 56–64. [Google Scholar] [CrossRef]
- Hetrick, C.E.; Patcas, F.; Amiridis, M.D. Effect of water on the oxidation of dichlorobenzene over V2O5/TiO2 catalysts. Appl. Catal. B Environ. 2011, 101, 622–628. [Google Scholar] [CrossRef]
- Ojala, S.; Pitkäaho, S.; Laitinen, T.; Koivikko, N.N.; Brahmi, R.; Gaálová, J.; Matejova, L.; Kucherov, A.; Päivärinta, S.; Hirschmann, C. Catalysis in VOC abatement. Top. Catal. 2011, 54, 1224. [Google Scholar] [CrossRef]
- Huang, H.; Xu, Y.; Feng, Q.; Leung, D.Y. Low temperature catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2015, 5, 2649–2669. [Google Scholar] [CrossRef]
- Pitkäaho, S.; Nevanperä, T.; Matejova, L.; Ojala, S.; Keiski, R.L. Oxidation of dichloromethane over Pt, Pd, Rh, and V2O5 catalysts supported on Al2O3, Al2O3-TiO2 and Al2O3-CeO2. Appl. Catal. B Environ. 2013, 138, 33–42. [Google Scholar] [CrossRef]
- Matějová, L.; Topka, P.; Kaluža, L.; Pitkäaho, S.; Ojala, S.; Gaálová, J.; Keiski, R.L. Total oxidation of dichloromethane and ethanol over ceria-zirconia mixed oxide supported platinum and gold catalysts. Appl. Catal. B Environ. 2013, 142, 54–64. [Google Scholar] [CrossRef]
- Scirè, S.; Liotta, L.F. Supported gold catalysts for the total oxidation of volatile organic compounds. Appl. Catal. B Environ. 2012, 125, 222–246. [Google Scholar] [CrossRef]
- López-Fonseca, R.; Gutiérrez-Ortiz, J.I.; Gutiérrez-Ortiz, M.A.; González-Velasco, J.R. Catalytic oxidation of aliphatic chlorinated volatile organic compounds over Pt/H-BETA zeolite catalyst under dry and humid conditions. Catal. Today 2005, 107, 200–207. [Google Scholar] [CrossRef]
- González-Velasco, J.; Aranzabal, A.; Gutiérrez-Ortiz, J.; López-Fonseca, R.; Gutiérrez-Ortiz, M. Activity and product distribution of alumina supported platinum and palladium catalysts in the gas-phase oxidative decomposition of chlorinated hydrocarbons. Appl. Catal. B Environ. 1998, 19, 189–197. [Google Scholar] [CrossRef]
- Khaleel, A.; Nawaz, M. Enhanced catalytic complete oxidation of 1,2-dichloroethane over mesoporous transition metal-doped γ-Al2O3. J. Environ. Sci. 2015, 29, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Durán, F.G.; Barbero, B.P.; Cadús, L.E.; Rojas, C.; Centeno, M.A.; Odriozola, J.A. Manganese and iron oxides as combustion catalysts of volatile organic compounds. Appl. Catal. B Environ. 2009, 92, 194–201. [Google Scholar] [CrossRef]
- Garcia, T.; Sellick, D.; Varela, F.; Vázquez, I.; Dejoz, A.; Agouram, S.; Taylor, S.H.; Solsona, B. Total oxidation of naphthalene using bulk manganese oxide catalysts. Appl. Catal. A Gen. 2013, 450, 169–177. [Google Scholar] [CrossRef]
- Liotta, L.F.; Wu, H.; Pantaleo, G.; Venezia, A.M. ChemInform Abstract: Co3O4 Nanocrystals and Co3O4—MOx Binary Oxides for CO, CH4 and VOC Oxidation at Low Temperatures: A Review. Cheminform 2014, 45, 3085–3102. [Google Scholar] [CrossRef]
- Solsona, B.; García, T.; Sanchis, R.; Soriano, M.D.; Moreno, M.; Rodríguez-Castellón, E.; Agouram, S.; Dejoz, A.; Nieto, J.M.L. Total oxidation of VOCs on mesoporous iron oxide catalysts: Soft chemistry route versus hard template method. Chem. Eng. J. 2016, 290, 273–281. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Liu, X.; Zhu, T.; Guo, Y.; Hao, Q. Catalytic oxidation of chlorinated benzenes over V2O5/TiO2 catalysts: The effects of chlorine substituents. Catal. Today 2015, 241, 92–99. [Google Scholar] [CrossRef]
- Tseng, T.K.; Wang, L.; Ho, C.T.; Chu, H. The destruction of dichloroethane over a γ-alumina supported manganese oxide catalyst. J. Hazard. Mater. 2010, 178, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Debecker, D.P.; Delaigle, R.; Bouchmella, K.; Eloy, P.; Gaigneaux, E.M.; Mutin, P.H. Total oxidation of benzene and chlorobenzene with MoO3- and WO3-promoted V2O5/TiO2 catalysts prepared by a nonhydrolytic sol-gel route. Catal. Today 2010, 157, 125–130. [Google Scholar] [CrossRef]
- Bertinchamps, F.; Poleunis, C.; Grégoire, C.; Eloy, P.; Bertrand, P.; Gaigneaux, E.M. Elucidation of deactivation or resistance mechanisms of CrOx, VOx and MnOx supported phases in the total oxidation of chlorobenzene via ToF-SIMS and XPS analyses. Surf. Interface Anal. 2010, 40, 231–236. [Google Scholar] [CrossRef]
- Abdullah, A.Z.; Bakar, M.Z.; Bhatia, S. Combustion of chlorinated volatile organic compounds (VOCs) using bimetallic chromium-copper supported on modified H-ZSM-5 catalyst. J. Hazard. Mater. 2006, 129, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Liotta, L.F. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B Environ. 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Brink, R.W.V.D.; Louw, R.; Mulder, P. Formation of polychlorinated benzenes during the catalytic combustion of chlorobenzene using a Pt/γ-Al2O3 catalyst. Appl. Catal. B Environ. 1998, 16, 219–226. [Google Scholar] [CrossRef]
- Huang, H.; Dai, Q.; Wang, X. Morphology effect of Ru/CeO2 catalysts for the catalytic combustion of chlorobenzene. Appl. Catal. B Environ. 2014, 158, 96–105. [Google Scholar] [CrossRef]
- Hetrick, C.E.; Lichtenberger, J.; Amiridis, M.D. Catalytic oxidation of chlorophenol over V2O5/TiO2 catalysts. Appl. Catal. B Environ. 2008, 77, 255–263. [Google Scholar] [CrossRef]
- Bertinchamps, F.; Grégoire, C.; Gaigneaux, E.M. Systematic investigation of supported transition metal oxide based formulations for the catalytic oxidative elimination of (chloro)-aromatics: Part II: Influence of the nature and addition protocol of secondary phases to VOx/TiO2. Appl. Catal. B Environ. 2006, 66, 10–22. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Zeng, J.; Zhu, T. Catalytic oxidation of trichloroethylene over TiO2 supported ruthenium catalysts. Catal. Commun. 2016, 76, 13–18. [Google Scholar] [CrossRef]
- Raróg-Pilecka, W.; Miśkiewicz, E.; Szmigiel, D.; Kowalczyk, Z. Structure sensitivity of ammonia synthesis over promoted ruthenium catalysts supported on graphitised carbon. J. Catal. 2005, 231, 11–19. [Google Scholar] [CrossRef]
- Siporin, S.E.; Davis, R.J. Use of kinetic models to explore the role of base promoters on Ru/MgO ammonia synthesis catalysts. J. Catal. 2004, 225, 359–368. [Google Scholar] [CrossRef]
- Öström, H.; Öberg, H.; Xin, H.; Larue, J.; Beye, M.; Dell’Angela, M.; Gladh, J.; Ng, M.L.; Sellberg, J.A.; Kaya, S. Surface chemistry. Probing the transition state region in catalytic CO oxidation on Ru. Science 2015, 347, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Qadir, K.; Sang, H.J.; Mun, B.S.; Butcher, D.R.; Renzas, J.R.; Aksoy, F.; Zhi, L.; Somorjai, G.A.; Park, J.Y. Intrinsic Relation between Catalytic Activity of CO Oxidation on Ru Nanoparticles and Ru Oxides Uncovered with Ambient Pressure XPS. Nano Lett. 2012, 12, 5761–5768. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Goodman, D.W. CO oxidation over ruthenium: Identification of the catalytically active phases at near-atmospheric pressures. Phys. Chem. Chem. Phys. 2012, 14, 6688–6697. [Google Scholar] [CrossRef] [PubMed]
- Crihan, D.; Knapp, M.; Zweidinger, S.; Lundgren, E.; Weststrate, C.J.; Andersen, J.N.; Seitsonen, A.P.; Over, H. Stable deacon process for HCl oxidation over RuO2. Angew. Chem. Int. Ed. 2008, 47, 2131–2134. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Liu, X.; Zeng, J.; Wang, J.; Wei, Y.; Zhu, T. Gas-solid catalytic reactions over ruthenium-based catalysts. Chin. J. Catal. 2016, 37, 1181–1192. [Google Scholar] [CrossRef]
- Xiang, G.; Shi, X.; Wu, Y.; Zhuang, J.; Wang, X. Size effects in atomic-level epitaxial redistribution process of RuO2 over TiO2. Sci. Rep. 2012, 2, 801. [Google Scholar] [CrossRef] [PubMed]
- Mondelli, C.; Amrute, A.P.; Krumeich, F.; Schmidt, T.; Pérez-Ramírez, J. Shaped RuO2/SnO2-Al2O3 Catalyst for Large-Scale Stable Cl2 Production by HCl Oxidation. Hemcatchem 2011, 3, 657–660. [Google Scholar] [CrossRef]
- López, N.; Gómez-Segura, J.; Marín, R.P.; Pérez-Ramírez, J. Mechanism of HCl oxidation (Deacon process) over RuO2. J. Catal. 2008, 255, 29–39. [Google Scholar] [CrossRef]
- Okal, J.; Zawadzki, M. Catalytic combustion of butane on Ru/γ-Al2O3 catalysts. Appl. Catal. B Environ. 2009, 89, 22–32. [Google Scholar] [CrossRef]
- Kamiuchi, N.; Mitsui, T.; Muroyama, H.; Matsui, T.; Kikuchi, R.; Eguchi, K. Catalytic combustion of ethyl acetate and nano-structural changes of ruthenium catalysts supported on tin oxide. Appl. Catal. B Environ. 2010, 97, 120–126. [Google Scholar] [CrossRef]
- Okal, J.; Zawadzki, M.; Tylus, W. Microstructure characterization and propane oxidation over supported Ru nanoparticles synthesized by the microwave-polyol method. Appl. Catal. B Environ. 2011, 101, 548–559. [Google Scholar] [CrossRef]
- Okal, J.; Zawadzki, M. Combustion of propane over novel zinc aluminate-supported ruthenium catalysts. Appl. Catal. B Environ. 2011, 105, 182–190. [Google Scholar] [CrossRef]
- Debecker, D.P.; Farin, B.; Gaigneaux, E.M.; Sanchez, C.; Sassoye, C. Total oxidation of propane with a nano-RuO2/TiO2 catalyst. Appl. Catal. A Gen. 2014, 481, 11–18. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, J.; Shi, W.; Wang, J.; Zhu, T.; Chen, Y. Catalytic oxidation of benzene over ruthenium–cobalt bimetallic catalysts and study of its mechanism. Catal. Technol. 2016, 7, 213–221. [Google Scholar] [CrossRef]
- Dai, Q.; Bai, S.; Wang, J.; Li, M.; Wang, X.; Lu, G. The effect of TiO2 doping on catalytic performances of Ru/CeO2 catalysts during catalytic combustion of chlorobenzene. Appl. Catal. B Environ. 2013, 142, 222–233. [Google Scholar] [CrossRef]
- Dai, Q.; Bai, S.; Wang, Z.; Wang, X.; Lu, G. Catalytic combustion of chlorobenzene over Ru-doped ceria catalysts. Appl. Catal. B Environ. 2012, 126, 64–75. [Google Scholar] [CrossRef]
- Miranda, B.; Díaz, E.; Ordóñez, S.; Díez, F.V. Catalytic combustion of trichloroethene over Ru/Al2O3: Reaction mechanism and kinetic study. Catal. Commun. 2006, 7, 945–949. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, J.; Wang, J.; Shi, W.; Zhu, T. Catalytic oxidation of methyl bromide using ruthenium-based catalysts. Catal. Sci. Technol. 2016, 6, 4337–4344. [Google Scholar] [CrossRef]
- Dai, Q.; Bai, S.; Wang, X.; Lu, G. Catalytic combustion of chlorobenzene over Ru-doped ceria catalysts: Mechanism study. Appl. Catal. B Environ. 2013, 129, 580–588. [Google Scholar] [CrossRef]
- Sreethawong, T.; Sukjit, D.; Ouraipryvan, P.; Schwank, J.W.; Chavadej, S. Oxidation of Oxygenated Volatile Organic Compound Over Monometallic and Bimetallic Ru-Au Catalysts. Catal. Lett. 2010, 138, 160–170. [Google Scholar] [CrossRef]
- Aouad, S.; Saab, E.; Abi-Aad, E.; Aboukaïs, A. Study of the Ru/Ce system in the oxidation of carbon black and volatile organic compounds. Kinet. Catal. 2007, 48, 835–840. [Google Scholar] [CrossRef]
- Jodaei, A.; Salari, D.; Niaei, A.; Khatamian, M.; Çaylak, N. Preparation of Ag-M (M: Fe, Co and Mn)-ZSM-5 bimetal catalysts with high performance for catalytic oxidation of ethyl acetate. Environ. Technol. 2011, 32, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Barakat, T.; Idakiev, V.; Cousin, R.; Shao, G.S.; Yuan, Z.Y.; Tabakova, T.; Siffert, S. Total oxidation of toluene over noble metal based Ce, Fe and Ni doped titanium oxides. Appl. Catal. B Environ. 2014, 146, 138–146. [Google Scholar] [CrossRef]
- Xie, S.; Deng, J.; Zang, S.; Yang, H.; Guo, G.; Arandiyan, H.; Dai, H. Au-Pd/3DOM Co3O4: Highly active and stable nanocatalysts for toluene oxidation. J. Catal. 2015, 322, 38–48. [Google Scholar] [CrossRef]
- Darif, B.; Ojala, S.; Pirault-Roy, L.; Bensitel, M.; Brahmi, R.; Keiski, R.L. Study on the catalytic oxidation of DMDS over Pt-Cu catalysts supported on Al2O3, AlSi20 and SiO2. Appl. Catal. B Environ. 2016, 181, 24–33. [Google Scholar] [CrossRef]
- Wang, X.; Stöver, J.R.; Zielasek, V.; Altmann, L.; Thiel, K.; Al-Shamery, K.; Bäumer, M.; Borchert, H.; Parisi, J.R.; Kolny-Olesiak, J. Colloidal synthesis and structural control of PtSn bimetallic nanoparticles. Langmuir 2011, 27, 11052–11061. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, K.; Jiang, S.; Li, J. Pd-Co based spinel oxides derived from Pd nanoparticles immobilized on layered double hydroxides for toluene combustion. Appl. Catal. B Environ. 2016, 181, 236–248. [Google Scholar] [CrossRef]


| Catalysts | Ru (at. %) | Ce (at. %) | Ru/Ce | Oads/Olatt |
|---|---|---|---|---|
| 1Ru/TiO2(P25) | 0.5 | - | - | 0.2 |
| 1Ru/TiO2(Anatase) | 0.2 | - | - | 0.1 |
| 1Ru/TiO2(Rutile) | 0.7 | - | - | 0.2 |
| 1Ru-5Ce/TiO2(P25) | 0.5 | 2.6 | 0.2 | 0.2 |
| 1Ru-5Ce/TiO2(Anatase) | 0.3 | 1.9 | 0.1 | 0.2 |
| 1Ru-5Ce/TiO2(Rutile) | 0.7 | 3.4 | 0.3 | 0.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, M.; Chen, L.; Liu, X.; Xu, W.; Zhu, T.; Chen, G. Catalytic Oxidation of Chlorobenzene over Ruthenium-Ceria Bimetallic Catalysts. Catalysts 2018, 8, 116. https://doi.org/10.3390/catal8030116
Ye M, Chen L, Liu X, Xu W, Zhu T, Chen G. Catalytic Oxidation of Chlorobenzene over Ruthenium-Ceria Bimetallic Catalysts. Catalysts. 2018; 8(3):116. https://doi.org/10.3390/catal8030116
Chicago/Turabian StyleYe, Meng, Li Chen, Xiaolong Liu, Wenqing Xu, Tingyu Zhu, and Guanyi Chen. 2018. "Catalytic Oxidation of Chlorobenzene over Ruthenium-Ceria Bimetallic Catalysts" Catalysts 8, no. 3: 116. https://doi.org/10.3390/catal8030116