A Comparative Study of Gold Impregnation Methods for Obtaining Metal/Semiconductor Nanophotocatalysts: Direct Turkevich, Inverse Turkevich, and Progressive Heating Methods
Abstract
:1. Introduction
2. Results and Discussion
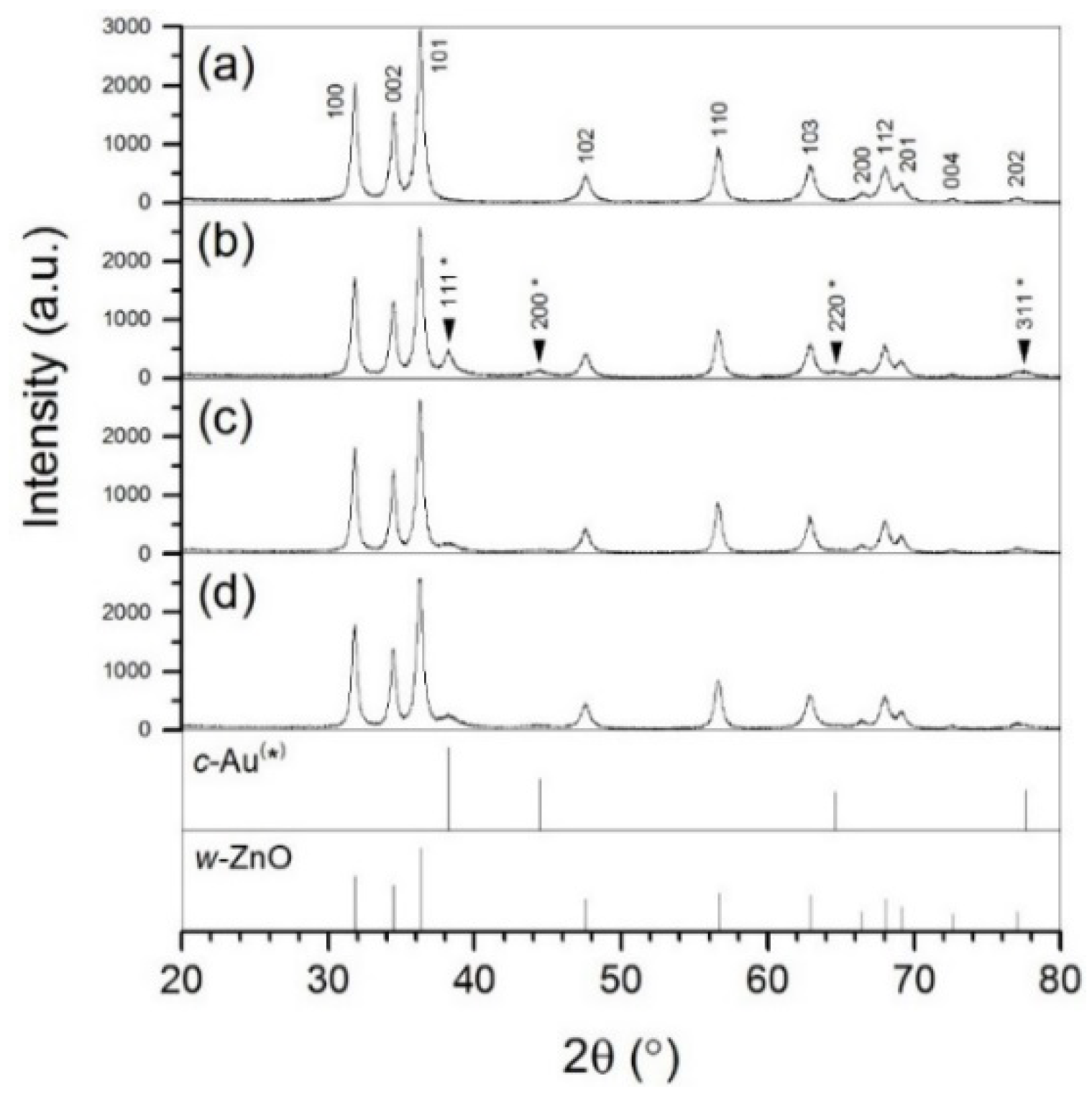
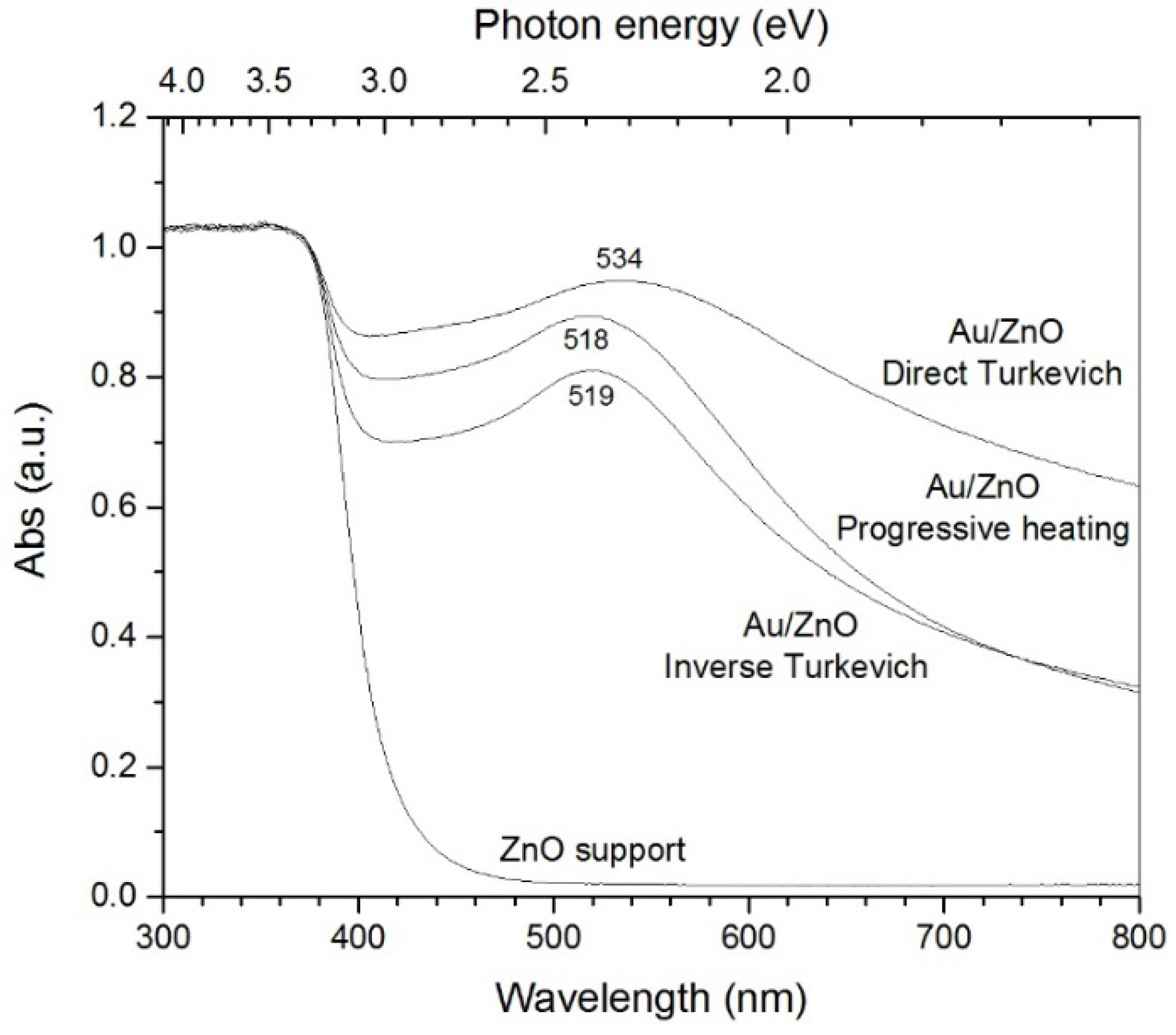
2.1. Microstructure and Optical Properties
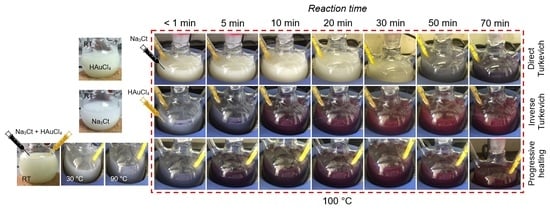
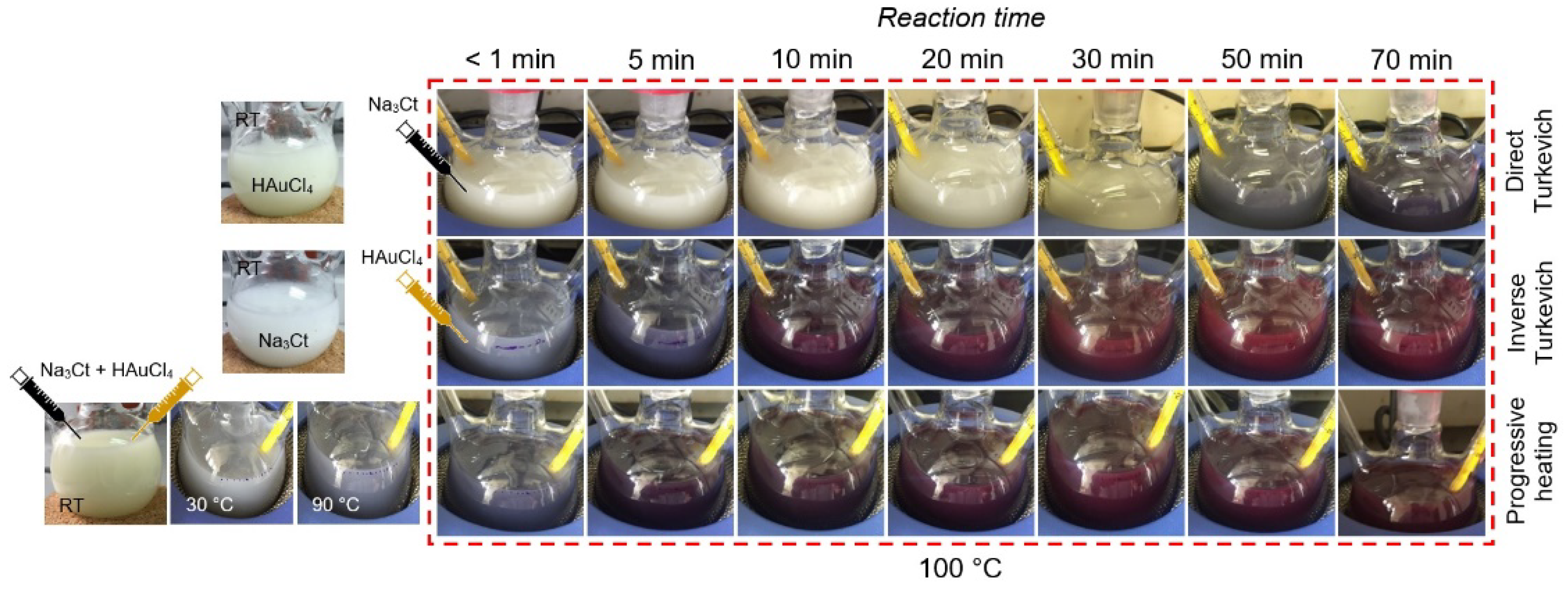
2.2. Reaction and Growth Mechanism of Au-NPs
2.3. Photocatalytic Activity
3. Materials and Methods
3.1. Synthesis of Materials
3.2. Materials Characterization
3.3. Photocatalytic Experiments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Teoh, W.Y.; Scott, J.A.; Amal, R. Progress in Heterogeneous Photocatalysis: From Classical Radical Chemistry to Engineering Nanomaterials and Solar Reactors. J. Phys. Chem. Lett. 2012, 3, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.A.; Dulay, M.T. Heterogeneous photocatalysis. Chem. Rev. 1993, 93, 341–357. [Google Scholar] [CrossRef]
- Mills, A.; Davies, R.H.; Worsley, D. Water purification by semiconductor photocatalysis. Chem. Soc. Rev. 1993, 22, 417. [Google Scholar] [CrossRef]
- Reddy, P.A.K.; Reddy, P.V.L.; Kwon, E.; Kim, K.H.; Akter, T.; Kalagara, S. Recent advances in photocatalytic treatment of pollutants in aqueous media. Environ. Int. 2016, 91, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.G.; Rao, K.S.R.K. Comparison of modification strategies towards enhanced charge carrier separation and photocatalytic degradation activity of metal oxide semiconductors (TiO2, WO3 and ZnO). Appl. Surf. Sci. 2017, 391, 124–148. [Google Scholar] [CrossRef]
- Mondal, C.; Pal, J.; Ganguly, M.; Sinha, A.K.; Jana, J.; Pal, T. A one pot synthesis of Au–ZnO nanocomposites for plasmon-enhanced sunlight driven photocatalytic activity. New J. Chem. 2014, 38, 2999. [Google Scholar] [CrossRef]
- Gu, X.; Li, C.; Yuan, S.; Ma, M.; Qiang, Y.; Zhu, J. ZnO based heterojunctions and their application in environmental photocatalysis. Nanotechnology 2016, 27, 402001. [Google Scholar] [CrossRef] [PubMed]
- Gerischer, H. A mechanism of electron hole pair separation in illuminated semiconductor particles. J. Phys. Chem. 1984, 88, 6096–6097. [Google Scholar] [CrossRef]
- Chen, Z.H.; Tang, Y.B.; Liu, C.P.; Leung, Y.H.; Yuan, G.D.; Chen, L.M.; Wang, Y.Q.; Bello, I.; Zapien, J.A.; Zhang, W.J.; et al. Vertically Aligned ZnO Nanorod Arrays Sentisized with Gold Nanoparticles for Schottky Barrier Photovoltaic Cells. J. Phys. Chem. C 2009, 113, 13433–13437. [Google Scholar] [CrossRef]
- Tung, R.T. The physics and chemistry of the Schottky barrier height. Appl. Phys. Rev. 2014, 1, 11304. [Google Scholar] [CrossRef]
- Tian, Y.; Tatsuma, T. Plasmon-induced photoelectrochemistry at metal nanoparticles supported on nanoporous TiO2. Chem. Commun. (Camb) 2004, 1810–1811. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Cronin, S.B. A Review of Surface Plasmon Resonance-Enhanced Photocatalysis. Adv. Funct. Mater. 2013, 23, 1612–1619. [Google Scholar] [CrossRef]
- Mubeen, S.; Hernandez-Sosa, G.; Moses, D.; Lee, J.; Moskovits, M. Plasmonic Photosensitization of a Wide Band Gap Semiconductor: Converting Plasmons to Charge Carriers. Nano Lett. 2011, 11, 5548–5552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, A.; Cao, S.-W.; Bosman, M.; Li, S.; Xue, C. Direct evidence of plasmon enhancement on photocatalytic hydrogen generation over Au/Pt-decorated TiO 2 nanofibers. Nanoscale 2014, 6, 5217–5222. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Z.; Zhao, W.; Zhao, C.; Yang, J.; Wang, Y. Zinc Oxide nanorod/Au composite arrays and their enhanced photocatalytic properties. J. Colloid Interface Sci. 2014, 432, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Wei, L.; Zhang, Z.; Zhang, X.; Pan, H.; Chen, J. Visible-Light Photocatalytic Application of Hierarchical Au-ZnO Flower-Rod Heterostructures via Surface Plasmon Resonance. Plasmonics 2013, 8, 1193–1202. [Google Scholar] [CrossRef]
- Hu, J.; You, N.; Yu, Z.; Zhou, G.; Xu, X. Two-dimensional ZnO ultrathin nanosheets decorated with Au nanoparticles for effective photocatalysis. J. Appl. Phys. 2016, 120, 74301. [Google Scholar] [CrossRef]
- Wu, S.; Chen, Z.; Wang, T.; Ji, X. A facile approach for the fabrication of Au/ZnO-hollow-sphere-monolayer thin films and their photocatalytic properties. Appl. Surf. Sci. 2017, 412, 69–76. [Google Scholar] [CrossRef]
- Hang, D.-R.; Islam, S.E.; Chen, C.-H.; Sharma, K.H. Full Solution-Processed Synthesis and Mechanisms of a Recyclable and Bifunctional Au/ZnO Plasmonic Platform for Enhanced UV/Vis Photocatalysis and Optical Properties. Chem. A Eur. J. 2016, 22, 14950–14961. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Kreider, P.B.; Chang, C.H.; Park, C.M.; Ahn, H.G. Visible-light-sensitive nanoscale Au-ZnO photocatalysts. J. Nanoparticle Res. 2013, 15. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef] [PubMed]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Subramanian, V.; Wolf, E.E.; Kamat, P.V. Catalysis with TiO2/Gold Nanocomposites. Effect of Metal Particle Size on the Fermi Level Equilibration. J. Am. Chem. Soc. 2004, 126, 4943–4950. [Google Scholar] [CrossRef] [PubMed]
- DeSario, P.A.; Pietron, J.J.; DeVantier, D.E.; Brintlinger, T.H.; Stroud, R.M.; Rolison, D.R. Plasmonic enhancement of visible-light water splitting with Au–TiO2 composite aerogels. Nanoscale 2013, 5, 8073–8083. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Shim, H.S.; Lee, M.; Song, J.K.; Lee, D. Size-controlled electron transfer and photocatalytic activity of ZnO-Au nanoparticle composites. J. Phys. Chem. Lett. 2011, 2, 2840–2845. [Google Scholar] [CrossRef]
- Qian, K.; Sweeny, B.C.; Johnston-Peck, A.C.; Niu, W.; Graham, J.O.; DuChene, J.S.; Qiu, J.; Wang, Y.-C.; Engelhard, M.H.; Su, D.; et al. Surface Plasmon-Driven Water Reduction: Gold Nanoparticle Size Matters. J. Am. Chem. Soc. 2014, 136, 9842–9845. [Google Scholar] [CrossRef] [PubMed]
- Carabineiro, S.A.C.; Machado, B.F.; Bacsa, R.R.; Serp, P.; Dražić, G.; Faria, J.L.; Figueiredo, J.L. Catalytic performance of Au/ZnO nanocatalysts for CO oxidation. J. Catal. 2010, 273, 191–198. [Google Scholar] [CrossRef]
- Ojea-Jiménez, I.; Bastús, N.G.; Puntes, V. Influence of the Sequence of the Reagents Addition in the Citrate-Mediated Synthesis of Gold Nanoparticles. J. Phys. Chem. C 2011, 115, 15752–15757. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Grösse und der inneren Struktur von Kolloidteilchen mittels Röntgensrahlen [Determination of the size and internal structure of colloidal particles using X-rays]. Göttinger Nachrichten Math. Phys. 1918, 2, 98–100. [Google Scholar]
- Escobedo Morales, A.; Sanchez Mora, E.; Pal, U. Use of diffuse reflectance spectroscopy for optical characterization of un-supported nanostructures. Rev. Mex. Fis. 2007, 53S, 18–22. [Google Scholar]
- Chen, P.; Liedberg, B. Curvature of the Localized Surface Plasmon Resonance Peak. Anal. Chem. 2014, 86, 7399–7405. [Google Scholar] [CrossRef] [PubMed]
- Escobedo-Morales, A.; Téllez-Flores, D.; Ruiz Peralta, M.D.L.; Garcia-Serrano, J.; Herrera-González, A.M.; Rubio-Rosas, E.; Sánchez-Mora, E.; Olivares Xometl, O.; De Lourdes, M.; Peralta, R. Green method for producing hierarchically assembled pristine porous ZnO nanoparticles with narrow particle size distribution. Mater. Chem. Phys. 2015, 151, 282–287. [Google Scholar] [CrossRef]
- Ji, X.; Song, X.; Li, J.; Bai, Y.; Yang, W.; Peng, X. Size Control of Gold Nanocrystals in Citrate Reduction: The Third Role of Citrate. J. Am. Chem. Soc. 2007, 129, 13939–13948. [Google Scholar] [CrossRef] [PubMed]
- Granqvist, C.G.; Buhrman, R.A. Size distributions for supported metal catalysts. Coalescence growth versus ostwald ripening. J. Catal. 1976, 42, 477–479. [Google Scholar] [CrossRef]
- Kumar, S.; Gandhi, K.S.; Kumar, R. Modeling of formation of gold nanoparticles by citrate method. Ind. Eng. Chem. Res. 2007, 46, 3128–3136. [Google Scholar] [CrossRef]
- Anumol, E.A.; Kundu, P.; Deshpande, P.A.; Madras, G.; Ravishankar, N. New Insights into Selective Heterogeneous Nucleation of Metal Nanoparticles on Oxides by Microwave-Assisted Reduction: Rapid Synthesis of High-Activity Supported Catalysts. ACS Nano 2011, 5, 8049–8061. [Google Scholar] [CrossRef] [PubMed]
- Thanh, N.T.K.; Maclean, N.; Mahiddine, S. Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, W.; Lian, H.; Liu, Q.; Jiang, D.; Wu, T. Effect of Au loading, H2O and CO concentration on the stability of Au/ZnO catalysts for room-temperature CO oxidation. React. Kinet. Catal. Lett. 2002, 75, 343–351. [Google Scholar] [CrossRef]
- Donkova, B.; Vasileva, P.; Nihtianova, D.; Velichkova, N.; Stefanov, P.; Mehandjiev, D. Synthesis, characterization, and catalytic application of Au/ZnO nanocomposites prepared by coprecipitation. J. Mater. Sci. 2011, 46, 7134–7143. [Google Scholar] [CrossRef]
- She, P.; Xu, K.; Zeng, S.; He, Q.; Sun, H.; Liu, Z. Investigating the size effect of Au nanospheres on the photocatalytic activity of Au-modified ZnO nanorods. J. Colloid Interface Sci. 2017, 499, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wen, W.; Qian, X.-Y.; Liu, J.-B.; Wu, J.-M. UV and visible light photocatalytic activity of Au/TiO2 nanoforests with Anatase/Rutile phase junctions and controlled Au locations. Sci. Rep. 2017, 7, 41253. [Google Scholar] [CrossRef] [PubMed]
- Narang, P.; Sundararaman, R.; Atwater, H.A. Plasmonic hot carrier dynamics in solid-state and chemical systems for energy conversion. Nanophotonics 2016, 5, 96–111. [Google Scholar] [CrossRef]
- Christopher, P.; Moskovits, M. Hot Charge Carrier Transmission from Plasmonic Nanostructures. Annu. Rev. Phys. Chem. 2017, 68, 379–398. [Google Scholar] [CrossRef] [PubMed]
- Brillson, L.J.; Lu, Y. ZnO Schottky barriers and Ohmic contacts. J. Appl. Phys. 2011, 109, 121301. [Google Scholar] [CrossRef]
- Rochkind, M.; Pasternak, S.; Paz, Y. Using dyes for evaluating photocatalytic properties: A critical review. Molecules 2015, 20, 88–110. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Quantum yields of active oxidative species formed on TiO2 photocatalyst. J. Photochem. Photobiol. A Chem. 2000, 134, 139–142. [Google Scholar] [CrossRef]
- Patil, S.S.; Mali, M.G.; Tamboli, M.S.; Patil, D.R.; Kulkarni, M.V.; Yoon, H.; Kim, H.; Al-Deyab, S.S.; Yoon, S.S.; Kolekar, S.S.; et al. Green approach for hierarchical nanostructured Ag-ZnO and their photocatalytic performance under sunlight. Catal. Today 2016, 260, 126–134. [Google Scholar] [CrossRef]
- Sarkar, S.; Makhal, A.; Bora, T.; Baruah, S.; Dutta, J.; Pal, S.K. Photoselective excited state dynamics in ZnO–Au nanocomposites and their implications in photocatalysis and dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2011, 13, 12488–12496. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.X.; Liu, L.H. Analysis of terrestrial solar radiation exergy. Sol. Energy 2009, 83, 1390–1404. [Google Scholar] [CrossRef]


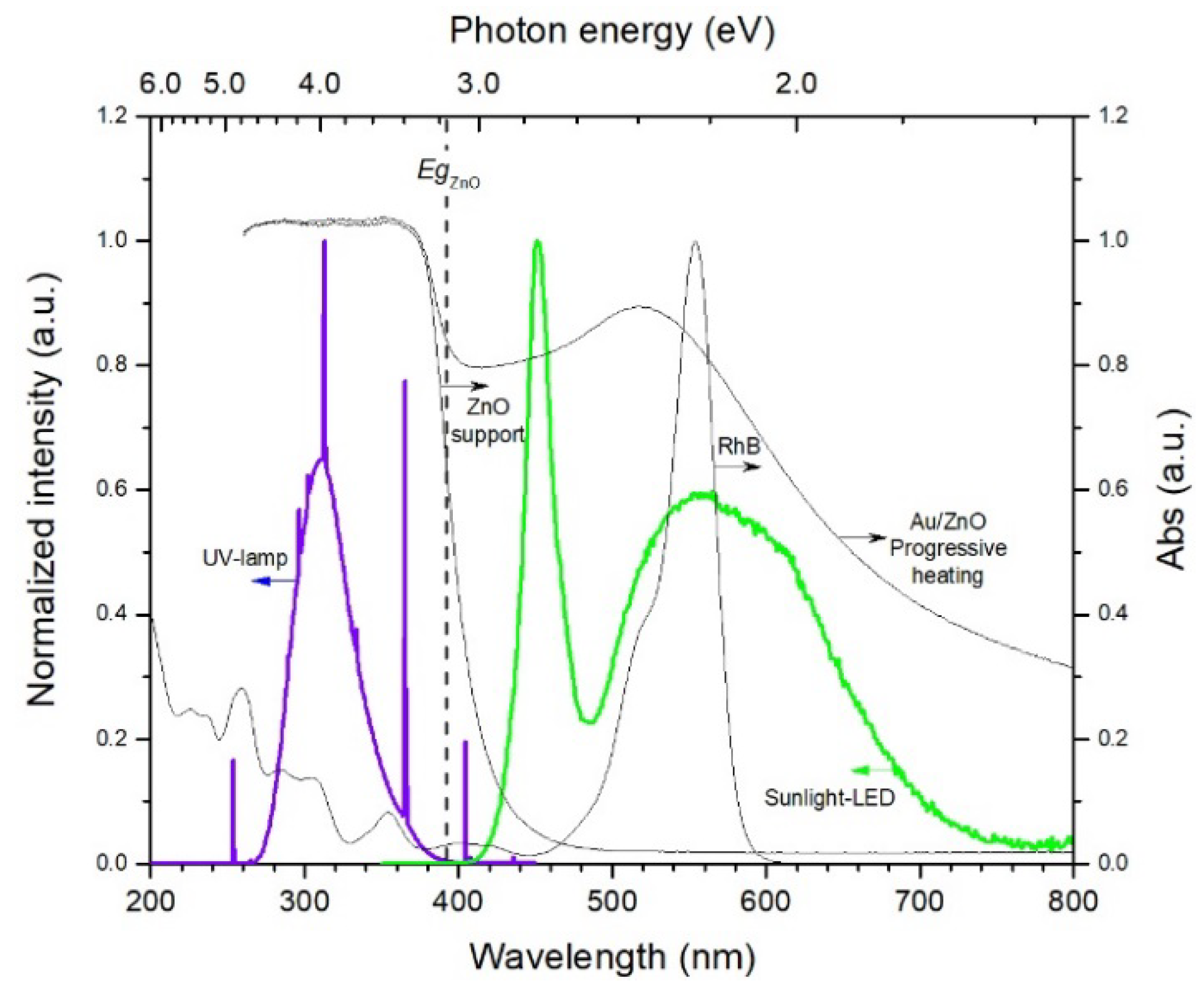
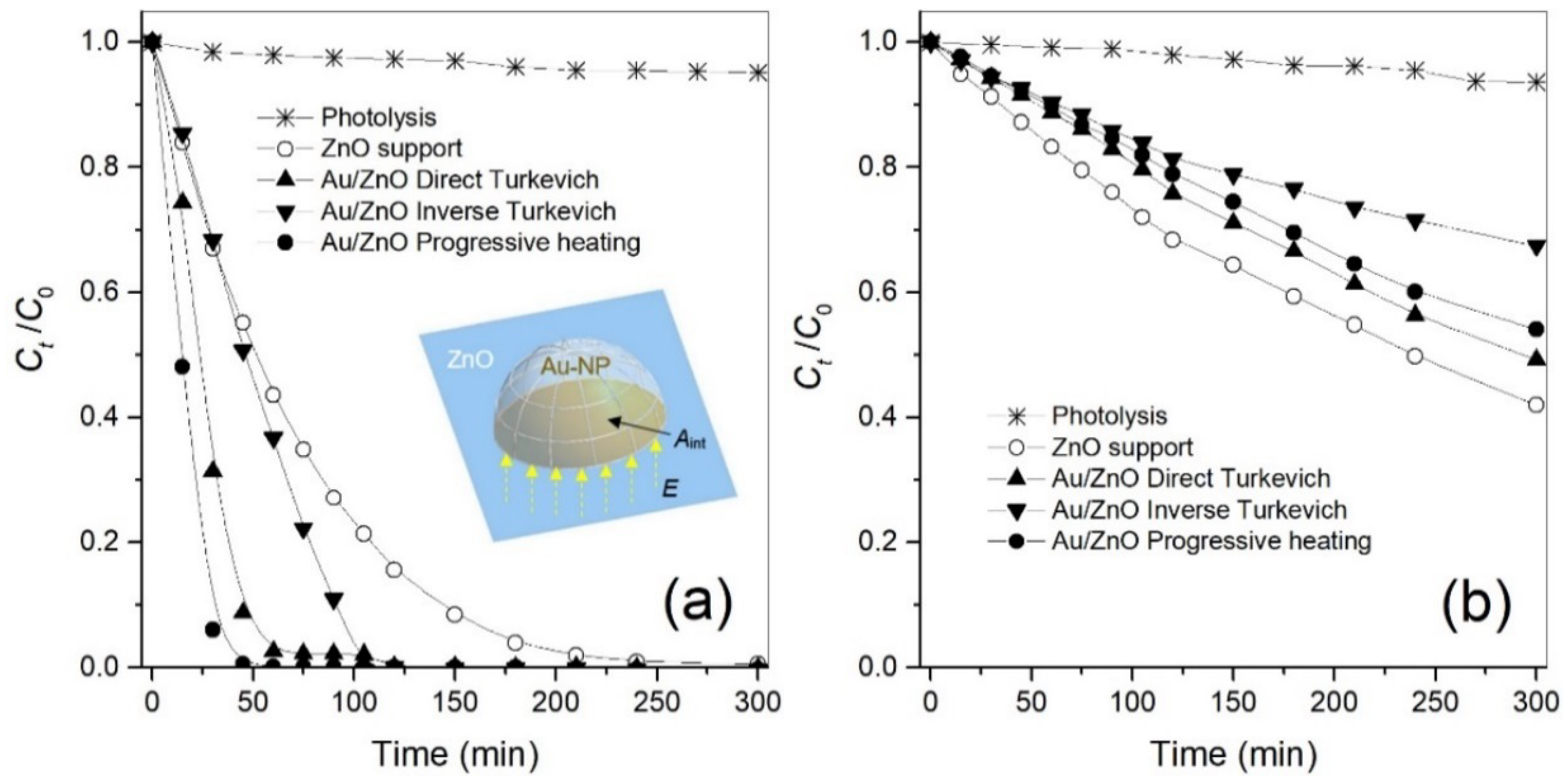
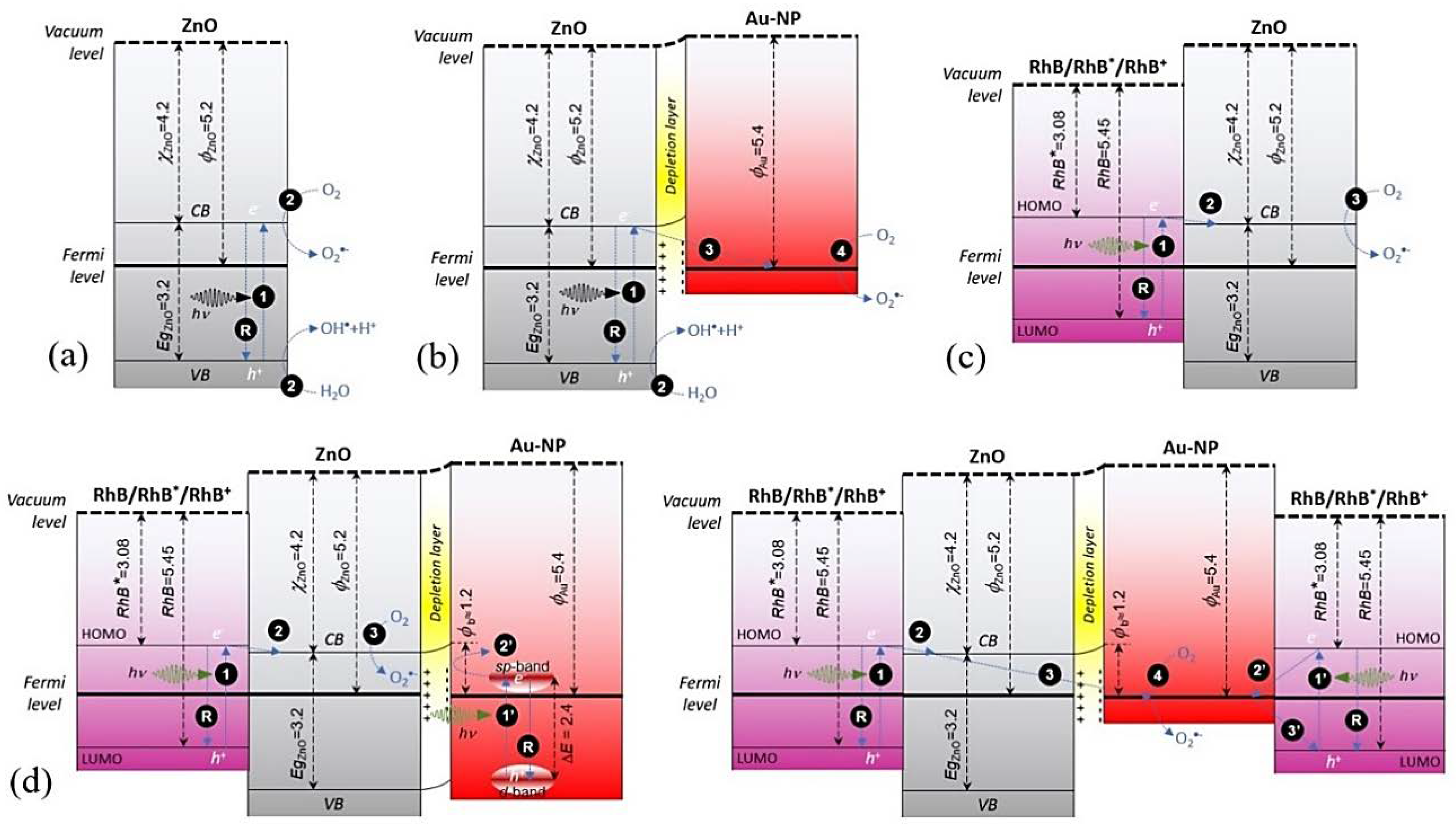
| ZnO Support | Au-NPs/ZnO Direct Turkevich | Au-NPs/ZnO Inverse Turkevich | Au-NPs/ZnO Progressive Heating | ||
|---|---|---|---|---|---|
| (I111)Au/(I101)ZnO (a.u.) | 0.00 | 0.19 | 0.07 | 0.10 | |
| Gold loading (Au wt.%) | --- | 5.3 | 1.9 | 3.8 | |
| τZnO:τAu (XRD) (nm:nm) | 17:--- | 18:12 | 18:9 | 18:7 | |
| Dm-Au-NPs (TEM) (nm) | --- | 11.7 ± 2.4 | 10.8 ± 1.6 | 9.7 ± 1.8 | |
| SPR-λmax (nm) | ---:--- | 534 | 519 | 518 | |
| SBET (m2 g−1) | 19.9 | 21.3 | 24.6 | 26.7 | |
| UV | η (t = 60 min):k (%: × 10−3 min−1) | 56.4:18.8 | 97.4:101.1 | 63.3:23.7 | 99.9:115.9 |
| Vis | η (t = 60 min):k (%: × 10−3 min−1) | 16.7:2.9 | 11.3:2.4 | 9.7:1.3 | 10.3:2.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matamoros-Ambrocio, M.; Ruiz-Peralta, M.D.L.; Chigo-Anota, E.; García-Serrano, J.; Pérez-Centeno, A.; Sánchez-Cantú, M.; Rubio-Rosas, E.; Escobedo-Morales, A. A Comparative Study of Gold Impregnation Methods for Obtaining Metal/Semiconductor Nanophotocatalysts: Direct Turkevich, Inverse Turkevich, and Progressive Heating Methods. Catalysts 2018, 8, 161. https://doi.org/10.3390/catal8040161
Matamoros-Ambrocio M, Ruiz-Peralta MDL, Chigo-Anota E, García-Serrano J, Pérez-Centeno A, Sánchez-Cantú M, Rubio-Rosas E, Escobedo-Morales A. A Comparative Study of Gold Impregnation Methods for Obtaining Metal/Semiconductor Nanophotocatalysts: Direct Turkevich, Inverse Turkevich, and Progressive Heating Methods. Catalysts. 2018; 8(4):161. https://doi.org/10.3390/catal8040161
Chicago/Turabian StyleMatamoros-Ambrocio, Mayra, María De Lourdes Ruiz-Peralta, Ernesto Chigo-Anota, Jesús García-Serrano, Armando Pérez-Centeno, Manuel Sánchez-Cantú, Efraín Rubio-Rosas, and Alejandro Escobedo-Morales. 2018. "A Comparative Study of Gold Impregnation Methods for Obtaining Metal/Semiconductor Nanophotocatalysts: Direct Turkevich, Inverse Turkevich, and Progressive Heating Methods" Catalysts 8, no. 4: 161. https://doi.org/10.3390/catal8040161
APA StyleMatamoros-Ambrocio, M., Ruiz-Peralta, M. D. L., Chigo-Anota, E., García-Serrano, J., Pérez-Centeno, A., Sánchez-Cantú, M., Rubio-Rosas, E., & Escobedo-Morales, A. (2018). A Comparative Study of Gold Impregnation Methods for Obtaining Metal/Semiconductor Nanophotocatalysts: Direct Turkevich, Inverse Turkevich, and Progressive Heating Methods. Catalysts, 8(4), 161. https://doi.org/10.3390/catal8040161