Intrinsic Catalytic Activity of Gold/Multi-Walled Carbon Nanotubes Composites in Squaric Acid-Iron(II/III) System
Abstract
:1. Introduction
2. Results and Discussion
2.1. Principle of Catalytic Activity by SQA-Iron(II/III) Chelate
2.2. Characteristics of Au/MWCNTs Composites
2.3. Optimized Synthesis of Au/MWCNTs Composites by Orthogonal Design
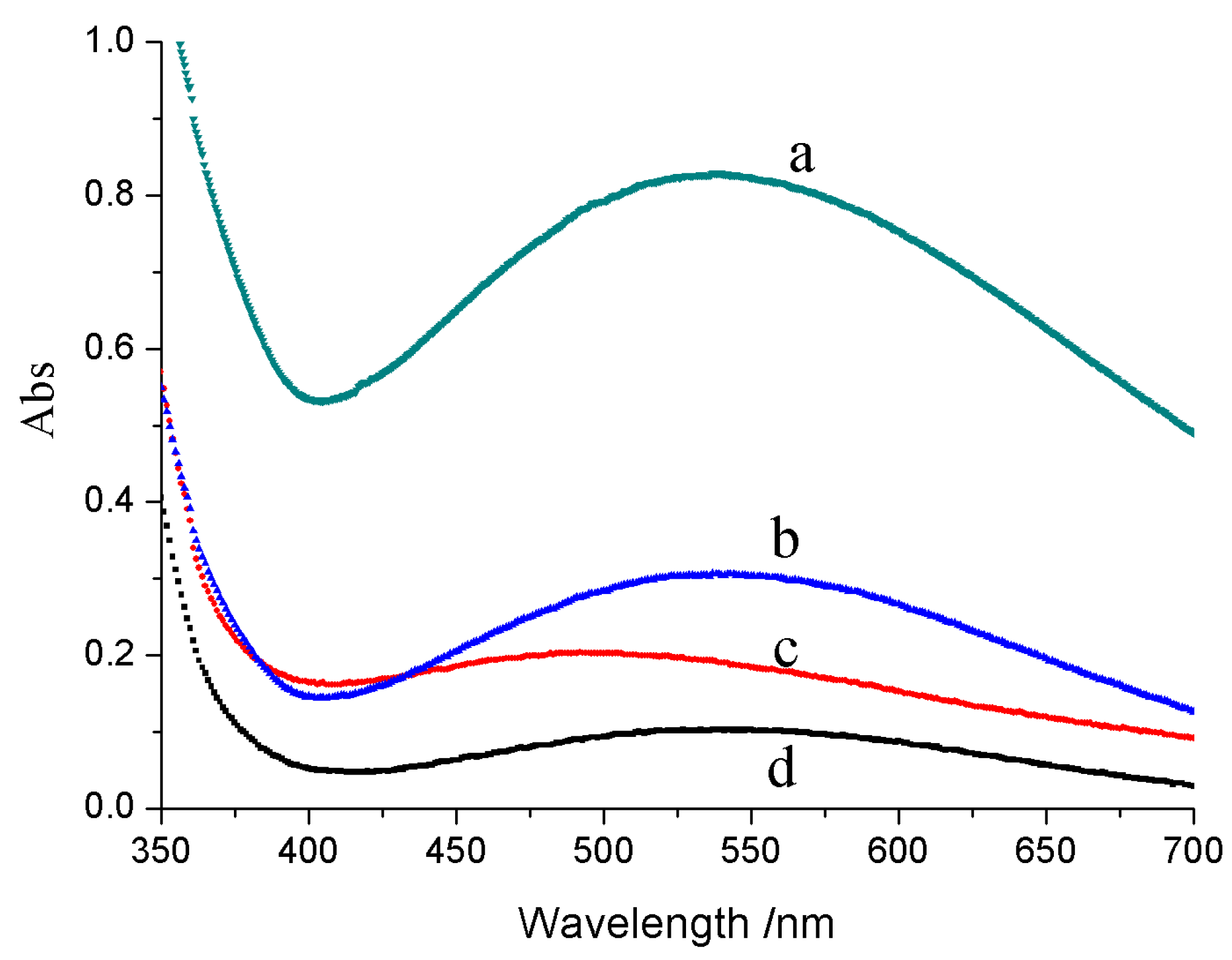
2.4. Performance of Catalytic Activity
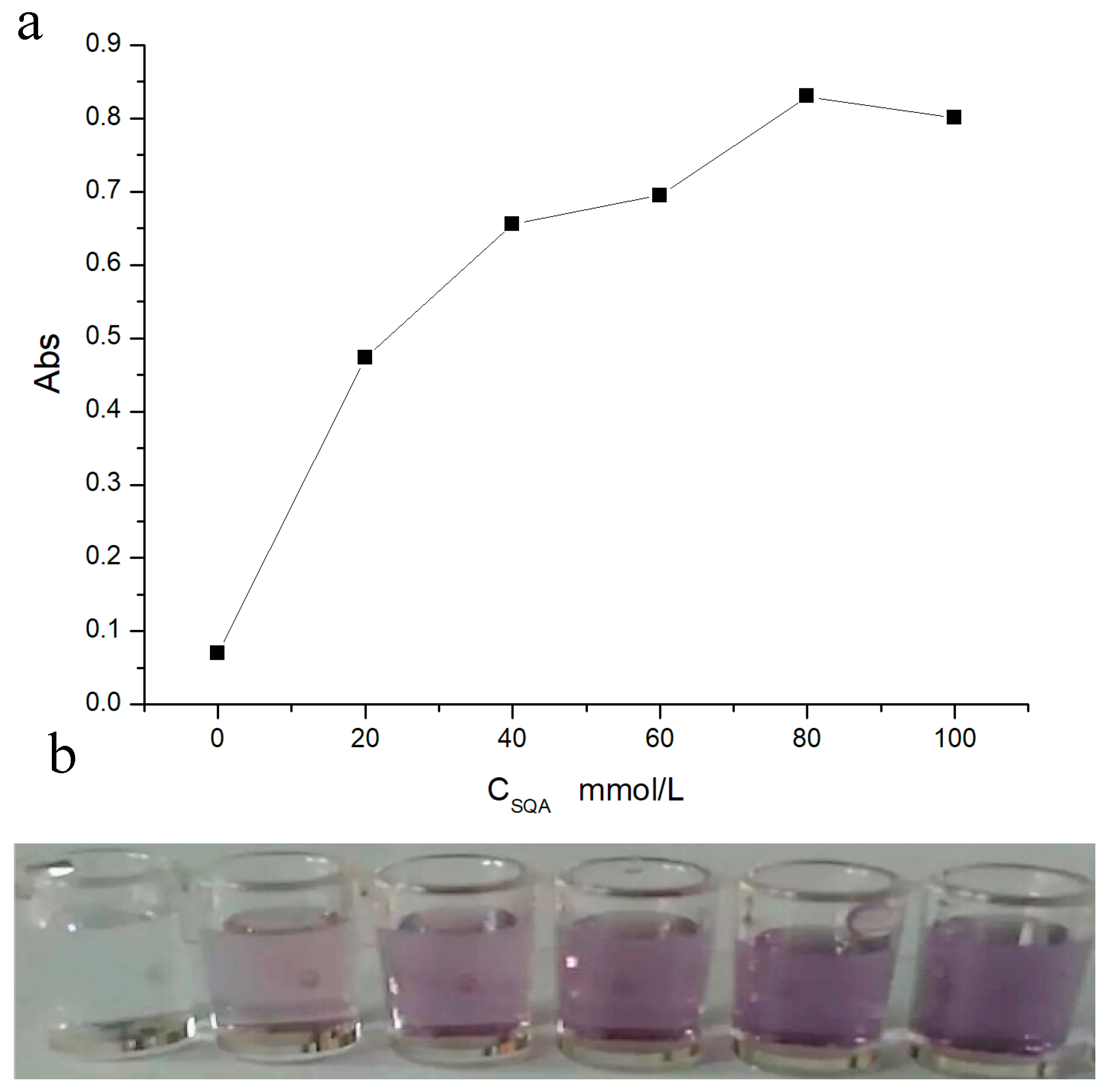
2.5. Optimization of SQA Concentration
2.6. The Conversion Rate of Iron(II)
2.7. Effects of pH in the Catalytic Process
2.8. Kinetics of Catalytic Reaction and Stability of Chelating Product
2.9. Catalytic Activity Compared with H2O2
3. Materials and Methods
3.1. Materials
3.2. Apparatus
3.3. Preparation of Au/MWCNTs Composites
3.4. Catalytic Activity Assay
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Li, J.; Cai, J.; Jia, H.; Zhang, L.; Lei, Y.; He, W. Formation of iron oxide/Pd hybrid nanostructures with enhanced peroxidase-like activity and catalytic reduction of 4-nitrophenol. J. Environ. Sci. Health C 2017, 35, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Li, Q.; Zhou, Z.; Ma, C.; Song, Y.; Xu, F.; Wang, L. A sensitive fluorescent assay for thiamine based on metal-organic frameworks with intrinsic peroxidase-like activity. Anal. Chim. Acta 2015, 856, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.R.; Takemeura, K.; Li, T.C.; Kitamoto, N.; Tanaka, T.; Suzuki, T.; Park, E.Y. Size-controlled preparation of peroxidase-like graphene-gold nanoparticle hybrids for the visible detection of norovirus-like particles. Biosensors Bioelectron. 2017, 87, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Yang, X.; Yang, Z.; Zhu, G.; Mao, H.; Wang, K. Multiwalled carbon nanotube@reduced graphene oxide nanoribbon heterostructure: Synthesis, intrinsic peroxidase-like catalytic activity, and its application in colorimetric biosensing. J. Mater. Chem. B 2015, 3, 1624–1632. [Google Scholar] [CrossRef]
- Hsu, C.W.; Lin, Z.Y.; Chan, T.Y.; Chiu, T.C.; Hu, C.C. Oxidized multiwalled carbon nanotubes decorated with silver nanoparticles for fluorometric detection of dimethoate. Food Chem. 2017, 224, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Pan, X.; Guo, S.; Ren, P.; Bao, X. Towards fundamentals of confined catalysis in carbon nanotubes. J. Am. Chem. Soc. 2015, 137, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Melchionna, M.; Marchesan, S.; Prato, M.; Fornasiero, P. Cheminform abstract: Carbon nanotubes and catalysis: The many facets of a successful marriage. Catal. Sci. Technol. 2015, 5, 3859–3875. [Google Scholar] [CrossRef] [Green Version]
- Cui, R.; Han, Z.; Zhu, J.J. Helical carbon nanotubes: Intrinsic peroxidase catalytic activity and its application for biocatalysis and biosensing. Chemistry 2011, 17, 9377–9384. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Li, C.; Zhang, X.; Hua, W.; Yang, D.; Hu, J.; Yue, Y.; Gao, Z. Poly(styrene sulfonic acid)–grafted carbon nanotube as a stable protonic acid catalyst. Catal. Commun. 2010, 12, 217–221. [Google Scholar] [CrossRef]
- Nahorny, S.; Zanin, H.; Christino, V.A.; Marciano, F.R.; Lobo, A.O.; Soares, L.E.S. Multi-walled carbon nanotubes/graphene oxide hybrid and nanohydroxyapatite composite: A novel coating to prevent dentin erosion. Mat. Sci. Eng. C 2017, 79, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Miao, L.; Wang, H.; Fu, Q. Highly dispersed Fe2O3 on carbon nanotubes for low-temperature selective catalytic reduction of NO with NH3. Chem. Commun. 2015, 51, 956–958. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Wang, H.; Xiang, X.; Li, F. Co–Al mixed metal oxides/carbon nanotubes nanocomposite prepared via a precursor route and enhanced catalytic property. J. Solid State Chem. 2013, 197, 14–22. [Google Scholar] [CrossRef]
- Shen, Y.; Lua, A.C. Synthesis of Ni and Ni–Cu supported on carbon nanotubes for hydrogen and carbon production by catalytic decomposition of methane. Appl. Catal. B Environ. 2015, 164, 61–69. [Google Scholar] [CrossRef]
- Aslan, E.; Akin, I.; Patir, I.H. Enhanced hydrogen evolution catalysis based on Cu nanoparticles deposited on carbon nanotubes at the liquid/liquid interface. Chemcatchem 2016, 8, 719–723. [Google Scholar] [CrossRef]
- Huff, C.; Dushatinski, T.; Abdel-Fattah, T.M. Gold nanoparticle/multi-walled carbon nanotube composite as novel catalyst for hydrogen evolution reactions. Int. J. Hydrog. Energy 2017, 42, 18985–18990. [Google Scholar] [CrossRef]
- Alves, L.; Ballesteros, B.; Boronat, M.; Cabrero-Antonino, J.R.; Concepción, P.; Corma, A.; Correa-Duarte, M.A. Synthesis and stabilization of subnanometric gold oxide nanoparticles on multiwalled carbon nanotubes and their catalytic activity. J. Am. Chem. Soc. 2011, 133, 10251–10261. [Google Scholar] [CrossRef] [PubMed]
- Cegłowski, M.; Pełech, I.; Schroeder, G. Functionalization of gold-coated carbon nanotubes with self-assembled monolayers of thiolates. J. Mater. Sci. 2012, 47, 3463–3467. [Google Scholar] [CrossRef]
- Alexeyeva, N.; Matisen, L.; Saar, A.; Laaksonen, P.; Kontturi, K.; Tammeveski, K. Kinetics of oxygen reduction on gold nanoparticle/multi-walled carbon nanotube hybrid electrodes in acid media. J. Electroanal. Chem. 2010, 642, 6–12. [Google Scholar] [CrossRef]
- Madhumati, R.; Mitali, P.; Rigved, E.; Yeoheung, Y.; Vasselin, S.; Mark, S.; William, R.H.; Moni, K.D.; Prashant, N.K. Gold-coated carbon nanotube electrode arrays: Immunosensors for impedimetric detection of bone biomarkers. Biosens. Bioelectron. 2016, 77, 580–588. [Google Scholar]
- Guo, W.; Zhang, A.; Zhang, X.; Huang, C.; Yang, D.; Jia, N. Multiwalled carbon nanotubes/gold nanocomposites-based electrochemiluminescent sensor for sensitive determination of bisphenol A. Anal. Bioanal. Chem. 2016, 408, 7173–7180. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.R.; Kim, J.; Suzuki, T.; Lee, J.; Park, E.Y. Enhanced catalytic activity of gold nanoparticle-carbon nanotube hybrids for influenza virus detection. Biosensors Bioelectron. 2016, 85, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fang, A.; He, L.; Zhang, Y.; Yao, S. Sensitive fluorescent detection of H2O2 and glucose in human serum based on inner filter effect of squaric acid-iron(III) on the fluorescence of upconversion nanoparticle. Talanta 2017, 164, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Kiskin, M.A.; Shvedenkov, Y.G.; Ikorskii, V.N.; Romanenko, G.V.; Ovcharenko, V.I. Molecular magnet based on the Fe II, III complex with squaric acid. Russ. Chem. Bull. 2003, 52, 265–266. [Google Scholar] [CrossRef]
- Sisley, M.J.; Jordan, R.B. Kinetic and equilibrium studies of complexes of aqueous iron (III) and squaric acid. Inorg. Chem. 2002, 30, 2190–2195. [Google Scholar] [CrossRef]
- Lai, W.; Tang, D.; Zhuang, J.; Chen, G.; Yang, H. Magnetic bead-based enzyme-chromogenic substrate system for ultrasensitive colorimetric immunoassay accompanying cascade reaction for enzymatic formation of squaric acid-iron (III) chelate. Anal. Chem. 2014, 86, 5061–5068. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y.T.; Huang, L.; Wang, X. Spectral analysis for trace glucose measurement based on the chelating system of Fe3+-squaric acid. Chin. J. Anal. Lab. 2017, 9, 1019–1022. [Google Scholar]
- Sugimori, H.; Kanzaki, Y.; Yokota, K.; Murakami, T. Nonlinear dependence of the oxidation rate of Fe (II) on dissolved oxygen under low-O2 conditions in aqueous solutions. J. Miner. Petrol. Sci. 2011, 106, 142–152. [Google Scholar] [CrossRef]
- Das, T.; Ghosh, P.; Shanavas, M.S.; Maity, A.; Mondal, S.; Purkayastha, P. Protein-templated gold nanoclusters: Size dependent inversion of fluorescence emission in the presence of molecular oxygen. Nanoscale 2012, 4, 6018–6024. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, Z.; Liu, W.; Zhang, H.; Zuo, W.; Tang, H.; Chen, F.; Wang, B. Size- and shape-dependent peroxidase-like catalytic activity of MnFe2O4 nanoparticles and their applications in highly efficient colorimetric detection of target cancer cells. Dalton Trans. 2015, 44, 12871–12877. [Google Scholar] [CrossRef] [PubMed]
- Herzing, A.A.; Kiely, C.J.; Carley, A.F.; Landon, P.; Hutchings, G.J. Identification of active gold nanoclusters on iron oxide supports for CO oxidation. Science 2008, 321, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Su, Y.; Lv, X.; Xia, H.; Wang, Y. Electrochemical acetylene sensor based on Au/MWCNTs. Sensors Actuators B Chem. 2010, 149, 427–431. [Google Scholar] [CrossRef]
- Matsubayashi, M.; Matsubayashi, M.; Morita, M. Decoloration reaction of peroxidase-H2O2 system to the tolerance for hydrogen peroxide. Fiber 2015, 71, 250–256. [Google Scholar] [CrossRef]
- Yang, L.; Liu, X.; Lu, Q.; Huang, N.; Liu, M.; Zhang, Y.; Yao, S. Catalytic and peroxidase-like activity of carbon based-AuPd bimetallic nanocomposite produced using carbon dots as the reductant. Anal. Chim. Acta 2016, 930, 23–30. [Google Scholar] [CrossRef] [PubMed]








| Levels | Ultrasonic Time (min) | Sodium Formate Concentration (mmol/L) | Reaction Time (h) |
|---|---|---|---|
| 1 | 5 | 200 | 1 |
| 2 | 30 | 400 | 2 |
| 3 | 60 | 600 | 4 |
| No. | Ultrasonic Time (min) | Sodium Formate Concentration (mmol/L) | Reaction Time (h) | Absorbance |
|---|---|---|---|---|
| 1 | 5 | 200 | 1 | 0.386 ± 0.017 |
| 2 | 30 | 200 | 2 | 0.464 ± 0.033 |
| 3 | 60 | 200 | 4 | 0.438 ± 0.028 |
| 4 | 5 | 400 | 2 | 0.358 ± 0.019 |
| 5 | 30 | 400 | 4 | 0.574 ± 0.024 |
| 6 | 60 | 400 | 1 | 0.509 ± 0.030 |
| 7 | 5 | 600 | 4 | 0.439 ± 0.021 |
| 8 | 30 | 600 | 1 | 0.433 ± 0.032 |
| 9 | 60 | 600 | 2 | 0.464 ± 0.020 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Jiang, C.; Lin, S.; Lai, J.; Zheng, L.; Xu, P. Intrinsic Catalytic Activity of Gold/Multi-Walled Carbon Nanotubes Composites in Squaric Acid-Iron(II/III) System. Catalysts 2018, 8, 187. https://doi.org/10.3390/catal8050187
Li Y, Jiang C, Lin S, Lai J, Zheng L, Xu P. Intrinsic Catalytic Activity of Gold/Multi-Walled Carbon Nanotubes Composites in Squaric Acid-Iron(II/III) System. Catalysts. 2018; 8(5):187. https://doi.org/10.3390/catal8050187
Chicago/Turabian StyleLi, Yanxia, Cheng Jiang, Shengheng Lin, Jiajia Lai, Liyun Zheng, and Pengjun Xu. 2018. "Intrinsic Catalytic Activity of Gold/Multi-Walled Carbon Nanotubes Composites in Squaric Acid-Iron(II/III) System" Catalysts 8, no. 5: 187. https://doi.org/10.3390/catal8050187





