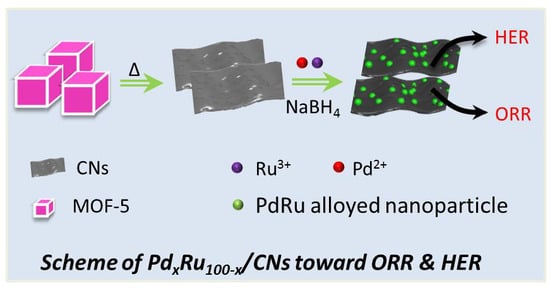
Oxygen Reduction Reaction and Hydrogen Evolution Reaction Catalyzed by Pd–Ru Nanoparticles Encapsulated in Porous Carbon Nanosheets
Abstract
:1. Introduction
2. Results and Discussions
2.1. Morphological Characterization
2.2. X-ray Diffraction (XRD) and XPS Analysis
2.3. ORR Performance
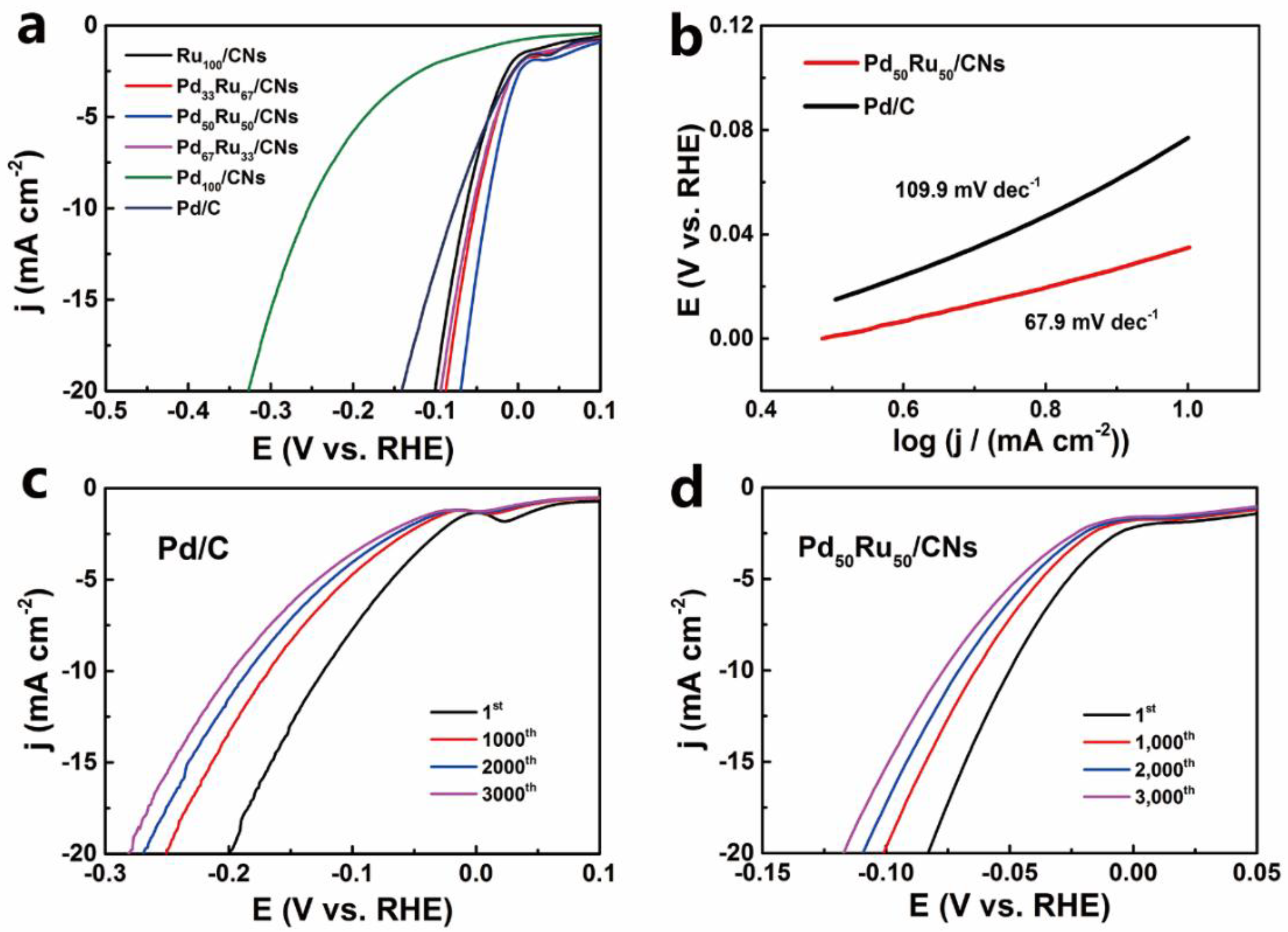
2.4. HER Performance
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Porous Carbon Nanosheets (CNs)
3.3. Preparation of PdxRu100−x/CNs
3.4. Morphological Characterizations
3.5. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.-W.; Li, W.-W.; Yu, H.-Q. Cathodic catalysts in bioelectrochemical systems for energy recovery from wastewater. Chem. Soc. Rev. 2014, 43, 7718–7745. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kraft, M.; Xu, R. Metal-free carbonaceous electrocatalysts and photocatalysts for water splitting. Chem. Soc. Rev. 2016, 45, 3039–3052. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gong, M.; Liang, Y.; Feng, J.; Kim, J.-E.; Wang, H.; Hong, G.; Zhang, B.; Dai, H. Advanced zinc-air batteries based on high-performance hybrid electrocatalysts. Nat. Commun. 2013, 4, 1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Zhang, R.; Chen, W. Graphene-Supported Nanoelectrocatalysts for Fuel Cells: Synthesis, Properties, and Applications. Chem. Rev. 2014, 114, 5117–5160. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wu, W.; Wang, K. Oxygen Reduction Reaction Catalyzed by Noble Metal Clusters. Catalysts 2018, 8, 65. [Google Scholar] [CrossRef]
- Wang, J.; Xu, F.; Jin, H.; Chen, Y.; Wang, Y. Non-Noble Metal-based Carbon Composites in Hydrogen Evolution Reaction: Fundamentals to Applications. Adv. Mater. 2017, 29, 1605838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Tang, Z.; Yan, W.; Wang, Q.; Yang, H.; Chen, S. Co@Pt Core@Shell nanoparticles encapsulated in porous carbon derived from zeolitic imidazolate framework 67 for oxygen electroreduction in alkaline media. J. Power Sources 2017, 343, 458–466. [Google Scholar] [CrossRef]
- Shao, M.; Chang, Q.; Dodelet, J.-P.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Chen, L.; Sun, L. Recent progress in electrochemical hydrogen production with earth-abundant metal complexes as catalysts. Energy Environ. Sci. 2012, 5, 6763–6778. [Google Scholar] [CrossRef]
- Antolini, E. Palladium in fuel cell catalysis. Energy Environ. Sci. 2009, 2, 915–931. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Xu, Q. Recent progress in synergistic catalysis over heterometallic nanoparticles. J. Mater. Chem. 2011, 21, 13705–13725. [Google Scholar] [CrossRef]
- Gilroy, K.D.; Ruditskiy, A.; Peng, H.-C.; Qin, D.; Xia, Y. Bimetallic Nanocrystals: Syntheses, Properties, and Applications. Chem. Rev. 2016, 116, 10414–10472. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Goodman, D.W. Pd-Au bimetallic catalysts: Understanding alloy effects from planar models and (supported) nanoparticles. Chem. Soc. Rev. 2012, 41, 8009–8020. [Google Scholar] [CrossRef] [PubMed]
- Tao, F. Synthesis, catalysis, surface chemistry and structure of bimetallic nanocatalysts. Chem. Soc. Rev. 2012, 41, 7977–7979. [Google Scholar] [CrossRef] [PubMed]
- He, L.-L.; Song, P.; Wang, A.-J.; Zheng, J.-N.; Mei, L.-P.; Feng, J.-J. A general strategy for the facile synthesis of AuM (M = Pt/Pd) alloyed flowerlike-assembly nanochains for enhanced oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 5352–5359. [Google Scholar] [CrossRef]
- Yan, W.; Tang, Z.; Wang, L.; Wang, Q.; Yang, H.; Chen, S. PdAu alloyed clusters supported by carbon nanosheets as efficient electrocatalysts for oxygen reduction. Int. J. Hydrogen Energy 2017, 42, 218–227. [Google Scholar] [CrossRef]
- Li, S.-S.; Wang, A.-J.; Hu, Y.-Y.; Fang, K.-M.; Chen, J.-R.; Feng, J.-J. One-step, seedless wet-chemical synthesis of gold@palladium nanoflowers supported on reduced graphene oxide with enhanced electrocatalytic properties. J. Mater. Chem. A 2014, 2, 18177–18183. [Google Scholar] [CrossRef]
- Chen, C.-W.; Hsieh, Y.-S.; Syu, C.-C.; Chen, H.-R.; Lee, C.-L. Displacement preparation-induced effects on structure of Ag–Pd nanobrushes for catalyzing oxygen reduction. J. Alloy. Compd. 2013, 580, S359–S363. [Google Scholar] [CrossRef]
- Sekol, R.C.; Li, X.; Cohen, P.; Doubek, G.; Carmo, M.; Taylor, A.D. Silver palladium core–shell electrocatalyst supported on MWNTs for ORR in alkaline media. Appl. Catal. B: Environ. 2013, 138–139, 285–293. [Google Scholar] [CrossRef]
- Xu, L.; Luo, Z.; Fan, Z.; Zhang, X.; Tan, C.; Li, H.; Zhang, H.; Xue, C. Triangular Ag-Pd alloy nanoprisms: Rational synthesis with high-efficiency for electrocatalytic oxygen reduction. Nanoscale 2014, 6, 11738–11743. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wu, J.; Zhang, H.; Jiang, Y.; Jin, C.; Fu, M.; Yang, H.; Yang, D. Facile synthesis of Rh-Pd alloy nanodendrites as highly active and durable electrocatalysts for oxygen reduction reaction. Nanoscale 2014, 6, 7012–7018. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Prakash, J. Oxygen reduction reaction on carbon supported Palladium–Nickel alloys in alkaline media. Electrochem. Commun. 2009, 11, 1162–1165. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Q.; Li, Y.; Han, M.; Gu, L.; Nan, C.; Bao, J.; Dai, Z. Five-Fold Twinned Pd2NiAg Nanocrystals with Increased Surface Ni Site Availability to Improve Oxygen Reduction Activity. J. Am. Chem. Soc. 2015, 137, 2820–2823. [Google Scholar] [CrossRef] [PubMed]
- Yun, M.; Ahmed, M.S.; Jeon, S. Thiolated graphene oxide-supported palladium cobalt alloyed nanoparticles as high performance electrocatalyst for oxygen reduction reaction. J. Power Sources 2015, 293, 380–387. [Google Scholar] [CrossRef]
- Kuttiyiel, K.A.; Sasaki, K.; Su, D.; Wu, L.J.; Zhu, Y.M.; Adzic, R.R. Gold-promoted structurally ordered intermetallic palladium cobalt nanoparticles for the oxygen reduction reaction. Nat. Commun. 2014, 5, 5185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Zhao, S.; Liu, S.; Yin, H.; Chen, Y.-Y.; Bao, J.; Han, M.; Dai, Z. Component-Controlled Synthesis and Assembly of Cu–Pd Nanocrystals on Graphene for Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2015, 7, 5347–5357. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, J.-E.; Momma, T.; Osaka, T. Synthesis of Pd–Sn nanoparticles by ultrasonic irradiation and their electrocatalytic activity for oxygen reduction. Electrochim. Acta 2009, 54, 3412–3418. [Google Scholar] [CrossRef]
- Hubkowska, K.; Łukaszewski, M.; Czerwiński, A. Pd–Ru electrodeposits with high hydrogen absorption capacity. Electrochem. Commun. 2012, 20, 175–177. [Google Scholar] [CrossRef]
- Peng, Y.; Lu, B.; Chen, L.; Wang, N.; Lu, J.E.; Ping, Y.; Chen, S. Hydrogen evolution reaction catalyzed by ruthenium ion-complexed graphitic carbon nitride nanosheets. J. Mater. Chem. A 2017, 5, 18261–18269. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, P.; Wang, G.; Zhang, P.P.; Zhuang, X.D.; Chen, M.W.; Weidinger, I.M.; Feng, X.L. Ruthenium/nitrogen-doped carbon as an electrocatalyst for efficient hydrogen evolution in alkaline solution. J. Mater. Chem. A 2017, 5, 25314–25318. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Zhu, Y.; Li, L.H.; Han, Y.; Chen, Y.; Jaroniec, M.; Qiao, S.-Z. High Electrocatalytic Hydrogen Evolution Activity of an Anomalous Ruthenium Catalyst. J. Am. Chem. Soc. 2016, 138, 16174–16181. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.H.; Huang, T.; Liu, P.; Zhang, J.; Sasaki, K.; Vukmirovic, M.B.; Adzic, R.R. Palladium Monolayer and Palladium Alloy Electrocatalysts for Oxygen Reduction. Langmuir 2006, 22, 10409–10415. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hsieh, Y.-C.; Chang, L.-C.; Wu, P.-W.; Lee, J.-F. Synthesis of Pd9Ru@Pt nanoparticles for oxygen reduction reaction in acidic electrolytes. J. Power Sources 2015, 277, 116–123. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Q.; Bao, J.; Li, Y.; Dai, Z.; Gu, L. Significantly Enhanced Hydrogen Evolution Activity of Freestanding Pd-Ru Distorted Icosahedral Clusters with less than 600 Atoms. Chem. Eur. J. 2017, 23, 18203–18207. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Tang, Z.; Li, L.; Wang, L.; Yang, H.; Wang, Q.; Wu, W.; Chen, S. Ultrasmall Palladium Nanoclusters Encapsulated in Porous Carbon Nanosheets for Oxygen Electroreduction in Alkaline Media. ChemElectroChem 2017, 4, 1349–1355. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L.; Tang, Z.; Wang, F.; Yan, W.; Yang, H.; Zhou, W.; Li, L.; Kang, X.; Chen, S. Oxygen reduction catalyzed by gold nanoclusters supported on carbon nanosheets. Nanoscale 2016, 8, 6629–6635. [Google Scholar] [CrossRef] [PubMed]
- Bin, D.; Yang, B.; Ren, F.; Zhang, K.; Yang, P.; Du, Y. Facile synthesis of PdNi nanowire networks supported on reduced graphene oxide with enhanced catalytic performance for formic acid oxidation. J. Mater. Chem. A 2015, 3, 14001–14006. [Google Scholar] [CrossRef]
- Xiong, Z.; Xu, H.; Li, S.; Gu, Z.; Yan, B.; Guo, J.; Du, Y. Concave Pd-Ru nanocubes bounded with high active area for boosting ethylene glycol electrooxidation. Appl. Surf. Sci. 2018, 427, 83–89. [Google Scholar] [CrossRef]
- Lange, F.; Armbruster, U.; Martin, A. Heterogeneously-Catalyzed Hydrogenation of Carbon Dioxide to Methane using RuNi Bimetallic Catalysts. Energy Technol. 2015, 3, 55–62. [Google Scholar] [CrossRef]
- Meku, E.; Du, C.; Wang, Y.; Du, L.; Sun, Y.; Kong, F.; Yin, G. Concentration Gradient Pd-Ir-Ni/C Electrocatalyst with Enhanced Activity and Methanol Tolerance for Oxygen Reduction Reaction in Acidic Medium. Electrochim. Acta 2016, 192, 177–187. [Google Scholar] [CrossRef] [Green Version]
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Moudler, J.F.; Muilenberg, G.E. Handbook of X-ray Photoelectron Spectroscopy: A Referencce Book of Standard Data for Use in X-ray Photoelectron Spectroscopy; Perkin-Elmer Corp., Physical Electronics Division: Eden Prairie, MN, USA, 1979; pp. 1–100. [Google Scholar]
- Li, D.; Tang, Z.; Chen, S.; Tian, Y.; Wang, X. Peptide-FlgA3-Based Gold Palladium Bimetallic Nanoparticles That Catalyze the Oxygen Reduction Reaction in Alkaline Solution. ChemCatChem 2017, 9, 2980–2987. [Google Scholar] [CrossRef]
- Li, T.; Tang, Z.; Wang, K.; Wu, W.; Chen, S.; Wang, C. Palladium nanoparticles grown on β-Mo2C nanotubes as dual functional electrocatalysts for both oxygen reduction reaction and hydrogen evolution reaction. Int. J. Hydrogen Energy 2018, 43, 4932–4941. [Google Scholar] [CrossRef]
- Deming, C.P.; Mercado, R.; Gadiraju, V.; Sweeney, S.W.; Khan, M.; Chen, S. Graphene Quantum Dots-Supported Palladium Nanoparticles for Efficient Electrocatalytic Reduction of Oxygen in Alkaline Media. ACS Sustainable Chem. Eng. 2015, 3, 3315–3323. [Google Scholar] [CrossRef]
- Deming, C.P.; Mercado, R.; Lu, J.E.; Gadiraju, V.; Khan, M.; Chen, S. Oxygen Electroreduction Catalyzed by Palladium Nanoparticles Supported on Nitrogen-Doped Graphene Quantum Dots: Impacts of Nitrogen Dopants. ACS Sustainable Chem. Eng. 2016, 4, 6580–6589. [Google Scholar] [CrossRef]
- Du, C.; Gao, X.; Zhuang, Z.; Cheng, C.; Zheng, F.; Li, X.; Chen, W. Epitaxial growth of zigzag PtAu alloy surface on Au nano-pentagrams with enhanced Pt utilization and electrocatalytic performance toward ethanol oxidation reaction. Electrochim. Acta 2017, 238, 263–268. [Google Scholar] [CrossRef]
- Zong, Z.; Xu, K.; Li, D.; Tang, Z.; He, W.; Liu, Z.; Wang, X.; Tian, Y. Peptide templated Au@Pd core-shell structures as efficient bi-functional electrocatalysts for both oxygen reduction and hydrogen evolution reactions. J. Catal. 2018, 361, 168–176. [Google Scholar] [CrossRef]
- Wu, W.; Tang, Z.; Wang, K.; Liu, Z.; Li, L.; Chen, S. Peptide templated AuPt alloyed nanoparticles as highly efficient bi-functional electrocatalysts for both oxygen reduction reaction and hydrogen evolution reaction. Electrochim. Acta 2018, 260, 168–176. [Google Scholar] [CrossRef]
- Wang, H.; Yang, N.; Li, W.; Ding, W.; Chen, K.; Li, J.; Li, L.; Wang, J.; Jiang, J.; Jia, F.; Wei, Z. Understanding the Roles of Nitrogen Configurations in Hydrogen Evolution: Trace Atomic Cobalt Boosts the Activity of Planar Nitrogen-Doped Graphene. ACS Energy Lett. 2018, 3, 1345–1352. [Google Scholar] [CrossRef]
- Jahan, M.; Bao, Q.; Loh, K.P. Electrocatalytically Active Graphene–Porphyrin MOF Composite for Oxygen Reduction Reaction. J. Am. Chem. Soc. 2012, 134, 6707–6713. [Google Scholar] [CrossRef] [PubMed]
- Łukaszewski, M.; Klimek, K.; Żurowski, A.; Kędra, T.; Czerwiński, A. Kinetics and mechanism of hydrogen electrosorption in palladium-based alloys. Solid State Ionics 2011, 190, 18–24. [Google Scholar] [CrossRef]
- Safavi, A.; Kazemi, S.H.; Kazemi, H. Electrocatalytic behaviors of silver–palladium nanoalloys modified carbon ionic liquid electrode towards hydrogen evolution reaction. Fuel 2014, 118, 156–162. [Google Scholar] [CrossRef]
- Ghasemi, S.; Hosseini, S.R.; Nabipour, S.; Asen, P. Palladium nanoparticles supported on graphene as an efficient electrocatalyst for hydrogen evolution reaction. Int. J. Hydrogen Energy 2015, 40, 16184–16191. [Google Scholar] [CrossRef]
- Kong, D.; Cha, J.J.; Wang, H.; Lee, H.R.; Cui, Y. First-row transition metal dichalcogenide catalysts for hydrogen evolution reaction. Energy Environ. Sci. 2013, 6, 3553–3558. [Google Scholar] [CrossRef]
- Yin, X.; Sun, G.; Wang, L.; Bai, L.; Su, L.; Wang, Y.; Du, Q.; Shao, G. 3D hierarchical network NiCo2S4 nanoflakes grown on Ni foam as efficient bifunctional electrocatalysts for both hydrogen and oxygen evolution reaction in alkaline solution. Int. J. Hydrogen Energy 2017, 42, 25267–25276. [Google Scholar] [CrossRef]
- Yin, X.; Sun, G.; Song, A.; Wang, L.; Wang, Y.; Dong, H.; Shao, G. A novel structure of Ni-(MoS2/GO) composite coatings deposited on Ni foam under supergravity field as efficient hydrogen evolution reaction catalysts in alkaline solution. Electrochim. Acta 2017, 249, 52–63. [Google Scholar] [CrossRef]
- Du, C.; Yang, L.; Yang, F.; Cheng, G.; Luo, W. Nest-like NiCoP for Highly Efficient Overall Water Splitting. ACS Catal. 2017, 7, 4131–4137. [Google Scholar] [CrossRef]
- Garrick, T.R.; Diao, W.; Tengco, J.M.; Stach, E.A.; Senanayake, S.D.; Chen, D.A.; Monnier, J.R.; Weidner, J.W. The Effect of the Surface Composition of Ru-Pt Bimetallic Catalysts for Methanol Oxidation. Electrochim. Acta 2016, 195, 106–111. [Google Scholar] [CrossRef] [Green Version]
- Miao, K.; Luo, Y.; Zou, J.; Yang, J.; Zhang, F.; Huang, L.; Huang, J.; Kang, X.; Chen, S. PdRu alloy nanoparticles of solid solution in atomic scale: outperformance towards formic acid electro-oxidation in acidic medium. Electrochim. Acta 2017, 251, 588–594. [Google Scholar] [CrossRef]
- Wang, W.; Lei, B.; Guo, S. Engineering Multimetallic Nanocrystals for Highly Efficient Oxygen Reduction Catalysts. Adv. Energy Mater. 2016, 6, 1600236. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.-J.; Baek, J.-B. Metal-Free Catalysts for Oxygen Reduction Reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, Z.; Yan, W.; Yang, H.; Wang, Q.; Chen, S. Porous Carbon-Supported Gold Nanoparticles for Oxygen Reduction Reaction: Effects of Nanoparticle Size. ACS Appl. Mater. Interfaces 2016, 8, 20635–20641. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Wu, W.; Tang, Z.; Wu, Y.; Burns, R.; Tichnell, B.; Liu, Z.; Chen, S. Oxygen Reduction Reaction and Hydrogen Evolution Reaction Catalyzed by Pd–Ru Nanoparticles Encapsulated in Porous Carbon Nanosheets. Catalysts 2018, 8, 329. https://doi.org/10.3390/catal8080329
Tian J, Wu W, Tang Z, Wu Y, Burns R, Tichnell B, Liu Z, Chen S. Oxygen Reduction Reaction and Hydrogen Evolution Reaction Catalyzed by Pd–Ru Nanoparticles Encapsulated in Porous Carbon Nanosheets. Catalysts. 2018; 8(8):329. https://doi.org/10.3390/catal8080329
Chicago/Turabian StyleTian, Juntai, Wen Wu, Zhenghua Tang, Yuan Wu, Robert Burns, Brandon Tichnell, Zhen Liu, and Shaowei Chen. 2018. "Oxygen Reduction Reaction and Hydrogen Evolution Reaction Catalyzed by Pd–Ru Nanoparticles Encapsulated in Porous Carbon Nanosheets" Catalysts 8, no. 8: 329. https://doi.org/10.3390/catal8080329