Valorization of Biomass Derived Terpene Compounds by Catalytic Amination
Abstract
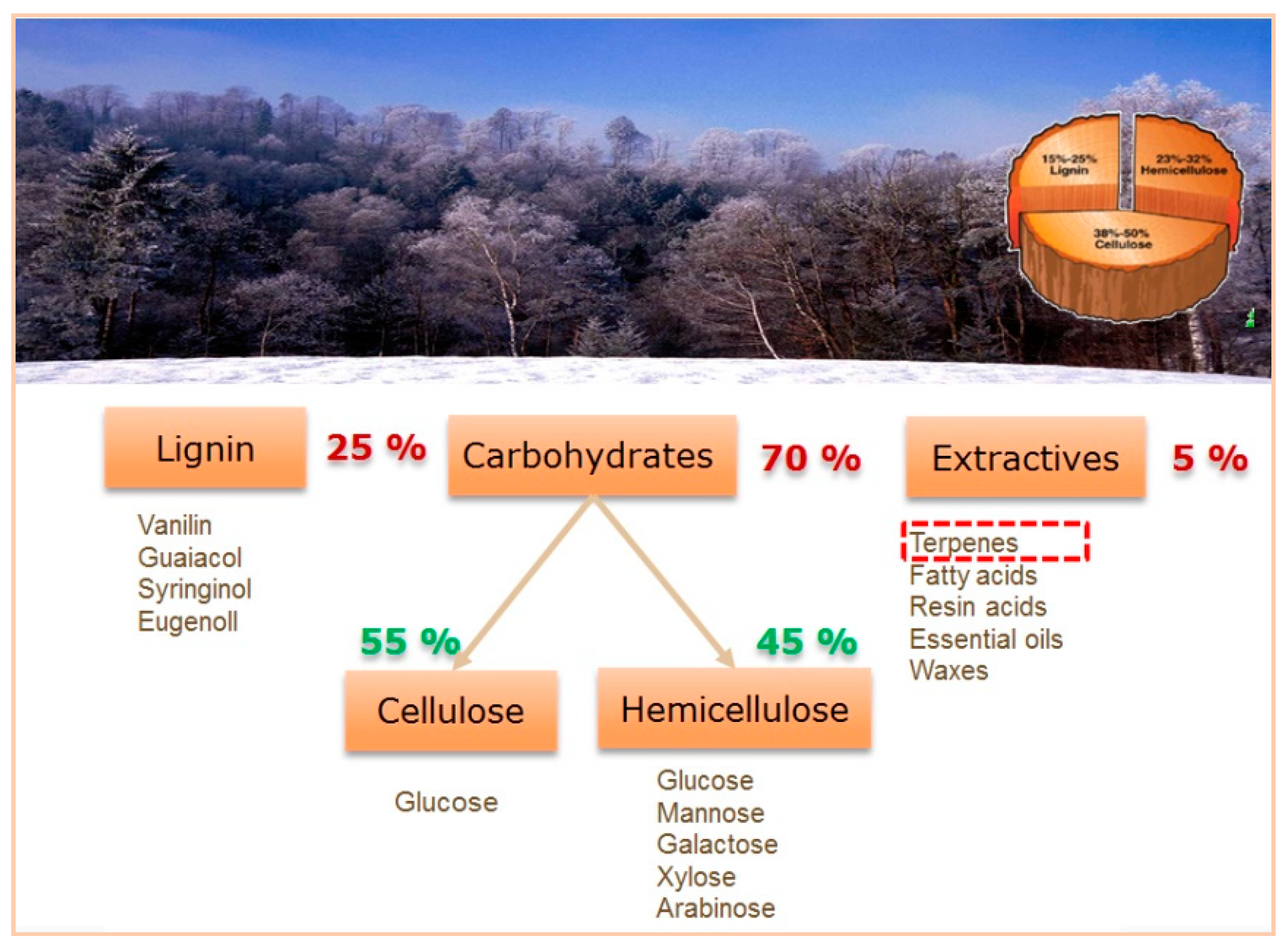
:1. Introduction
2. Terpenes Valorization into Valuable Amines
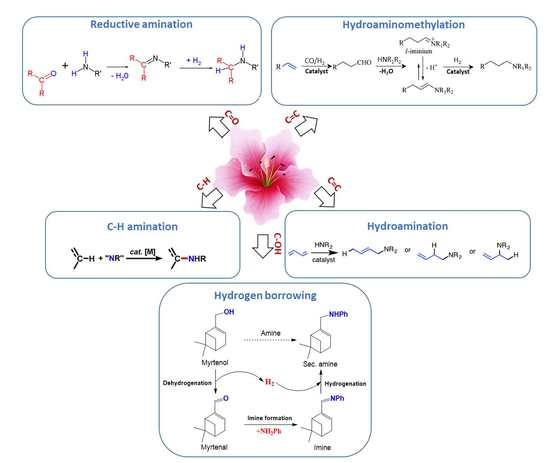
3. Possible Catalytic Tools for Synthesis of Terpene-Based Amines
- (1)
- reductive amination of aldehydes and ketones
- (2)
- hydroaminomethylation
- (3)
- hydroamination of double C=C bonds
- (4)
- hydrogen borrowing methodology for amination of alcohols
- (5)
- C–H amination of terpenes, which is a very specific case.
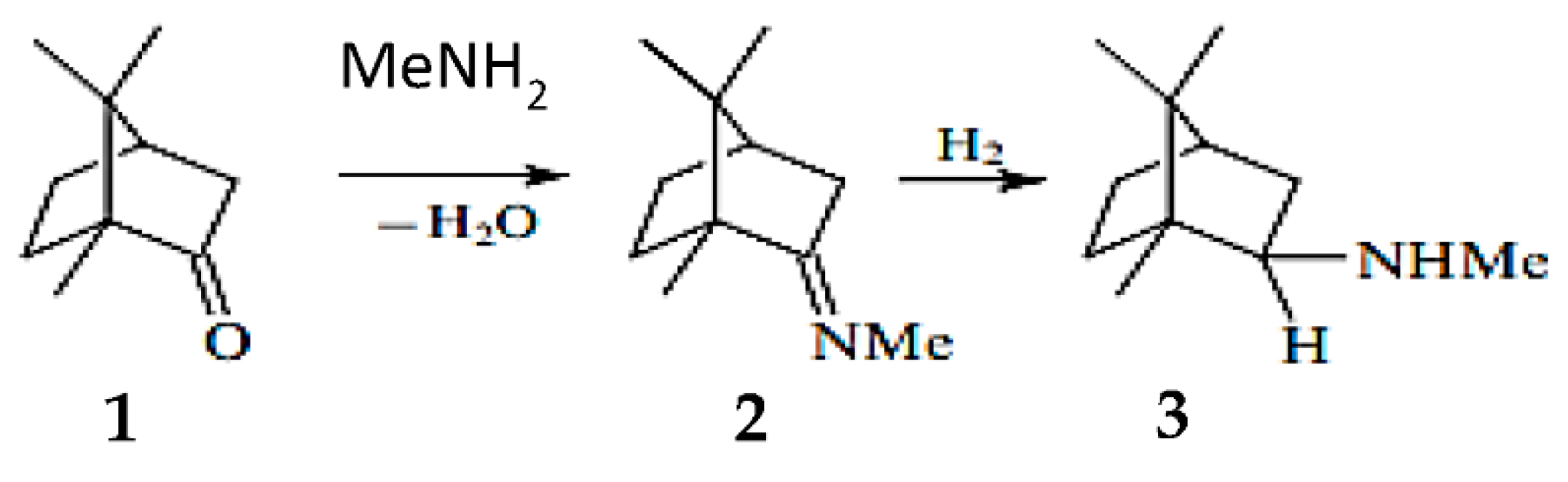
3.1. Reductive Amination of Terpenes with Carbonyl Moiety
3.1.1. Reductive Amination of Aldehydes
3.1.2. Reductive Amination of Ketones
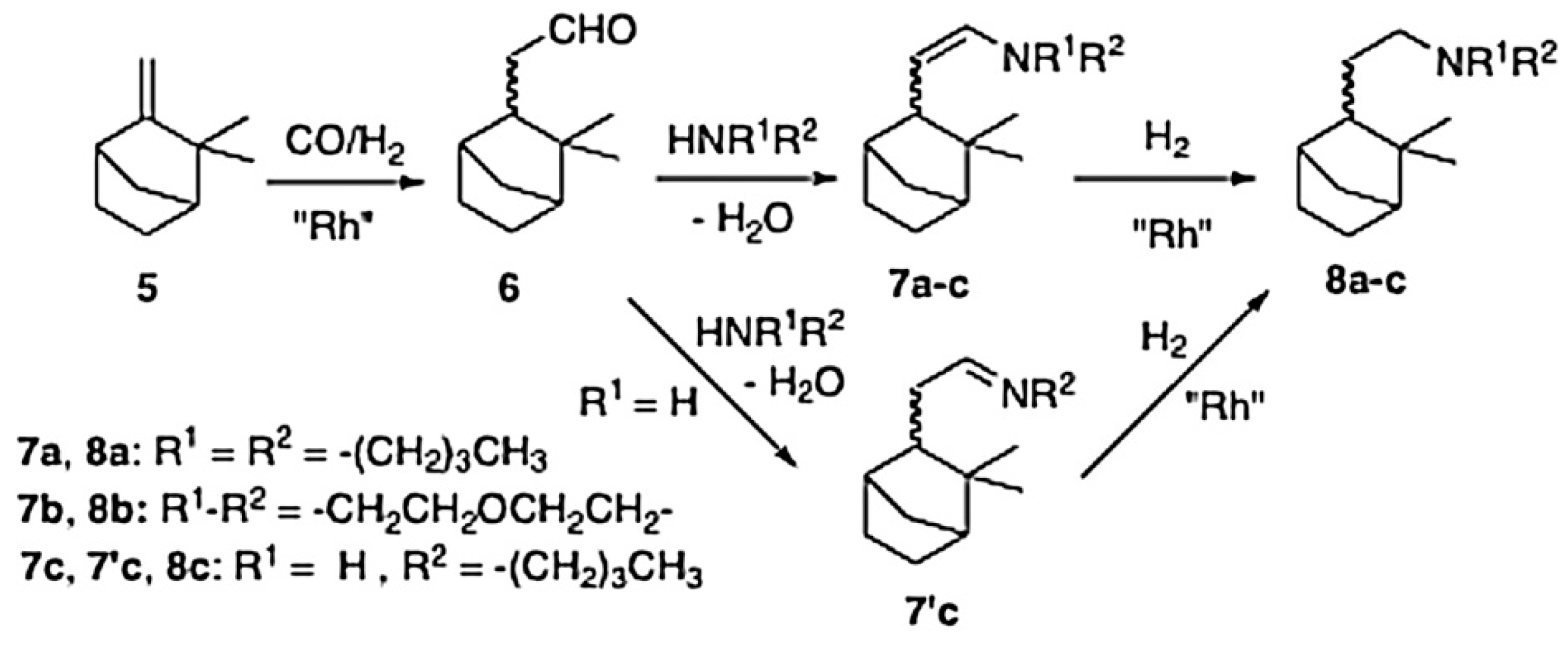
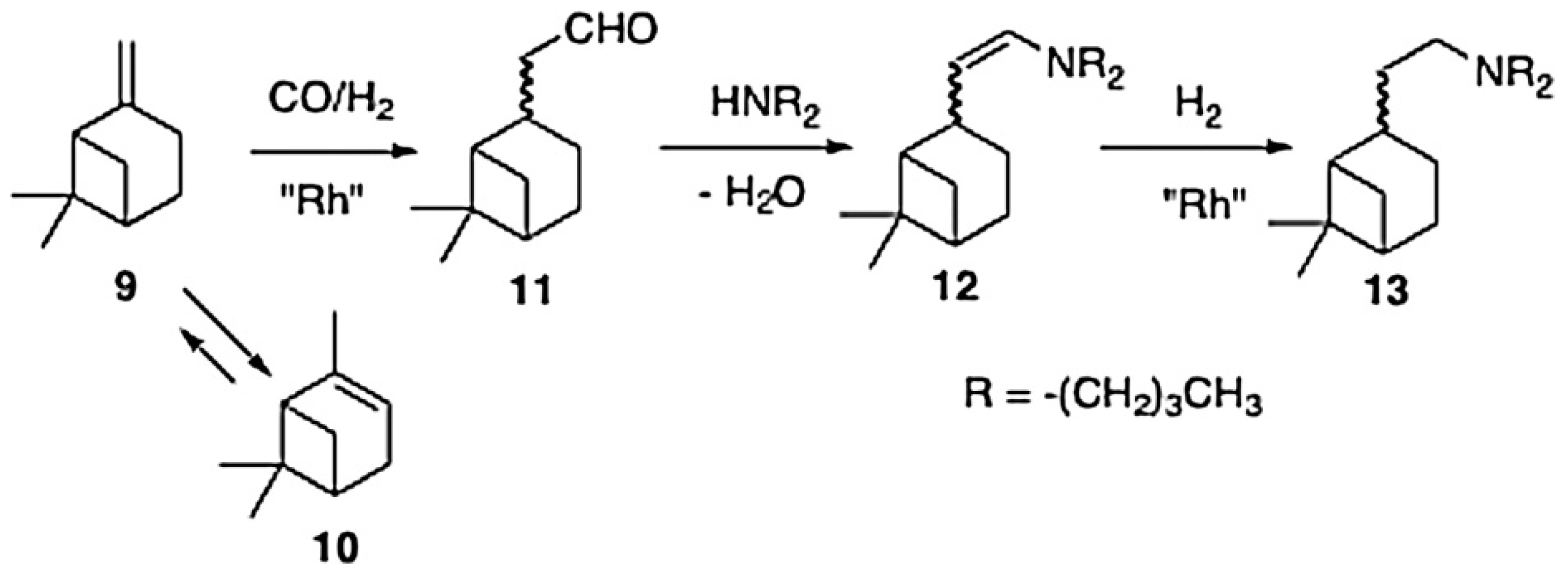
3.2. Hydroaminomethylation of Olefin Bonds in Terpenes
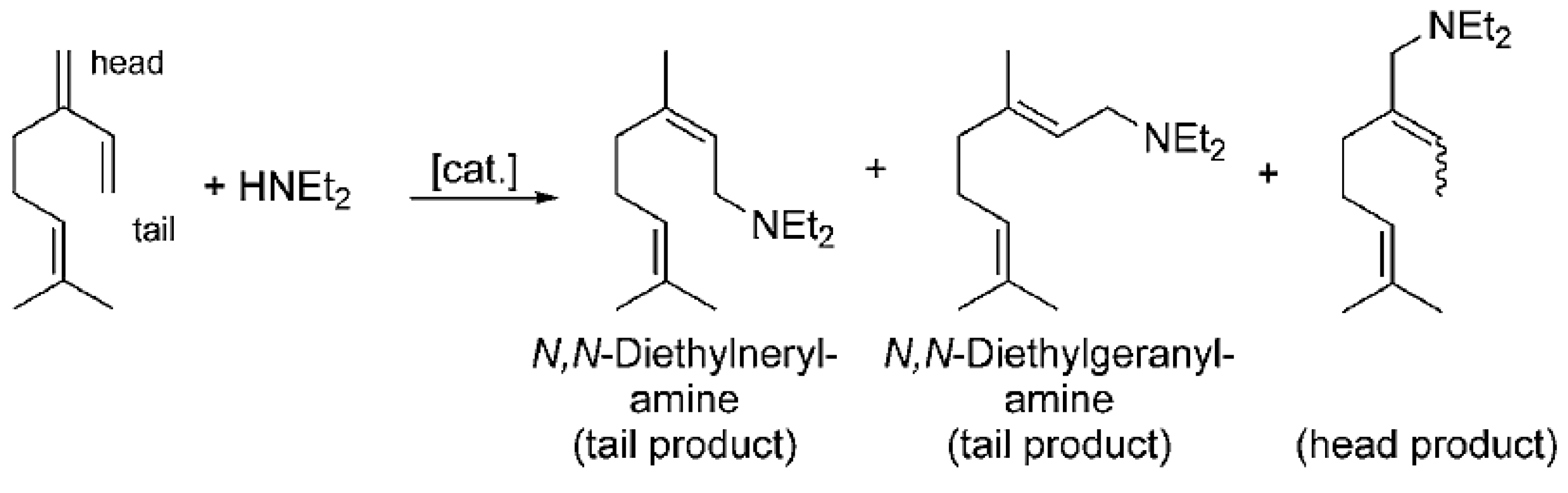
3.3. Hydroamination on Olefin Bonds of Terpenes
3.4. Amination of Terpene Alcohols
3.4.1. Homogeneous Catalysts
3.4.2. Heterogeneous Catalysts
3.5. C–H Amination of Terpenes
4. Learning from Homogeneous Catalysis and Future Outlook for Heterogeneous Catalysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lawrence, S.A. Amines: Synthesis, Properties and Applications; Cambridge University Press: Cambridge, UK, 2004; p. 372. [Google Scholar]
- Shi, F.; Cui, X. Catalytic Amination for N-Alkylamine Synthesis; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Maxwell, G.R. Synthetic Nitrogen Products: A Practical Guide to the Products and Processes; Kluwer Academic Publishers: New York, NY, USA, 2004. [Google Scholar]
- Pelckmans, M.; Renders, T.; Van de Vyver, S.; Sels, B.F. Bio-based amines through sustainable heterogeneous catalysis. Green Chem. 2017, 19, 5303–5331. [Google Scholar] [CrossRef]
- Eller, K.; Henkes, E.; Rossbacher, R.; Höke, H. Amines, aliphatic. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2012; pp. 647–698. [Google Scholar]
- Frauenkron, M.; Melder, J.-P.; Ruider, G.; Rossbacher, R.; Höke, H. Ethanolamines and Propanolamines. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2012; pp. 405–431. [Google Scholar]
- Bähn, S.; Imm, S.; Neubert, L.; Zhang, M.; Neumann, H.; Beller, M. The catalytic amination of alcohols. ChemCatChem 2011, 3, 1853–1864. [Google Scholar] [CrossRef]
- Kimura, H. Progress in one-step amination of long-chain fatty alcohols with dimethylamine: Development of key technologies for industrial applications, innovations, and future outlook. Catal. Rev. Sci. Eng. 2011, 53, 1–90. [Google Scholar] [CrossRef]
- Pera-Titus, M.; Shi, F. Catalytic amination of biomass-based alcohols. ChemSusChem 2014, 7, 720–722. [Google Scholar] [CrossRef] [PubMed]
- Froidevaux, V.; Negrell, C.; Caillol, S.; Pascault, J.-P.; Boutevin, B. Biobased amines: From synthesis to polymers; present and future. Chem. Rev. 2016, 116, 14181–14224. [Google Scholar] [CrossRef] [PubMed]
- Geboers, J.; Van de Vyver, S.; Carpentier, K.; de Blochouse, K.; Jacobs, P.; Sels, B. Efficient catalytic conversion of concentrated cellulose feeds to hexitols with heteropoly acids and Ru on carbon. Chem. Commun. 2010, 46, 3577–3579. [Google Scholar] [CrossRef] [PubMed]
- Geboers, J.; Van de Vyver, S.; Carpentier, K.; Jacobs, P.; Sels, B. Efficient hydrolytic hydrogenation of cellulose in the presence of Ru-loaded zeolites and trace amounts of mineral acid. Chem. Commun. 2011, 47, 5590–5592. [Google Scholar] [CrossRef] [PubMed]
- Geboers, J.A.; Van de Vyver, S.; Ooms, R.; Op de Beeck, B.; Jacobs, P.A.; Sels, B.F. Chemocatalytic conversion of cellulose: Opportunities, advances and pitfalls. Catal. Sci. Technol. 2011, 1, 714–726. [Google Scholar] [CrossRef]
- Van de Vyver, S.; Geboers, J.; Jacobs, P.A.; Sels, B.F. Recent Advances in the Catalytic Conversion of Cellulose. ChemCatChem 2011, 3, 82–94. [Google Scholar] [CrossRef]
- Tinikul, R.; Chenprakhon, P.; Maenpuen, S.; Chaiyen, P. Biotransformation of plant-derived phenolic acids. Biotechnol. J. 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, J.; Lindström, M.E. Modification of industrial softwood kraft lignin using Mannich reaction with and without phenolation pretreatment. Ind. Crop. Prod. 2014, 52, 729–735. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, S.; Schutyser, W.; Vanholme, R.; Driessen, T.; Koelewijn, S.F.; Renders, T.; De Meester, B.; Huijgen, W.J.J.; Dehaen, W.; Courtin, C.M.; et al. Reductive lignocellulose fractionation into soluble lignin-derived phenolic monomers and dimers and processable carbohydrate pulps. Energy Environ. Sci. 2015, 8, 1748–1763. [Google Scholar] [CrossRef] [Green Version]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chena, T.-Y.; Wanga, H.-M.; Li, H.-Y.; Liub, C.-F.; Wena, J.-L. Amination of biorefinery technical lignins using Mannich reaction synergy with subcritical ethanol depolymerization. Int. J. Biol. Macromol. 2018, 107, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Sun, G.; Zhao, T. Synthesis and characterization of aminated lignin. Int. J. Biol. Macromol. 2013, 59, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H.; Kondo, A.; Noda, H. Biodiesel fuel production by transesterification of oils. J. Biosci. Bioeng. 2001, 92, 405–416. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A review on chitin and chitosan polymers: Structure, chemistry, solubility, derivatives. ChemBioEng. Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A Sustainable platform for production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Behr, A.; Wintzer, A. From terpenoids to amines: A critical review. In New Developments in Terpenes Research; Hu, J., Ed.; Nova Science Publishers: New York, NY, USA, 2014; Chapter 6; pp. 113–134. [Google Scholar]
- Kroutil, W.; Fischereder, E.-M.; Fuchs, C.S.; Lechner, H.; Mutti, F.G.; Pressnitz, D.; Rajagopalan, A.; Sattler, J.H.; Simon, R.C.; Siirola, E. Asymmetric preparation of prim-, sec-, and tert-amines employing selected biocatalysts. Org. Process Res. Dev. 2013, 17, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.J.; Truppo, M.D. Biocatalytic routes to nonracemic chiral amines. In Chiral Amine Synthesis: Methods, Developments and Applications, 2nd ed.; Nugent, T.C., Ed.; Wiley: Hoboken, NJ, USA, 2010; p. 523. [Google Scholar]
- Schrewe, M.; Ladkau, N.; Buehler, B.; Schmid, A. Direct terminal alkylamino-functionalization via multistep biocatalysis in one recombinant whole-cell catalyst. Adv. Synth. Catal. 2013, 355, 1693–1697. [Google Scholar] [CrossRef]
- Song, J.-W.; Lee, J.-H.; Bornscheuer, U.T.; Park, J.-B. Microbial synthesis of medium-chain α,ω-dicarboxylic acids and ω-aminocarboxylic acids from renewable long-chain fatty acids. Adv. Synth. Catal. 2014, 356, 178–1788. [Google Scholar] [CrossRef]
- Singh, R.; Kolev, J.N.; Sutera, P.A.; Fasan, R. Enzymatic C(sp3)-H amination: P450-catalyzed conversion of carbonazidates into oxazolidinones. ACS Catal. 2015, 5, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Deutschmann, O.; Knozinger, H.; Kochloefl, K.; Turek, T. Heterogeneous catalysis and solid catalysts. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2009; pp. 1–110. [Google Scholar]
- Harrewijn, P.; van Oosten, A.M.; Piron, P.G.M. Natural Terpenoids as Messengers. A Multidisciplinary Study of their Production, Biological Functions and Practical Applications; Kluwer Academic Publishers: South Holland, The Netherlands, 2001. [Google Scholar]
- Behr, A.; Johnen, L. Myrcene as a natural base chemical in sustainable chemistry: A critical review. ChemSusChem 2009, 2, 1072–1095. [Google Scholar] [CrossRef] [PubMed]
- Mäki-Arvela, P.; Simakova, I.L.; Salmi, T.; Murzin, D.Y. Catalytic transformations of extractives. In Catalytic Process Development for Renewable Materials; Hardcover Handbook; Wiley: Weinheim, Germany, 2013; Chapter 13; 450p. [Google Scholar]
- Murzin, D.Y.; Simakova, I.L. Catalysis in biomass conversion. In Comprehensive Inorganic Chemistry II; Schlogl, R., Niemantsverdriet, J.W., Eds.; Elsevier: New York, NY, USA, 2013; Chapter 7.27; pp. 2–32. [Google Scholar]
- Murzin, D.Y.; Simakova, I.L. Catalysis in biomass processing. Catal. Ind. 2011, 3, 218–249. [Google Scholar] [CrossRef]
- Murzin, D.Y.; Demidova, Y.; Hasse, B.; Etzold, B.; Simakova, I.L. Synthesis of fine chemicals using catalytic nanomaterials: Structure sensitivity. In Producing Fuels and Fine Chemicals from Biomass Using Nanomaterials; Luque, R., Balu, A.M., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 267–281. [Google Scholar]
- Salakhutdinov, N.F.; Volcho, K.P.; Yarovaya, O.I. Monoterpenes as a renewable source of biologically active compounds. Pure Appl. Chem. 2017, 89, 1105–1118. [Google Scholar] [CrossRef]
- Kapitsa, I.G.; Suslov, E.V.; Teplov, G.V.; Korchagina, D.V.; Komarova, N.I.; Volcho, K.P.; Voronina, T.A.; Shevela, A.I.; Salakhutdinov, N.F. Synthesis and anxiolytic activity of 2-aminoadamantane derivatives containing monoterpene fragments. Pharm. Chem. J. 2012, 46, 263–265. [Google Scholar] [CrossRef]
- Teplov, G.V.; Suslov, E.V.; Zarubaev, V.V.; Shtro, A.A.; Karpinskaya, L.A.; Rogachev, A.D.; Korchagina, D.V.; Volcho, K.P.; Salakhutdinov, N.F.; Kisilev, O.I. Synthesis of new compounds combining adamantanamine and monoterpene fragments and their antiviral activity against influenza virus A(H1N1)pdm09. Lett. Drug Des. Discov. 2013, 10, 477–485. [Google Scholar] [CrossRef]
- Volcho, K.P.; Laev, S.S.; Ashraf, G.M.; Aliev, G.; Salakhutdinov, N.F. Application of monoterpenoids and their derivatives for treatment of neurodegenerative disorders. Curr. Med. Chem. 2017, 24, 3283–3309. [Google Scholar]
- Silva, R.O.; Salvadori, M.S.; Sousa, F.B.M.; Santos, M.S.; Carvalho, N.S.; Sousa, D.P.; Gomes, B.S.; Oliveira, F.A.; Barbosa, A.L.R.; Frietas, R.M.; et al. Evaluation of the anti-inflammatory and antinociceptive effects of myrtenol, a plant-derived monoterpene alcohol, in mice. Flavour Fragr. J. 2014, 29, 184–192. [Google Scholar] [CrossRef]
- Sarmento-Neto, J.F.; do Nascimento, L.G.; Felipe, C.F.B.; de Sousa, D.P. Analgesic potential of essential oils. Molecules 2016, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- Lochynski, S.; Kuldo, J.; Frackowiak, B.; Holband, J.; Wojcik, G. Stereochemistry of terpene derivatives. Part 2: Synthesis of new chiral amino acids with potential neuroactivity. Tetrahedron Asymmetry 2000, 11, 1295–1302. [Google Scholar] [CrossRef]
- Gajcy, K.; Pekala, J.; Frackowiak-Wojtasek, B.; Librowski, T.; Lochynski, S. Stereochemistry of terpene derivatives. Part 7: Novel rigidified amino acids from (+)-3-carene designed as chiral GABA analogues. Tetrahedron Asymmetry 2010, 21, 2015–2020. [Google Scholar] [CrossRef]
- Ferrarini, S.R.; Graebin, C.S.; Limberger, J.; Canto, R.F.S.; Dias, D.O.; da Rosa, R.G.; Madeira, M.D.F.; Eifler-Lima, V.L. Synthesis of limonene β-amino alcohol derivatives in support of new antileishmanial therapies. Mem. Inst. Oswaldo Cruz 2008, 103, 773–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrarini, S.R.; Duarte, M.O.; da Rosa, R.G.; Rolim, V.; Eifler-Lima, V.L.; von Poser, G.; Ribeiro, V.L.S. Acaricidal activity of limonene, limonene oxide and β-amino alcohol derivatives on Rhipicephalus (Boophilus) microplus. Vet. Parasitol. 2008, 157, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Strong, J. N-[3-(4-methyl-3-cyclohexenyl)butyl]amines. U.S. Patent 3,890,384, 27 January 1975. [Google Scholar]
- Strong, J. N-[3-(4-methyl-3-cyclohexenyl)butyl]amines and Their Use as Plant Growth Regulators. U.S. Patent 4,030,908, 27 January 1972. [Google Scholar]
- Keim, W.; Kurtz, K.R.; Roeper, M. Palladium catalyzed telomerization of isoprene with secondary amines and conversion of the resulting terpene amines to terpenols. J. Mol. Catal. 1983, 20, 129–138. [Google Scholar] [CrossRef]
- Watts, C.C.; Thoniyot, P.; Cappuccio, F.; Verhagen, J.; Gallagher, B.; Singaram, B. Catalytic asymmetric transfer hydrogenation of ketones using terpene-based chiral β-amino alcohols. Tetrahedron Asymmetry 2006, 17, 1301–1307. [Google Scholar] [CrossRef]
- Watts, C.C.; Thoniyot, P.; Hirayama, L.C.; Romano, T.; Singaram, B. Enantioselective alkynylations of aromatic and aliphatic aldehydes catalyzed by terpene derived chiral amino alcohols. Tetrahedron Asymmetry 2005, 16, 1829–1835. [Google Scholar] [CrossRef]
- Alves, M.-H.; Sfeir, H.; Tranchant, J.-F.; Gombart, E.; Sagorin, G.; Caillol, S.; Billon, L.; Save, M. Terpene and dextran renewable resources for the synthesis of amphiphilic biopolymers. Biomacromolecules 2014, 15, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Ajikumar, P.K.; Tyo, K.; Carlsen, S.; Mucha, O.; Phon, T.H.; Stephanopoulos, G. Terpenoids: Opportunities for biosynthesis of natural product drugs using engineered microorganisms. Mol. Pharm. 2008, 5, 167–190. [Google Scholar] [CrossRef] [PubMed]
- Lyubimov, S.E.; Kuchurov, I.V.; Verbitskaya, T.A.; Rastorguev, E.A.; Kalinin, V.N.; Zlotin, S.G.; Davankov, V.A. Pd-catalyzed allylic amination in supercritical carbon dioxide: Synthesis of carborane-containing terpenoids. J. Supercrit. Fluids 2010, 54, 218–221. [Google Scholar] [CrossRef]
- Armstrong, A.F.; Valliant, J.F. The bioinorganic and medicinal chemistry of carboranes: From new drug discovery to molecular imaging and therapy. Dalton Trans. 2007, 4240–4251. [Google Scholar] [CrossRef] [PubMed]
- Tietze, L.F.; Griesbach, U.; Bothe, U.; Nakamura, H.; Yamamoto, Y. Novel carboranes with a DNA binding unit for the treatment of cancer by boron neutron capture therapy. ChemBioChem 2002, 3, 219–225. [Google Scholar] [CrossRef]
- Di Meo, C.; Panza, L.; Capitani, D.; Mannina, L.; Banzato, A.; Rondina, M.; Renier, D.; Rosato, A.; Crescenzi, V. Hyaluronan as carrier of carboranes for tumor targeting in boron neutron capture therapy. Biomacromolecules 2007, 8, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.F.; Coderre, J.A.; Vicente, M.G.H.; Blue, T.E. Boron neutron capture therapy of cancer: Current status and future prospects. Clin. Cancer Res. 2005, 11, 3987–4002. [Google Scholar] [CrossRef] [PubMed]
- Nageshwar, D.; Rao, D.M.; Acharyulu, P.V.R. Terpenes to ionic liquids: Synthesis and characterization of citronellal-based chiral ionic liquids. Synth. Commun. 2009, 39, 3357–3368. [Google Scholar] [CrossRef]
- Bordenca, C.; Dorschner, K.P.; Johnson, R.P. Insect Repellent Compositions and Process Having an N-substituted Hydroxyalkyl Amine as an Active Ingredient. U.S. Patent 3,933,915, 23 June 1972. [Google Scholar]
- Behr, A.; Wintzer, A.; Lübke, C.; Müller, M. Synthesis of primary amines from the renewable compound citronellal via biphasic reductive amination. J. Mol. Catal. A Chem. 2015, 404–405, 74–82. [Google Scholar] [CrossRef]
- Kukula, P.; Koprivova, K. Structure-selectivity relationship in the chemoselective hydrogenation of unsaturated nitriles. J. Catal. 2005, 234, 161–171. [Google Scholar] [CrossRef]
- Bahn, S.; Imm, S.; Neubert, L.; Zhang, M.; Neumann, H.; Beller, M. Selective ruthenium-catalyzed alkylation of indoles by using amines. Chem. Eur. J. 2011, 17, 4705–4708. [Google Scholar] [PubMed]
- Fuchs, S.; Rösler, T.; Grabe, B.; Kampwerth, A.; Meier, G.; Strutz, H.; Behr, A.; Vorholt, A.J. Synthesis of primary amines via linkage of hydroaminomethylation of olefins and splitting of secondary amines. Appl. Cat. A Gen. 2018, 550, 198–205. [Google Scholar] [CrossRef]
- Donetti, A.; Casadio, S.; Bonardi, G.; Omodei-Sale, A. Terpene compounds as drugs. 13. o-Terpenylaminomethylphenols and their N-methyl derivatives. J. Med. Chem. 1972, 15, 1089–1091. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, N.G.; Kalechits, G.V.; Vyalimyae, T.K. Terpene amines. IV. Synthesis and study of the structure of amines from d-fenchone. Khimiya Prirodnych Soedinenii (Chem. Nat. Comp.) 1983, 4, 483–488. [Google Scholar] [CrossRef]
- Tarasevich, V.A.; Kozlov, N.G. Reductive amination of oxygen-containing organic compounds. Russ. Chem. Rev. 1999, 68, 55–72. [Google Scholar] [CrossRef]
- Kalck, P.; Urrutigoïty, M. Tandem hydroaminomethylation reaction to synthesize amines from alkenes. Chem. Rev. 2018, 118, 3833–3861. [Google Scholar] [CrossRef] [PubMed]
- Eilbracht, P.; Barfacker, L.; Buss, C.; Hollmann, C.; Kitsos-Rzychon, B.E.; Kranemann, C.L.; Rische, T.; Roggenbuck, R.; Schmidt, A. Tandem reaction sequences under hydroformylation conditions: new synthetic applications of transition metal catalysis. Chem. Rev. 1999, 99, 3329–3366. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Seayad, A.M.; Jackstell, R.; Beller, M. Highly selective synthesis of enamines from olefins. Angew. Chem. Int. Ed. 2003, 42, 5615–5619. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Seayad, A.M.; Jackstell, R.; Beller, M. Amines made easily: a highly selective hydroaminomethylation of olefins. J. Am. Chem. Soc. 2003, 125, 10311–10318. [Google Scholar] [CrossRef] [PubMed]
- Fogg, D.E.; dos Santos, E.N. Tandem catalysis: A taxonomy and illustrative review. Coord. Chem. Rev. 2004, 248, 2365–2379. [Google Scholar] [CrossRef]
- Melo, D.S.; Pereira-Júniora, S.S.; dos Santosa, E.N. An efficient method for the transformation of naturally occurring monoterpenes into amines through rhodium-catalyzed hydroaminomethylation. Appl. Catal. A Gen. 2012, 411–412, 70–76. [Google Scholar] [CrossRef]
- Börner, A.; Franke, R. Hydroformylation: Fundamentals, Processes, and Applications in Organic Synthesis; Wiley: Hoboken, NJ, USA, 2016; 736p. [Google Scholar]
- Graebin, C.S.; Eifler-Lima, V.L.; da Rosa, R.G. One-pot synthesis of secondary and tertiary amines from R(+)-limonene by tandem hydroformylation/reductive amination (hydroaminomethylation). Catal. Commun. 2008, 9, 1066–1070. [Google Scholar] [CrossRef]
- Behr, A.; Wintzer, A. Hydroaminomethylation of the renewable limonene with ammonia in an aqueous biphasic solvent system. Chem. Eng. Technol. 2015, 38, 2299–2304. [Google Scholar] [CrossRef]
- Faßbach, T.A.; Gaide, T.; Terhorst, M.; Behr, A.; Vorholt, A.J. Renewable surfactants through the hydroaminomethylation of terpenes. ChemCatChem 2017, 9, 1359–1362. [Google Scholar] [CrossRef]
- Oliveira, K.C.B.; Santos, A.G.; dos Santos, E.N. Hydroaminomethylation of eugenol with di-n-butylamine catalyzed by rhodium complexes: Bringing light on the promoting effect of Bronsted acids. Appl. Catal. A Gen. 2012, 445–446, 204–208. [Google Scholar] [CrossRef]
- Oliveira, K.C.B.; Carvalho, S.N.; Duarte, M.F.; Gusevskaya, E.V.; dos Santos, E.N.; Karroumi, J.E.; Gouygou, M.; Urrutigoïty, M. Phospholes as efficient ancillaries for the rhodium-catalyzed hydroformylation and hydroaminomethylation of estragole. Appl. Catal. A Gen. 2015, 497, 10–16. [Google Scholar] [CrossRef]
- Behr, A.; Reyer, S.; Manz, V. Hydroaminomethylation of isoprene: Recycling of the homogeneous rhodium catalyst in aqueous biphasic systems. Chem. Ing. Tech. 2012, 84, 108–113. [Google Scholar] [CrossRef]
- Sirol, S.; Kalck, P. Hydroformylation of optically pure monoterpenes catalyzed by dinuclear thiolato-bridged rhodium complexes. New J. Chem. 1997, 21, 1129–1137. [Google Scholar]
- Foca, C.M.; Barros, H.J.V.; dos Santos, E.N.; Gusevskaya, E.V.; Bayon, J.C. Hydroformylation of myrcene: Metal and ligand effects in the hydroformylation of conjugated dienes. New J. Chem. 2003, 27, 533–539. [Google Scholar] [CrossRef]
- Halligudi, S.B.; Bhatt, K.N.; Venkatasubramanian, K. Hydroformylation of olefins catalyzed by rhodium complex anchored on clay matrices. React. Kinet. Catal. Lett. 1993, 51, 459–464. [Google Scholar] [CrossRef]
- Barros, H.J.V.; Ospina, M.L.; Arguello, E.; Rocha, W.R.; Gusevskaya, E.V.; dos Santos, E.N. Rhodium catalyzed hydroformylation of β-pinene and camphene: Effect of phosphorous ligands and reaction conditions on diastereoselectivity. J. Organomet. Chem. 2003, 671, 150–157. [Google Scholar] [CrossRef]
- Estragol. Available online: https://en.wikipedia.org/wiki/Estragole#cite_note-2 (accessed on 28 June 2018).
- Haggin, J. Chemists seek greater recognition for catalysis. Chem. Eng. News 1993, 71, 23–27. [Google Scholar] [CrossRef]
- Beller, M.; Seavad, J.; Tillack, A.; Jiao, H. Catalytic Markovnikov and anti-Markovnikov functionalization of alkenes and alkynes: Recent developments and trends. Angew. Chem. Int. Ed. 2004, 43, 3368–3398. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Junge, K.; Beller, M. Palladium-catalysed regioselective hydroamination of 1,3-dienes: Synthesis of allylic amines. Org. Chem. Front. 2014, 1, 368–372. [Google Scholar] [CrossRef]
- Watson, I.D.G.; Yudin, A.K. New insights into the mechanism of palladium-catalyzed allylic amination. J. Am. Chem. Soc. 2005, 127, 17516–17529. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Fujita, T.; Sakamoto, M.; Haga, T.; Kuramochi, T. Palladium-catalyzed addition of dialkylamines to linalyl acetate and related compounds. J. Essent. Oil Res. 1994, 9, 441–445. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Urrutigoïty, M.; Fihri, A.; Hierso, J.-C.; Meunier, P.; Kalck, P. Efficient palladium-ferrocenylphosphine catalytic systems for allylic amination of monoterpene derivatives. Appl. Organomet. Chem. 2006, 20, 845–850. [Google Scholar] [CrossRef]
- Fihri, A.; Meunier, P.; Hierso, J.-C. Performances of symmetrical achiral ferrocenylphosphine ligands in palladium-catalyzed cross-coupling reactions: A review of syntheses, catalytic applications and structural properties. Coord. Chem. Rev. 2007, 251, 2017–2055. [Google Scholar] [CrossRef]
- Mignani, G.; Morel, D. Processes Amination of Conjugated Dienes. Patent FR 2,569,403, 23 August 1986. [Google Scholar]
- Akutagawa, S. Asymmetric synthesis by metal BINAP catalysts. Appl. Catal. A Gen. 1995, 128, 171–207. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Kumar, N.; Nieminen, V.; Sjöholm, R.; Salmi, T.; Murzin, D.Y. Cyclization of citronellal over zeolites and mesoporous materials for production of isopulegol. J. Catal. 2004, 225, 155–169. [Google Scholar] [CrossRef]
- Kumobayashi, H.; Mitsuhashi, S.; Akutagawa, S.; Ohtsuka, S. A practical synthesis of myrcenol by palladium complex-catalyzed elimination reaction. Chem. Lett. 1986, 15, 157–160. [Google Scholar] [CrossRef]
- Chalk, A.J.; Magennis, S.A.; Wertheimer, V.S.; Naipawer, R.E. Process for the Catalytic Synthesis of Conjugated Dienes from Dialkylallylamines. U.S. Patent 4,467,118, 21 August 1984. [Google Scholar]
- Chalk, A.J.; Wertheimer, V.; Magennis, S.A. A new palladium catalyzed equivalent of hofmann elimination for allylic amines. J. Mol. Catal. 1983, 19, 189–200. [Google Scholar] [CrossRef]
- Hata, G.; Tanaka, M. Terpene Hydrocarbons. JP Patent 50,123,605, 29 September 1975. [Google Scholar]
- Murata, A.; Tsuchiya, S.; Suzuki, H.; Ikeda, H. A Method for Producing of Chain Terpene Alcohols. DE Patent 2720839, 14 May 1977. [Google Scholar]
- Behr, A.; Johnen, L.; Rentmeister, N. Novel Palladium-catalysed hydroamination of myrcene and catalyst separation by thermomorphic solvent systems. Adv. Synth. Catal. 2010, 352, 2062–2072. [Google Scholar] [CrossRef]
- Hamid, M.H.S.A.; Slatford, P.A.; Williams, J.M.J. Borrowing hydrogen in the activation of alcohols. Adv. Synth. Catal. 2007, 349, 1555–1575. [Google Scholar] [CrossRef]
- Gunanathan, C.; Milstein, D. Applications of acceptorless dehydrogenation and related transformations in chemical synthesis. Science 2013, 341, 1229712. [Google Scholar] [CrossRef] [PubMed]
- Guillena, G.; Ramon, D.J.; Yus, M. Hydrogen autotransfer in the N-alkylation of amines and related compounds using alcohols and amines as electrophiles. Chem. Rev. 2010, 110, 1611–1641. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.-I.; Kon, K.; Onodera, W.; Yamazaki, H.; Kondo, J.N. Heterogeneous Ni catalyst for direct synthesis of primary amines from alcohols and ammonia. ACS Catal. 2013, 3, 112–117. [Google Scholar] [CrossRef]
- Dang, T.T.; Ramalingam, B.; Shan, S.P.; Seayad, A.M. Reductive N-Alkylation of nitro compounds to N-alkyl and N,N-dialkyl amines with glycerol as the hydrogen source. ACS Catal. 2013, 3, 2536–2540. [Google Scholar] [CrossRef]
- Murzin, D.Y. Chemical Reaction Technology; De Gruyter: Berlin, Germany, 2015; 428p. [Google Scholar]
- Ma, X.; Su, C.; Xu, Q. N-Alkylation by hydrogen autotransfer reactions. In Hydrogen Transfer Reactions: Reductions and Beyond; Guillena, G., Ramon, D.J., Eds.; Springer International Publishing: Basel, Switzerland, 2016; pp. 291–364. [Google Scholar]
- Imm, S.; Bahn, S.; Neubert, L.; Neumann, H.; Beller, M. An efficient and general synthesis of primary amines by ruthenium catalyzed amination of secondary alcohols with ammonia. Angew. Chem. Int. Ed. 2010, 49, 8126–8129. [Google Scholar] [CrossRef] [PubMed]
- Imm, S.; Bahn, S.; Zhang, M.; Neubert, L.; Neumann, H.; Klasovsky, F.; Pfeffer, J.; Haas, T.; Beller, M. Improved ruthenium-catalyzed amination of alcohols with ammonia: Synthesis of diamines and amino esters. Angew. Chem. Int. Ed. 2011, 50, 7599–7603. [Google Scholar] [CrossRef] [PubMed]
- Lamb, G.W.; Williams, J.M.J. Borrowing hydrogen-C-N bond formation from alcohols. Chim. Oggi-Chem. Today 2008, 26, 17–19. [Google Scholar]
- Pingen, D.; Muller, C.; Vogt, D. Direct amination of secondary alcohols using ammonia. Angew. Chem. Int. Ed. 2010, 49, 8130–8133. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Navas, J.; Sabater, M.J. Advances in one-pot synthesis through borrowing hydrogen catalysis. Chem. Rev. 2018, 118, 1410–1459. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.J.A.; Maxwell, A.C.; Williams, J.M.J. Borrowing hydrogen methodology for amine synthesis under solvent-free microwave conditions. J. Org. Chem. 2011, 76, 2328–2331. [Google Scholar] [CrossRef] [PubMed]
- Saidi, O.; Blacker, A.J.; Farah, M.M.; Marsden, S.P.; Williams, J.M.J. Iridium-catalysed amine alkylation with alcohols in water. Chem. Commun. 2010, 46, 1541–1543. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, R.; Fujita, K.; Yamaguchi, R. N-Alkylation of Amines with Alcohols catalyzed by a water soluble Cp*Iridium complex: An efficient method for the synthesis of amines in aqueous media. Adv. Synth. Catal. 2011, 353, 1161–1168. [Google Scholar] [CrossRef]
- Hollmann, D.; Tillack, A.; Michalik, D.; Jackstell, R.; Beller, M. An improved ruthenium catalyst for the environmentally benign amination of primary and secondary alcohols. Chem. Asian J. 2007, 2, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Asencio, A.; Ramon, D.J.; Yus, M. N-alkylation of poor nucleophilic amines and derivatives with alcohols by a hydrogen autotransfer process catalyzed by copper (II) acetate: Scope and mechanistic considerations. Tetrahedron 2011, 67, 3140–3149. [Google Scholar] [CrossRef]
- Martinez-Asencio, A.; Yus, M.; Ramon, D.J. Palladium (II) acetate as a catalyst for the N-alkylation of aromatic amines, sulfonamides and related nitrogenated compounds with alcohols by a hydrogen autotransfer process. Synthesis 2011, 3730–3740. [Google Scholar] [CrossRef]
- Blank, B.; Kempe, R. Catalytic alkylation of methyl-N-heteroaromatics with alcohols. J. Am. Chem. Soc. 2010, 132, 924–925. [Google Scholar] [CrossRef] [PubMed]
- Michlik, S.; Hille, T.; Kempe, R. The Iridium-catalyzed synthesis of symmetrically and unsymmetrically alkylated diamines under mild reaction conditions. Adv. Synth. Catal. 2012, 354, 847–862. [Google Scholar] [CrossRef]
- Demidova, Y.S.; Simakova, I.L.; Estrada, M.; Beloshapkin, S.; Suslov, E.V.; Korchagina, D.V.; Volcho, K.P.; Salakhutdinov, N.F.; Simakov, A.V.; Murzin, D.Y. One-pot myrtenol amination over Au nanoparticles supported on different metal oxides. Appl. Catal. A Gen. 2013, 464–465, 348–356. [Google Scholar] [CrossRef]
- Houssame, S.E.; Anane, H.; Firdoussi, L.E.; Karim, A. Palladium(0)-catalyzed amination of allylic natural terpenic functionalized olefins. Cent. Eur. J. Chem. 2008, 6, 470–476. [Google Scholar] [CrossRef] [Green Version]
- Pingen, D.; Diebolt, O.; Vogt, D. Direct amination of bio-alcohols using ammonia. ChemCatChem 2013, 5, 2905–2912. [Google Scholar] [CrossRef]
- Valkenberg, M.H.; Holderich, W.F. Preparation and use of hybrid organic–inorganic catalysts. Catal. Rev. 2002, 44, 321–374. [Google Scholar] [CrossRef]
- Huang, X.; Wu, H.; Liao, X.P.; Shi, B. Liquid phase hydrogenation of olefins using heterogenized ruthenium complexes as high active and reusable catalyst. Catal. Commun. 2010, 11, 487–492. [Google Scholar] [CrossRef]
- Cao, Y.; Hu, J.C.; Yang, P.; Dai, W.L.; Fan, K.N. CuCl catalyst heterogenized on diamide immobilized SBA-15 for efficient oxidative carbonylation of methanol to dimethylcarbonate. Chem. Commun. 2003, 908–909. [Google Scholar] [CrossRef]
- Mukhopadhyay, K.; Chaudhari, R.V. Heterogenized HRh(CO)(PPh3)3 on zeolite Y using phosphotungstic acid as tethering agent: A novel hydroformylation catalyst. J. Catal. 2003, 213, 73–77. [Google Scholar] [CrossRef]
- Johnson, B.F.G.; Raynor, S.A.; Shephard, D.S.; Mashmeyer, T.; Thomas, J.M.; Sankar, G.; Bromley, S.; Oldroyd, R.; Gladden, L.; Mantle, M.D. Superior performance of a chiral catalyst confined within mesoporous silica. Chem. Commun. 1999, 1167–1168. [Google Scholar] [CrossRef]
- Dyal, A.; Loos, K.; Noto, M.; Chang, S.W.; Spagnoli, C.; Shafi, K.V.P.M.; Ulman, A.; Cowman, M.; Gross, R.A. Activity of Candida rugose lipase immobilized on γ-Fe2O3 Magnetic Nanoparticles. J. Am. Chem. Soc. 2003, 125, 1684–1685. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, Y.; Wang, D.I.C.; Li, Z. Recyclable nanobiocatalyst for enantioselective sulfoxidation: Facile fabrication and high performance of chloroperoxidase-coated magnetic nanoparticles with iron oxide core and polymer shell. J. Am. Chem. Soc. 2009, 131, 12892–12893. [Google Scholar] [CrossRef] [PubMed]
- Shylesh, S.; Schuenemann, V.; Thiel, W.R. Magnetically separable nanocatalysts: Bridges between homogeneous and heterogeneous catalysis. Angew. Chem. Int. Ed. 2010, 49, 3428–3459. [Google Scholar] [CrossRef] [PubMed]
- Campelo, M.; Luna, D.; Luque, R.; Marinas, J.M.; Romero, A.A. Sustainable preparation of supported metal nanoparticles and their applications in catalysis. ChemSusChem 2009, 2, 18–45. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Tse, M.; Zhou, S.; Pohl, M.-M.; Radnik, J.; Hubner, S.; Jahnisch, K.; Bruckner, A.; Beller, M. Green and efficient synthesis of sulfonamides catalyzed by nano-Ru/Fe3O4. J. Am. Chem. Soc. 2009, 131, 1775–1779. [Google Scholar] [CrossRef] [PubMed]
- Demidova, Y.S.; Simakova, I.L.; Warne, J.; Simakov, A.; Murzin, D.Y. Kinetic modeling of one-pot myrtenol amination over Au/ZrO2 catalyst. Chem. Eng. J. 2014, 238, 164–171. [Google Scholar] [CrossRef]
- Simakova, I.L.; Demidova, Y.S.; Estrada, M.; Beloshapkin, S.; Suslov, E.V.; Volcho, K.P.; Salakhutdinov, N.F.; Murzin, D.Y.; Simakov, A. Gold catalyzed one-pot myrtenol amination: Effect of catalyst redox activation. Catal. Today 2017, 279, 63–70. [Google Scholar] [CrossRef]
- Demidova, Y.S.; Suslov, E.V.; Simakova, I.L.; Korchagina, D.V.; Mozhajcev, E.S.; Volcho, K.P.; Salakhutdinov, N.F.; Simakov, A.; Murzin, D.Y. Selectivity control in one-pot amination of Au/ZrO2 by molecular hydrogen addition. J. Mol. Catal. A Chem. 2017, 426, 60–67. [Google Scholar] [CrossRef]
- Demidova, Y.S.; Suslov, E.V.; Simakova, I.L.; Volcho, K.P.; Salakhutdinov, N.F.; Simakov, A.; Murzin, D.Y. Promoting effect of alcohols and formic acid on Au-catalyzed one-pot alcohol amination. Mol. Catal. 2017, 433, 414–419. [Google Scholar] [CrossRef]
- Demidova, Y.S.; Suslov, E.V.; Simakova, I.L.; Korchagina, D.V.; Mozhajcev, E.S.; Volcho, K.P.; Salakhutdinov, N.F.; Simakov, A.; Murzin, D.Y. One-pot monoterpene alcohol amination over Au/ZrO2 catalyst: Effect of the substrates structure. J. Catal. 2018, 360, 127–134. [Google Scholar] [CrossRef]
- Cherng, Y.-J.; Fang, J.-M.; Lu, T.-J. A new pinane-type tridentate modifier for asymmetric reduction of ketones with lithium aluminum hydride. Tetrahedron Asymmetry 1995, 6, 89–92. [Google Scholar] [CrossRef]
- Ishida, T.; Takamura, R.; Takei, T.; Akita, T.; Haruta, M. Support effects of metal oxides on gold-catalyzed one-pot N-alkylation of amine with alcohol. Appl. Catal. A Gen. 2012, 413–414, 261–266. [Google Scholar] [CrossRef]
- Lescot, C.; Darses, B.; Collet, F.; Retailleau, P.; Dauban, P. Intermolecular C–H amination of complex molecules: Insights into the factors governing the selectivity. J. Org. Chem. 2012, 77, 7232–7240. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; van Vranken, D.L. Asymmetric transition metal-catalyzed allylic alkylations. Chem. Rev. 1996, 96, 395–422. [Google Scholar] [CrossRef] [PubMed]
- Butt, N.A.; Zhang, W. Transition metal-catalyzed allylic substitution reactions with unactivated allylic substrates. Chem. Soc. Rev. 2015, 44, 7929–7967. [Google Scholar] [CrossRef] [PubMed]
- Piechaczyk, O.; Thoumazet, C.; Jean, Y.; Le Floch, P. DFT study on the palladium-catalyzed allylation of primary amines by allylic alcohol. J. Am. Chem. Soc. 2006, 128, 14306–14317. [Google Scholar] [CrossRef] [PubMed]



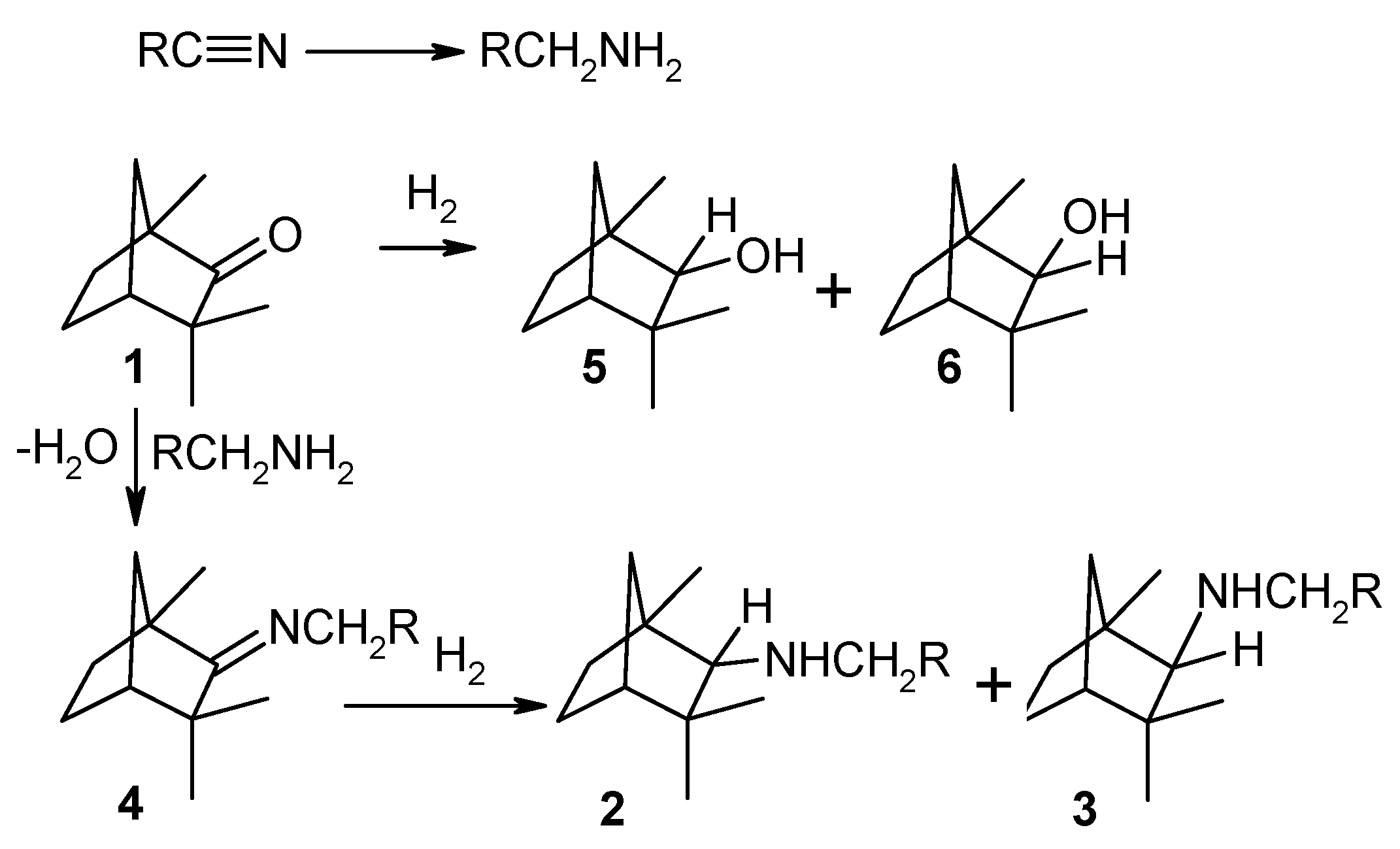








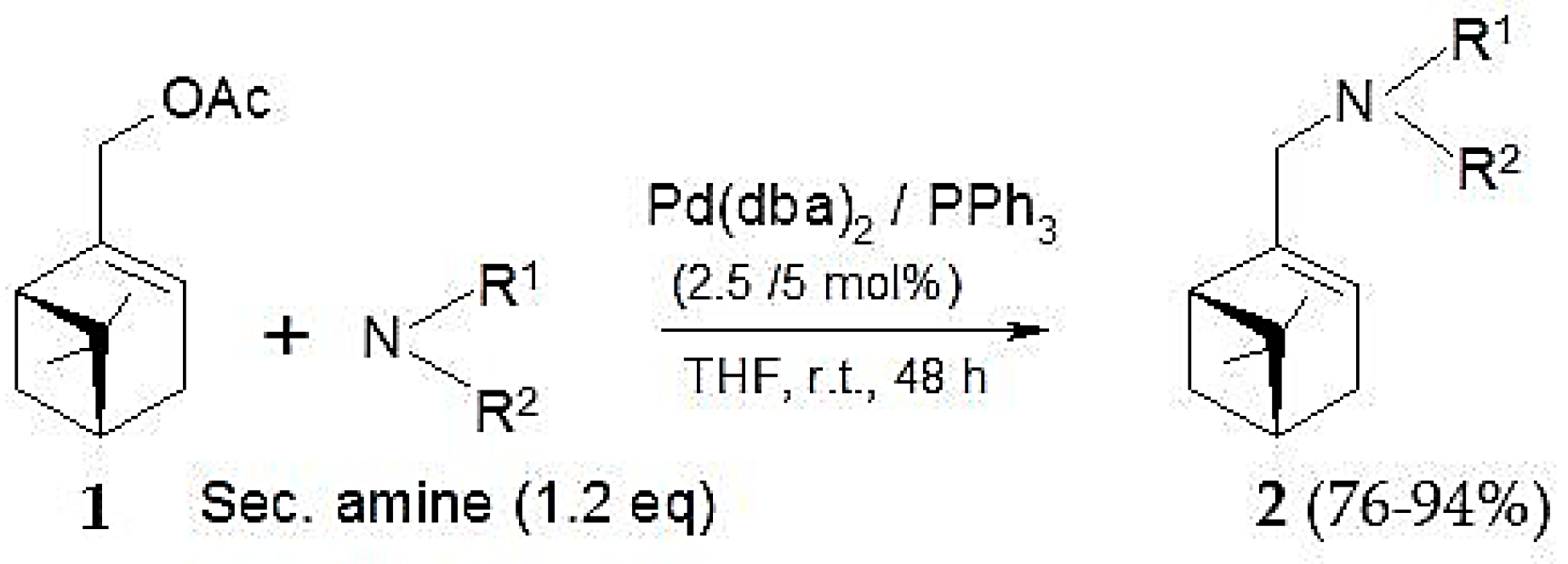





 ), myrtanal (►), 1 (●), 2(▲), 3 (
), myrtanal (►), 1 (●), 2(▲), 3 (  ), 4 (▼). The reaction conditions: T = 180 °C, myrtenol 1 mmol, aniline 1 mmol, toluene 10 mL, catalyst 1.4 mol% Au. Reprinted from [137] with permission from Elsevier.
), 4 (▼). The reaction conditions: T = 180 °C, myrtenol 1 mmol, aniline 1 mmol, toluene 10 mL, catalyst 1.4 mol% Au. Reprinted from [137] with permission from Elsevier.
 ), myrtanal (►), 1 (●), 2(▲), 3 (
), myrtanal (►), 1 (●), 2(▲), 3 (  ), 4 (▼). The reaction conditions: T = 180 °C, myrtenol 1 mmol, aniline 1 mmol, toluene 10 mL, catalyst 1.4 mol% Au. Reprinted from [137] with permission from Elsevier.
), 4 (▼). The reaction conditions: T = 180 °C, myrtenol 1 mmol, aniline 1 mmol, toluene 10 mL, catalyst 1.4 mol% Au. Reprinted from [137] with permission from Elsevier.
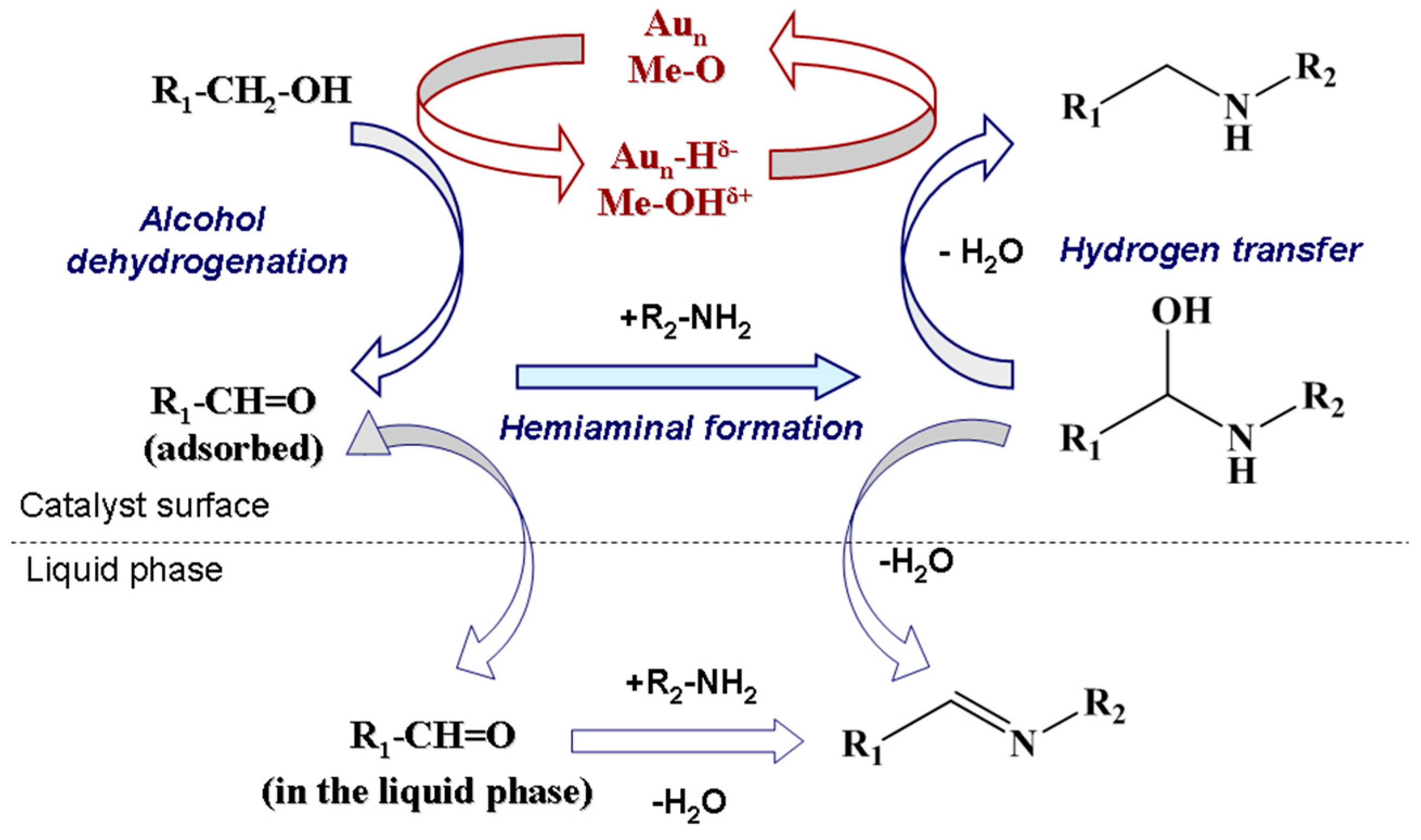


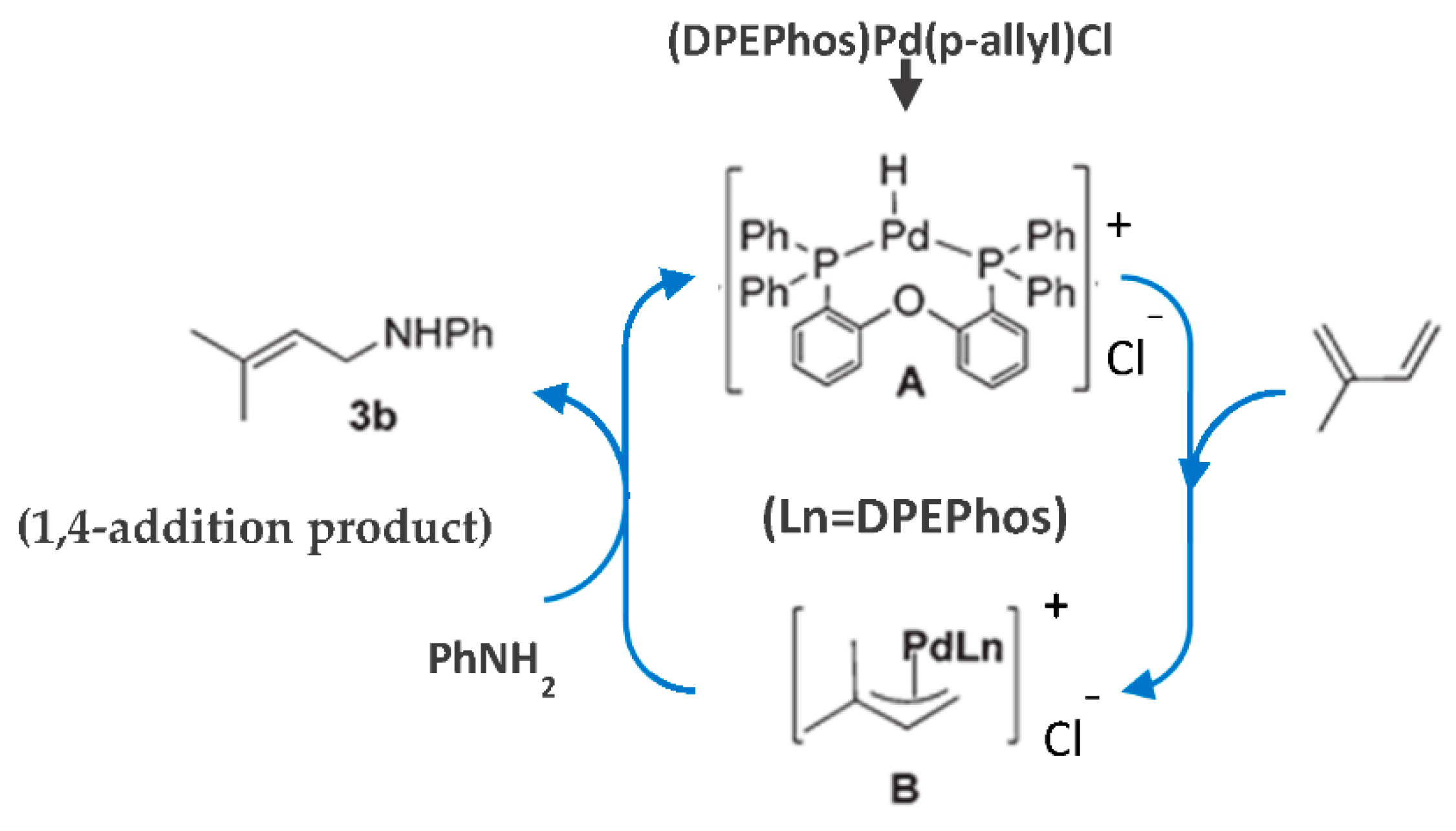
| Ligand | P/Rh b | Con. (%) | Product Distribution (%) | Regioselectivity (%) c | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | Aldehydes | Enamines | Amines | 9 | 10 | 11 | |||
| None | 0 | 100 d | 4 | 1 | 0 | 90 | 61 | 33 | 6 |
| PPh3 | 2 | 100 | 0 | 24 | 3 | 73 | 96 | 4 | 0 |
| PPh3 | 10 | 100 | 0 | 32 | 10 | 58 | 94 | 5 | 1 |
| NAPHOS | 2 | 34 | 3 | 34 | 56 | 7 | >99 | 0 | 0 |
| NAPHOS e | 2 | 100 | 10 | 21 | 5 | 64 | 97 | 3 | 0 |
| Ligand | P/Rh b | Acid | T (°C) | Con. (%) | Product Distribution (%) | Regioselectivity (%) c | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | Aldehydes | Enamines | Amines | 9 | 10 | 11 | |||||
| PPh3 | 2 | None | 120 | 100 d | 0 | 17 | 0 | 83 | 96 | 4 | 0 |
| PPh3 | 2 | H2SO4 d | 120 | 86 | 13 | 76 | 9 | 2 | 94 | 6 | 0 |
| PPh3 | 2 | HOTs d | 120 | 100 | 0 | 23 | 0 | 75 | 89 | 11 | 0 |
| PPh3 | 2 | HOTf d | 120 | 100 | 1 | 11 | 0 | 88 | 84 | 14 | 2 |
| NAPHOS | 2 | HOTf e | 100 | 100 | 5 | 9 | 0 | 86 | 99 | 1 | 0 |
| NAPHOS | 20 | HOTf e | 100 | 100 | 2 | 5 | 0 | 93 | 99 | 1 | 0 |
| NAPHOS | 40 | HOTf e | 100 | 100 | 2 | 3 | 0 | 95 | 98 | 2 | 0 |
| Catalyst/Conditions a | Amine | Geranylamine Selectivity (%) | Amine Yield (%) | TON c | TOF d (h−1) |
|---|---|---|---|---|---|
| 20.0 mol% BuLi, 50 °C, 4 h | HNEt2 | 95 | 77–83 | 4 | 1.04 |
| 33.3 mol% Na/16.7 mol% naphthalene, 20 °C, 1 h | HNEt2 | 80 | 53 | 2 | 1.60 |
| 36.0 mol% Li, 50 °C, 20 h | HNiPr2 | n.m. | 80 | 2 | 0.11 |
| 100 mol% Li, 50 mol% naphthalene, | HNEt2, morpholine, pyrrolidine, piperidine, HNnBu2 | geranylamine | 72 b | 1 b | 0.18 b |
| 100 mol% TMDAP, 20 °C, 4 h | 88 b | ||||
| 10 mol% BuLi, 50 °C, 2.5 h | HNMe2 | 88 | 79 | 8 | 3.18 |
| 1.7 mol% Na, 50 °C, 4 h | HNMe2, HNEt2, HNiPr2, HNnBu2 | 90 b | 83 b | 49 b | 12.21 b |
| 3.6 mol% Li, 25 °C, 72 h | HNMe2, HNEt2, HNiPr2, HNnBu2, piperidine, 2-methylpi- | 96 b | 87 b | 24 b | 0.34 b |
| peridine, 2,6-dimethyl-piperidine | |||||
| 36.0 mol% Li, 55 °C, 5 h | HNEt2 | 92 | 74–77 | 2 | 0.43 |
| 15.0 mol% BuLi, 55 °C, 12 h | HNEt2 | 95 | 85 | 6 | 0.47 |
| 0.1 mol% [RhCl(cod)]2, 2.3 mol% TPPTS, 100 °C, 21.5 h (biphasic) | morpholine | 53 | 59 | 590 | 27.44 |
| 0.2 mol% Pd(CF3CO2)2, 1.6 mol% DPPB, 100 °C, 4 h | morpholine | 57 | 98 | 490 | 123.50 |
| Substrate | Time (h) | T (°C) | Conversion b (%) | Selectivity c,d (%) | Ketones (%) | Product |
|---|---|---|---|---|---|---|
Menthol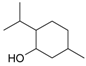 | 21 | 150 | 25.3 | 18.0 | 82 | Menthylamine |
| 21 | 170 | 31.6 | 70.8 | 29.2 | ||
| 63 | 150 | 32.7 | 24.3 | 75.7 | ||
Carveol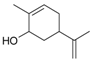 | 21 | 150 | 67.3 | 74.7 | Carvylamine | |
| 21 | 170 | 99.1 | 55.2 | |||
| 65.5 | 150 | 82.0 | 56 | |||
| 42 | 170 | 99.3 | 50.4 | |||
Verbenol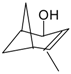 | 21 | 150 | 93.5 | 39.7 | 44.2 | Verbenylamine |
| 21 | 170 | 96.4 | 27.7 | 62.2 | ||
| 63 | 170 | 98.8 | 25.0 | 47.7 | ||
Borneol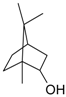 | 21 | 150 | 58.5 | 73.2 | Bornylamine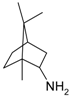 | |
| 21 | 170 | 76.8 | 83.9 | |||
| 65.5 | 150 | 72.2 | 78.7 | |||
| 63 | 170 | 79.1 | 81.2 | |||
Fenchol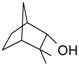 | 21 | 150 | 50.6 | 0 | 34.8 | Fenchylamine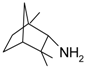 |
| 21 | 170 | 59.2 | 0 | 46.0 | ||
| 63 | 150 | 54.5 | 0 | 38.2 | ||
| 117 | 170 | 63.9 | 0 | 44.3 |
 .
.| Substrate | Time (h) | T (°C) | Conversion b (%) | Selectivity c,d (%) | Product |
|---|---|---|---|---|---|
Citronellol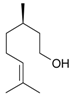 | 21 | 150 | 33.8 | 64.8 | Citronellylamine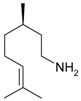 |
| 21 | 170 | 47.4 | 61.2 | ||
| 63 | 150 | 47.9 | 71.4 | ||
| 152 | 170 | 82.9 | 65.9 | ||
Geraniol | 21 | 150 | 98.9 | 79.9 | Geranylamine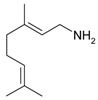 |
| 21 | 170 | 99.3 | 71 | ||
| 63 | 150 | 99.7 | 70.3 | ||
| 42 | 170 | 99.4 | 67.4 | ||
Nerol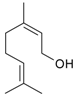 | 21 | 150 | 66.7 | 92.9 | Nerylamine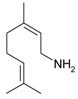 |
| 21 | 170 | 85.5 | 75.1 | ||
| 120 | 150 | 85.8 | 87 | ||
| 49 | 170 | 96 | 69.8 | ||
Farnesol | 21 | 150 | 83.8 | 77.4 | Farnesylamine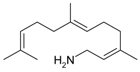 |
| 21 | 170 | 94.8 | 73.8 | ||
| 95 | 150 | 93,2 | 72.5 | ||
| 45 | 170 | 99.4 | 76.1 |
| Amine | Solvent | Ligand | T (°C) | Molar Ratio Amine/(3) | Yield of (4) (%) a | E:Z |
|---|---|---|---|---|---|---|
| (C2H5)2NH | THF | PPh3 | 20 | 2.0 | 40 | 60:40 |
| (C2H5)2NH | THF | PPh3 | 50 | 2.0 | 14 | 62:38 |
| (C2H5)2NH | IPE | PPh3 | 20 | 2.0 | 48 | 60:40 |
| (C2H5)2NH | IPE | PPh3 | 50 | 2.0 | 8 | 62:38 |
| (C2H5)2NH | Benzene | PPh3 | 20 | 2.0 | 35 | 76:24 |
| (C2H5)2NH | DMF | PPh3 | 20 | 2.0 | 70 | 52:48 |
| (C2H5)2NH | CH2Cl2 | PPh3 | 20 | 2.0 | 52 | 54:46 |
| (C2H5)2NH | (C2H5)2NH | PPh3 | 20 | Excess | 57 | 59:41 |
| Amine | Solvent | Ligand | T (°C) | Molar Ratio Amine/(5) | Yield of (2) (%) a | E:Z |
|---|---|---|---|---|---|---|
| (C2H5)2NH | THF | PPh3 | 20 | 2.0 | 20 | 91:9 |
| (C2H5)2NH | IPE | PPh3 | 20 | 2.0 | 21 | 92:8 |
| (C2H5)2NH | Benzene | PPh3 | 20 | 2.0 | 15 | 94:6 |
| (C2H5)2NH | DMF | PPh3 | 20 | 2.0 | 42 | 86:14 |
| (C2H5)2NH | 1,2-C2H4Cl2 | PPh3 | 20 | 2.0 | 30 | 86:14 |
| (C2H5)2NH | (C2H5)2NH | PPh3 | 20 | Excess | 48 | 91:9 |
| Aniline | THF | PPh3 | 20 | 2.0 | 52 | 100:0 b |
| Aniline | IPE | PPh3 | 20 | 2.0 | 60 | 100:0 |
| N-Me-Aniline | THF | PPh3 | 20 | 2.0 | 50 | 100:0 c |
| N-Me-Aniline | IPE | PPh3 | 20 | 2.0 | 59 | 100:0 |
| Morpholine | THF | PPh3 | 20 | 2.0 | 47 | 100:0 d |
| Morpholine | IPE | PPh3 | 20 | 2.0 | 45 | 100:0 |
| Pyrrolidine | THF | PPh3 | 20 | 2.0 | 32 | 85:15 e |
| Pyrrolidine | IPE | PPh3 | 20 | 2.0 | 49 | 94:6 |
| Secondary Amine | Product b | [a]D c | With 2.5 Equiv of Et3N | Without Et3N | ||
|---|---|---|---|---|---|---|
| Yield d (%) | Conv. (%) | Yield d (%) | Conv. e (%) | |||
| Pyrrolidine | 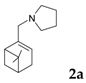 | −4.0 | 86 | 94 | 76 | 93 |
| Morpholine | 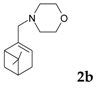 | −18.4 | 90 | 96 | 91 | 95 |
| Me2NH |  | −3.5 | 94 | 100 | 92 | 96 |
| Ligand b | Yield c (%) | Conversion d (%) |
|---|---|---|
| None | - | 2 |
| PPh3 | 90 | 96 |
| dppe | 88 | 94 |
| P(o-tolyl)3 | 13 | 22 |
| 2,2′-dipyridyl | 5 | 14 |
| Terpenic Allylic Esters | Secondary Amine | Product | Yield b (%) | Conv c (%) |
|---|---|---|---|---|
| Allyl acetate | ||||
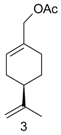 | Pyrrolidine | 4a | 48 | 60 |
| Morpholine | 4b | 48 | 56 | |
| Me2NH | 4c | 54 | 62 | |
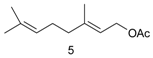 | Pyrrolidine | 6a | 39 | 58 |
| Morpholine | 6b | 40 | 56 | |
| Me2NH | 6c | 42 | 62 | |
| Allyl carbonate | ||||
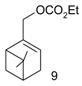 | Pyrrolidine | 2a | 94 | 96 |
| Morpholine | 2b | 94 | 96 | |
| Me2NH | 2c | 96 | 98 | |
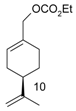 | Pyrrolidine | 4a | 50 | 68 |
| Morpholine | 4b | 52 | 62 | |
| Me2NH | 4c | 62 | 68 | |
 | Pyrrolidine | 6a | 43 | 64 |
| Morpholine | 6b | 44 | 62 | |
| Me2NH | 6c | 47 | 66 | |
| Conditions | trans-(%) | cis-(%) | de(%) |
|---|---|---|---|
| 1 bar H2, 8 bar N2, 180 °C | 65 | 35 | 30 |
| 2 bar H2, 7 bar N2, 180 °C | 62 | 38 | 24 |
| 9 bar N2, 180 °C/1 bar H2, 8 bar N2, 180 °C | 80 | 20 | 60 |
| Alcohol | R a (mol∙L−1∙h−1) | Time (h) | Alcohol Conversion (%) | Selectivity (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Sec. Amine | Imine | Aldehyde b | ||||||
| 1 c | 2 d | 1 c | 2 d | |||||
| Myrtenol | 2.1 × 10−2 | 2 | 44 | 39 | 5 | 50 | 3 | 0 |
| 8 | 87 | 52 | 6 | 34 | 2 | 0 | ||
| 16 | 98 | 53 | 7 | 33 | 2 | 0 | ||
| Nopol | 2.4 × 10−3 | 8 | 10 | 74 | 0 | 22 | 0 | 0 |
| 16 | 40 | 76 | 0 | 19 | 0 | 0 | ||
| Perillyl alcohol | 3.1 × 10−2 | 2 | 70 | Complicated mixture of the products | ||||
| 8 | 99 | |||||||
| Perillyl alcohol e | 1.3 × 10−2 | 8 | 98 | 1 | 19 | 28 | 11 | 2 |
| Amine | R a (mol∙L−1∙h−1) | Time (h) | Alcohol Conversion (%) | Selectivity (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Sec. Amine | Imine | Myrtenal/Myrtanal | ||||||
| 1 b | 2 c | 1 b | 2 c | |||||
| Aniline | 2.1 × 10−2 | 2 | 44 | 39 | 5 | 50 | 3 | 0/0 |
| 8 | 87 | 52 | 6 | 34 | 2 | 0/0 | ||
| 16 | 98 | 53 | 7 | 33 | 2 | 0/0 | ||
| Aniline d | 2.0 × 10−2 | 16 | 98 | 69 | 9 | 10 | 2 | 5/2 |
| 4-Methylaniline | 1.6 × 10−2 | 2 | 34 | 19 | 1 | 73 | 0 | 4/9 × 10−1 |
| 8 | 89 | 37 | 1 | 53 | 0 | 8/2 | ||
| 16 | 98 | 43 | 2 | 49 | 0 | 5/1 | ||
| 4-Methylaniline e | 1.4 × 10−2 | 16 | 95 | 51 | 4 | 30 | 2 | 7/2 |
| 4-Bromoaniline | 3.4 × 10−2 | 2 | 60 | 0 | 14 | 0 | 43 | 14/0 |
| 8 | 99 | 0 | 22 | 0 | 49 | 13/0 | ||
| Benzylamine | 2.3 × 10−2 | 2 | 48 | 36 | 13 | 26 | 14 | 1 × 10−1/0 |
| 8 | 98 | 3 | 35 | 0 | 49 | 0/0 | ||
| Phenethylamine | 3.3 × 10−3 | 8 | 40 | 63 | 0 | 36 | 0 | 0/0 |
| 16 | 53 | 51 | 0 | 47 | 0 | 0/0 | ||
| 24 | 70 | 46 | 1 | 52 | 0 | 0/0 | ||
| Phenethylamine e | 3.0 × 10−3 | 16 | 48 | 69 | 3 | 20 | 0 | 3/2 |
| 3,4-Dimethoxy-phenethylamine | 7.0 × 10−4 | 16 | 13 | 72 | 0 | 27 | 0 | 0/0 |
| 3,4-Dimethoxy-phenethylamine e | 6.9 × 10−4 | 16 | 12 | 80 | 0 | 15 | 0 | 2/1 |
| 3-Aminopyridine | 1.9 × 10−3 | 8 | 19 | 21 | 0 | 74 | 0 | 2/1 |
| 16 | 31 | 20 | 1 | 60 | 0 | 11/3 | ||
| 24 | 44 | 23 | 2 | 58 | 1 | 12/3 | ||
| 3-Aminopyridine e | 2.0 × 10−3 | 16 | 33 | 30 | 2 | 49 | 0 | 14/2 |

| Product | Yield | d.r. b | Product | Yield | d.r. b |
|---|---|---|---|---|---|
 | 91 | 39:1 | 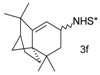 | 85 | |
 | 71 | 49:1 | 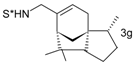 | 34(63) c | |
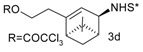
 | 73 | 49:1 | 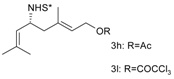 | 90 (3h) 89 (3I) | 19:1 >20:1 |
 | 98 | >20:1 | 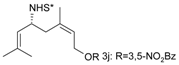 | 81 | 5:1 |
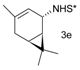 | 27 | 10:1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
L. Simakova, I.; V. Simakov, A.; Yu. Murzin, D. Valorization of Biomass Derived Terpene Compounds by Catalytic Amination. Catalysts 2018, 8, 365. https://doi.org/10.3390/catal8090365
L. Simakova I, V. Simakov A, Yu. Murzin D. Valorization of Biomass Derived Terpene Compounds by Catalytic Amination. Catalysts. 2018; 8(9):365. https://doi.org/10.3390/catal8090365
Chicago/Turabian StyleL. Simakova, Irina, Andrey V. Simakov, and Dmitry Yu. Murzin. 2018. "Valorization of Biomass Derived Terpene Compounds by Catalytic Amination" Catalysts 8, no. 9: 365. https://doi.org/10.3390/catal8090365