Review of Anodic Catalysts for SO2 Depolarized Electrolysis for “Green Hydrogen” Production
Abstract
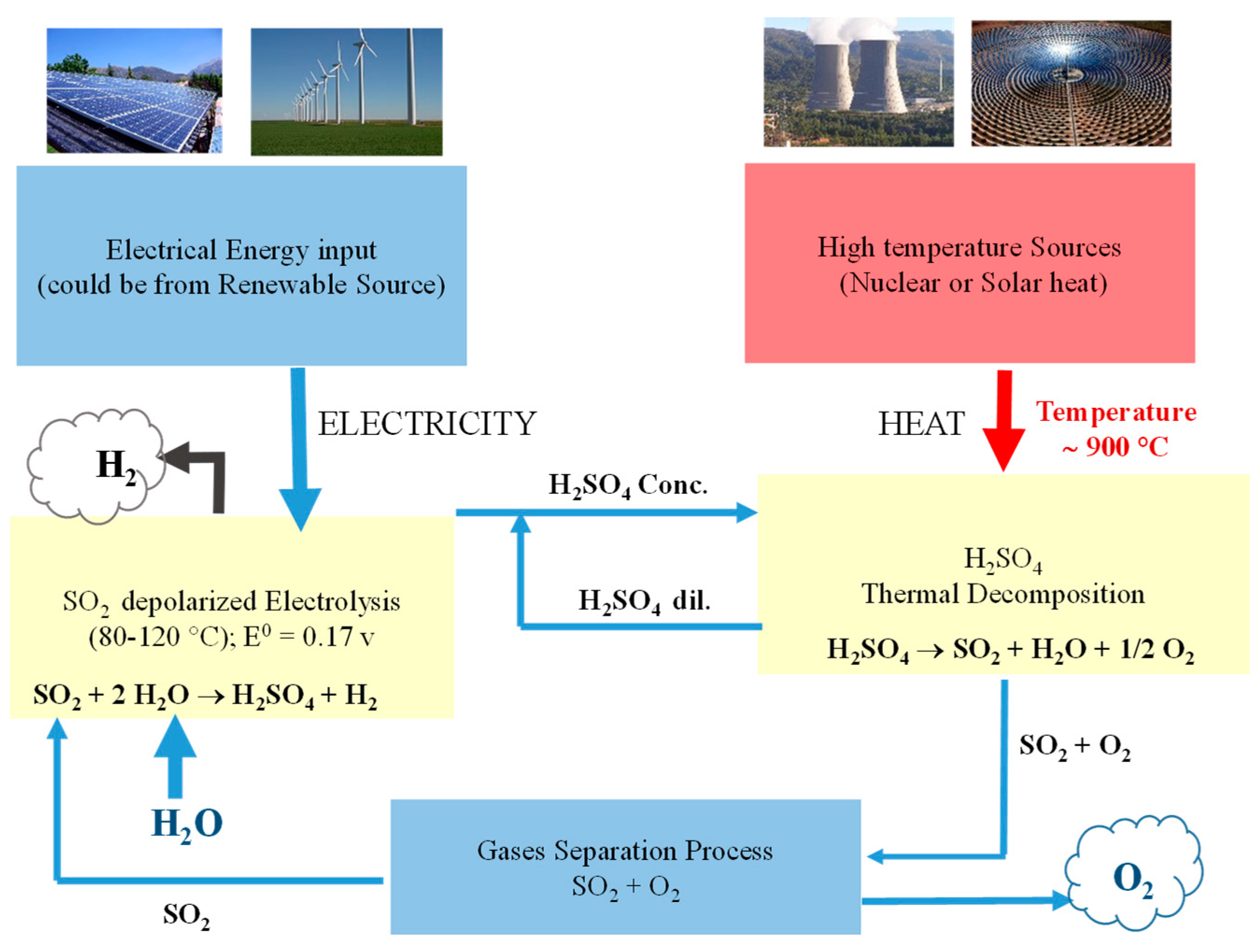
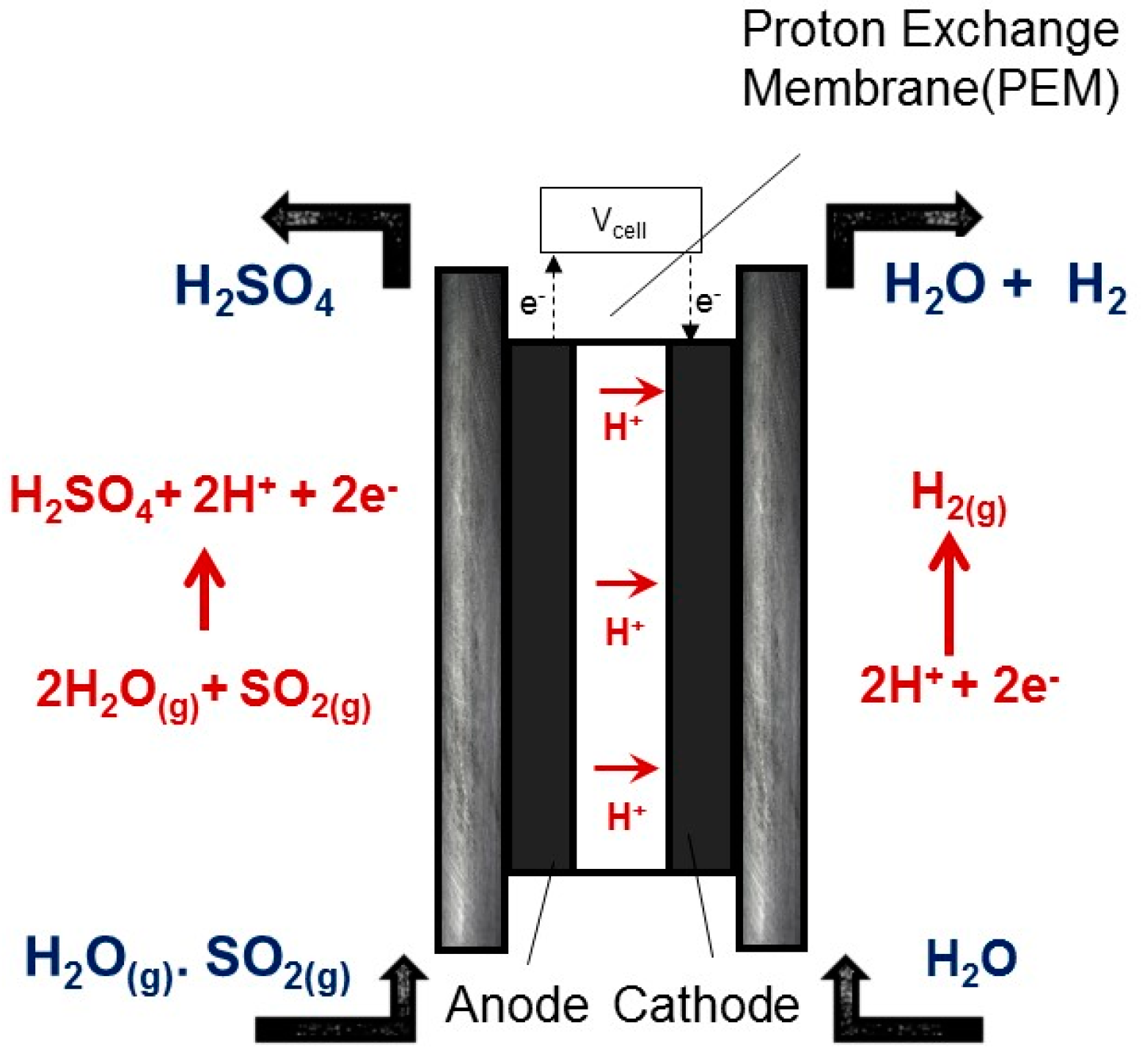
1. Introduction
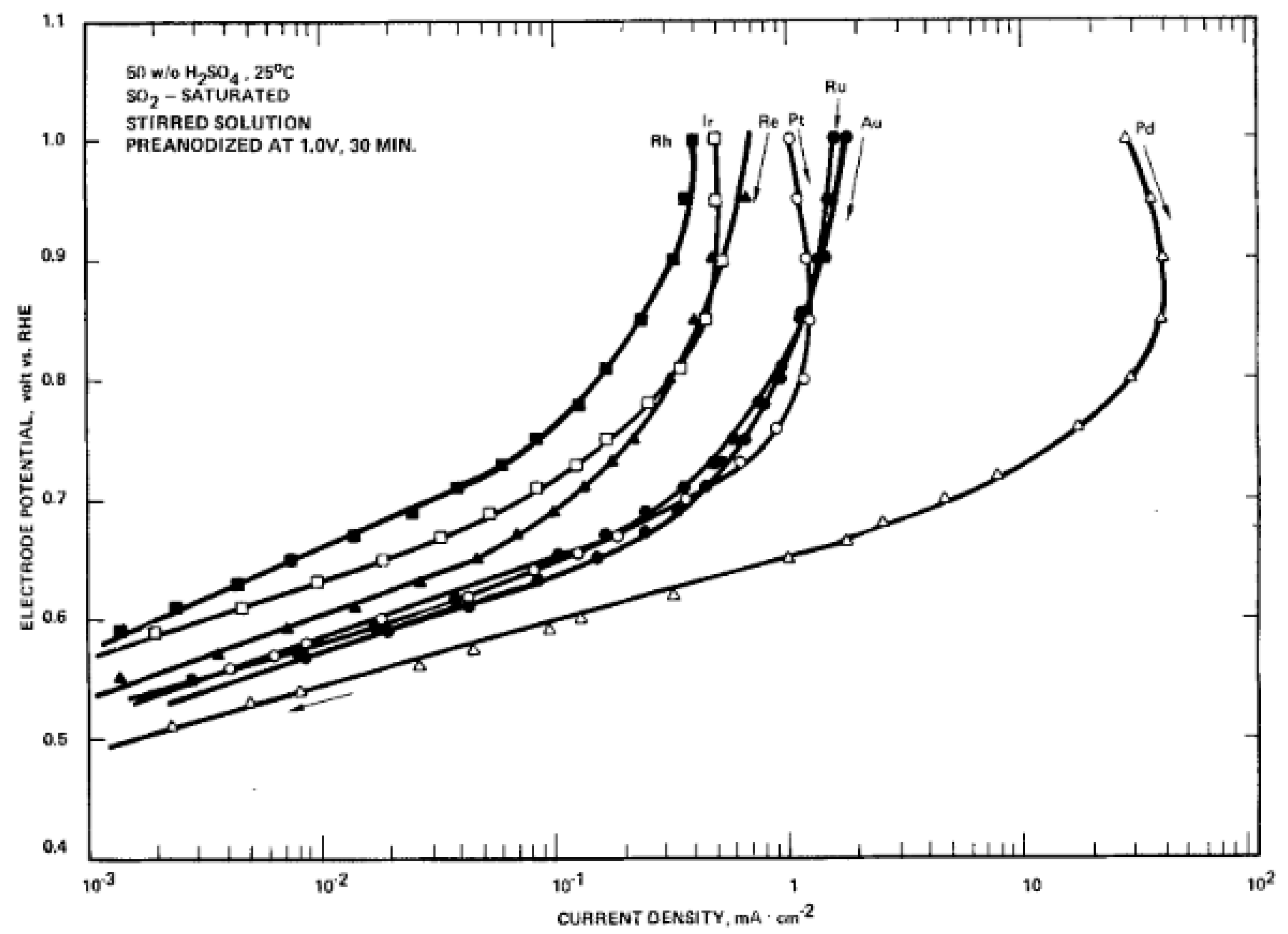
2. Platinum-Based Catalysts
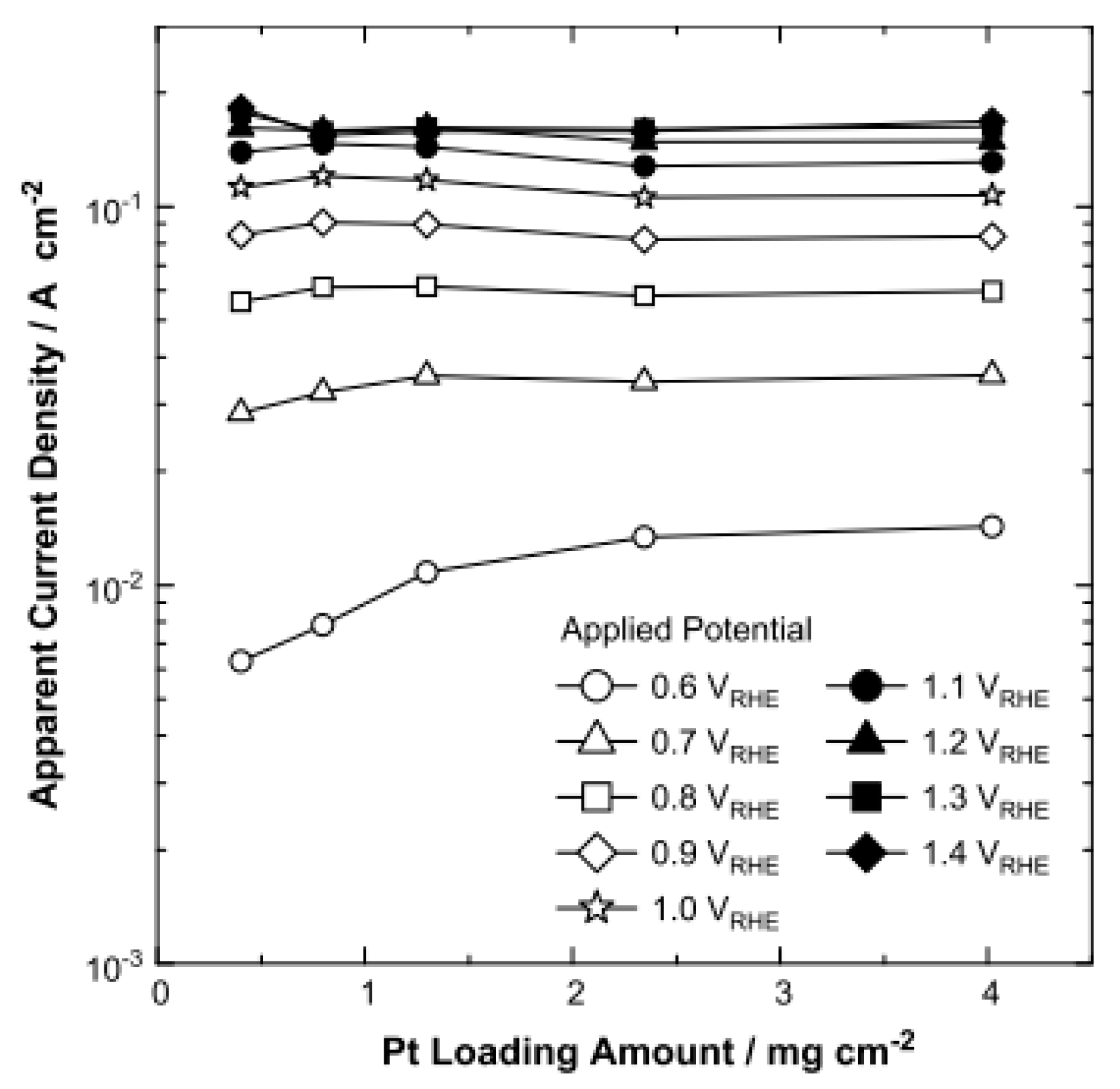
2.1. Effect of Platinum Loading
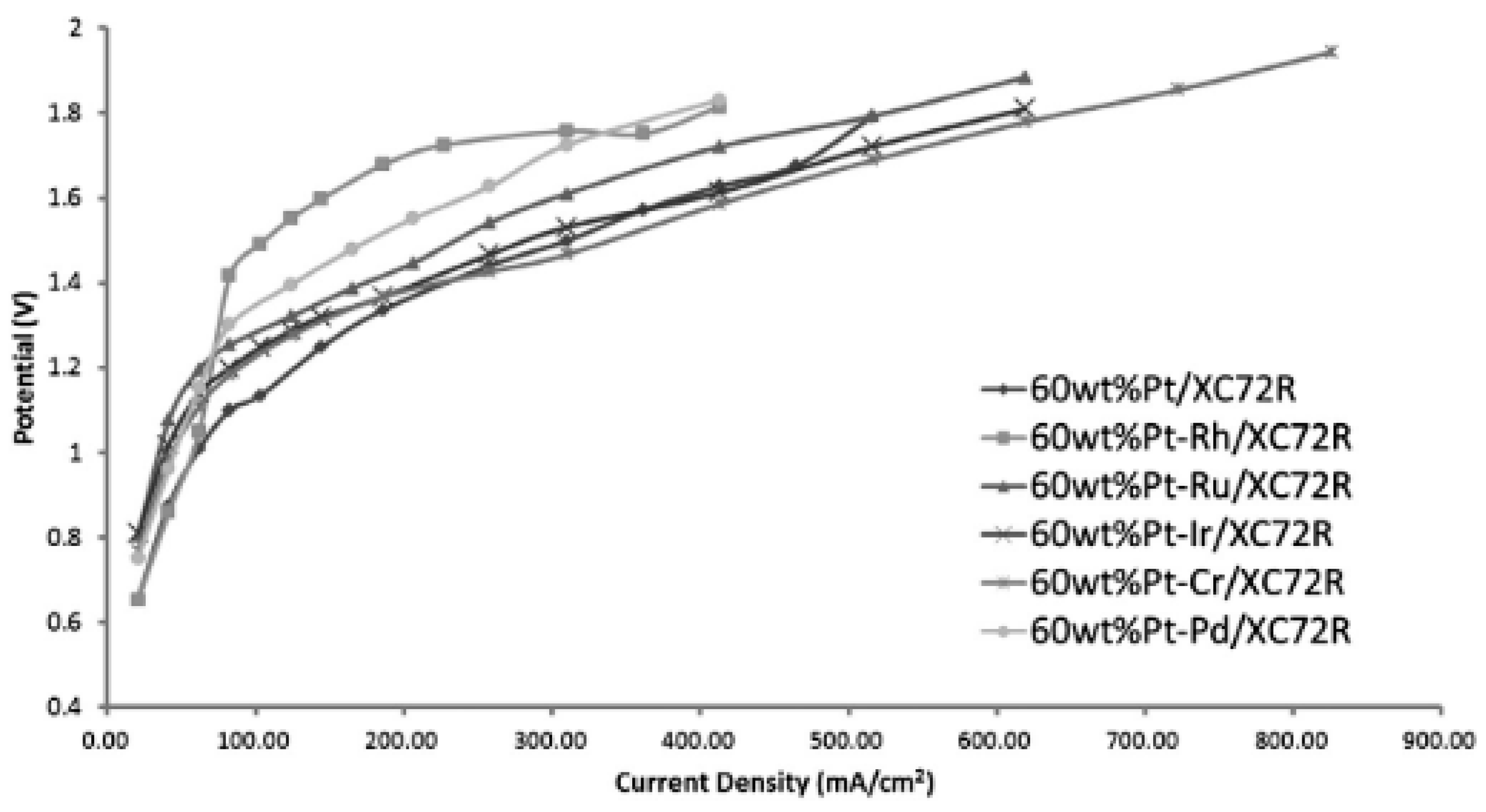
2.2. Combination of Platinum with Other Metals
3. Gold-Based Catalysts
4. Palladium-Based Catalysts
5. Catalysts Based on Other Compounds
6. New Tendencies
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Albrecht, U.; Altmann, M.; Barth, F.; Bünger, U.; Fraile, D.; Lanoix, J.-C.; Pschorr-Schoberer, E.; Vanhoudt, W.; Weindorf, W.; Zerta, M.; et al. Study on Hydrogen from Renewable Resources in the EU; Final Report; Ludwig-Bölkow-Systemtechnik GmbH & Hinicio S.A.: Brussels, 2015; Volume 39, ISBN 9789292461386. [Google Scholar]
- Winter, C.J.; Nitsch, J. Hydrogen as an Energy Carrier: Technologies, Systems, Economy; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 9783642615610. [Google Scholar]
- Sattler, C.; Roeb, M.; Agrafiotis, C.; Thomey, D. Solar hydrogen production via sulphur based thermochemical water-splitting. Sol. Energy 2017, 156, 30–47. [Google Scholar] [CrossRef]
- Masudy-Panah, S.; Moakhar, R.S.; Chua, C.S.; Kushwaha, A.; Dalapati, G.K. Stable and E ffi cient CuO Based Photocathode through Oxygen-Rich Composition and Au—Pd Nanostructure Incorporation for Solar- Hydrogen Production. ACS Appl. Mater. Interfaces 2017. [Google Scholar] [CrossRef] [PubMed]
- Masudy-Panah, S.; Eugene, Y.-J.K.; Khiavi, N.D.; Katal, R.; Gong, X. Aluminum-incorporated p-CuO/n-ZnO photocathode coated with nanocrystal-engineered TiO2 protective layer for photoelectrochemical water splitting and hydrogen generation. J. Mater. Chem. A 2018, 6, 11951–11965. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Low, J.; Fang, Y.; Xiao, J.; Chen, X. Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A Mater. Energy Sustain. 2014, 1–50. [Google Scholar] [CrossRef]
- Xinxin, W.; Kaoru, O. Thermochemical Water Splitting for Hydrogen Production Utilizing Nuclear Heat from an HTGR *. Tsinghua Sci. Technol. 2005, 10, 270–276. [Google Scholar]
- Bhosale, R.R.; Kumar, A.; Van Den Broeke, L.J.P.; Gharbia, S.; Dardor, D.; Jilani, M.; Folady, J.; Al-fakih, M.S. Solar hydrogen production via thermochemical iron oxide-iron sulfate water splitting cycle. Int. J. Hydrogen Energy 2014, 40, 1639–1650. [Google Scholar] [CrossRef]
- Bilgen, E. Solar hydrogen production by hybrid thermochemical processes. Sol. Energy 1988, 41, 199–206. [Google Scholar] [CrossRef]
- Gorensek, M.B. Hybrid sulfur cycle flowsheets for hydrogen production using high-temperature gas-cooled reactors. Int. J. Hydrogen Energy 2011, 36, 12725–12741. [Google Scholar] [CrossRef]
- Gorensek, M.B.; Edwards, T.B. Energy Efficiency Limits for a Recuperative Bayonet Sulfuric Acid Decomposition Reactor for Sulfur Cycle Thermochemical Hydrogen Production. Ind. Eng. Chem. Res. 2009, 48, 7232–7245. [Google Scholar] [CrossRef]
- Brecher, L.E.; Wu, C.K.; Enlectrolytic decomposition of water. (Westinghouse Electric Corporation, Pittsburg, PA, USA). Personal communication, 1975.
- Staser, J.; Ramasamy, R.P.; Sivasubramanian, P.; Weidner, J.W. Effect of Water on the Electrochemical Oxidation of Gas-Phase SO2 in a PEM Electrolyzer for H2 Production. Electrochem. Solid-State Lett. 2007, 10, E17–E19. [Google Scholar] [CrossRef]
- Sivasubramanian, P.; Ramasamy, R.; Freire, F.; Holland, C.; Weidner, J. Electrochemical hydrogen production from thermochemical cycles using a proton exchange membrane electrolyzer. Int. J. Hydrogen Energy 2007, 32, 463–468. [Google Scholar] [CrossRef]
- Leybros, J.; Saturnin, A.; Mansilla, C.; Gilardi, T.; Carles, P. Plant sizing and evaluation of hydrogen production costs from advanced processes coupled to a nuclear heat source: Part II: Hybrid-sulphur cycle. Int. J. Hydrogen Energy 2010, 35, 1019–1028. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Kazimi, M.S. Optimization of the Hybrid Sulfur Cycle for Nuclear Hydrogen Generation. Nucl. Technol. 2007, 159, 147–157. [Google Scholar] [CrossRef]
- Lu, P.W.T. Technological aspects of sulfur dioxide depolarized electrolysis for hydrogen production. Int. J. Hydrogen Energy 1983, 8, 773–781. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, P.; Chen, S.; Wang, L. Pt-based bimetallic catalysts for SO2-depolarized electrolysis reaction in the hybrid sulfur process. Int. J. Hydrogen Energy 2014, 39, 14196–14203. [Google Scholar] [CrossRef]
- Kriek, R.J.; Rossmeisl, J.; Siahrostami, S.; Björketun, M.E. H2 production through electro-oxidation of SO2: Identifying the fundamental limitations. Phys. Chem. Chem. Phys. 2014, 16, 9572–9579. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.W.T.; Ammon, R.L. An Investigation of Electrode Materials for the Anodic Oxidation of Sulfur Dioxide in Concentrated Sulfuric Acid. J. Electrochem. Soc. 1980, 127, 2610. [Google Scholar] [CrossRef]
- Appleby, A.J.; Pinchon, B. Electrochemical aspects of the H2SO4 SO2 thermoelectrochemical cycle for hydrogen production. Int. J. Hydrogen Energy 1980, 5, 253–267. [Google Scholar] [CrossRef]
- Seo, E.T. Determination of Sulfur Dioxide in Solution By Voltammetry. J. Electroanal. Chem. 1964, 7, 184–189. [Google Scholar]
- Seo, E.T.; Sawyer, D.T. Electrochemical oxidation of dissolved sulphur dioxide at platinum and gold electrodes. Electrochim. Acta 1965, 10, 239–252. [Google Scholar] [CrossRef]
- Wiesener, K. The electrochemical oxidation of sulphur dioxide at porous catalysed carbon electrodes in sulphuric acid. Electrochim. Acta 1973, 18, 185–189. [Google Scholar] [CrossRef]
- Lu, P.W.T.; Ammon, R.L. Sulfur dioxide depolarized electrolysis for hydrogen production: Development status. Int. J. Hydrogen Energy 1982, 7, 563–575. [Google Scholar] [CrossRef]
- Scott, K.; Taama, W.M. Investigation of anode materials in the anodic oxidation of sulphur dioxide in sulphuric acid solutions. Electrochim. Acta 1999, 44, 3421–3427. [Google Scholar] [CrossRef]
- Weidner, J.W.; Sivasubramanian, P.; Holland, C.E.; Freire, F.J. Electrochemical Generation of Hydrogen via Gas Phase Oxidation of Sulfur Dioxide and Hydrogen Bromide Hydrogen using Thermal and/or Electrical Energy. In Proceedings of the 2005 AIChE Annual Meeting, Cincinnati, OH, USA, 30 October–4 November 2005. [Google Scholar]
- Steimke, J.L.; Steeper, T.J. Characterization Testing of H2O-SO2 Electrolyzer at Ambient Pressure; Technical Report; U.S. Department of Energy: Washington, DC, USA, 2005. [CrossRef]
- Report of Savannah Westinghouse Company, 2006, by Steimke, J.L.; Steeper, T.J. Available online: https://classic.ntis.gov/assets/pdf/st-on-cd/DE2006892717.pdf (accessed on 7 January 2019).
- Colón-Mercado, H.R.; Hobbs, D.T. Catalyst evaluation for a sulfur dioxide-depolarized electrolyzer. Electrochem. Commun. 2007, 9, 2649–2653. [Google Scholar] [CrossRef]
- Allen, J.A.; Rowe, G.; Hinkley, J.T.; Donne, S.W. Electrochemical aspects of the Hybrid Sulfur Cycle for large scale hydrogen production. Int. J. Hydrogen Energy 2014, 39, 11376–11389. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, C.H.; Cho, W.C.; Kang, K.S.; Park, C.S.; Bae, K.K. The effect of Pt loading amount on SO2 oxidation reaction in an SO2-depolarized electrolyzer used in the hybrid sulfur (HyS) process. Int. J. Hydrogen Energy 2009, 34, 4701–4707. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, P.; Chen, S.; Wang, L.; Wang, J. Sensitivity study of process parameters in membrane electrode assembly preparation and SO2 depolarized electrolysis. Int. J. Hydrogen Energy 2013, 38, 11017–11022. [Google Scholar] [CrossRef]
- Krüger, A.J.; Krieg, H.M.; Van Der Merwe, J.; Bessarabov, D. Evaluation of MEA manufacturing parameters using EIS for SO2 electrolysis. Int. J. Hydrogen Energy 2014, 39, 18173–18181. [Google Scholar] [CrossRef]
- Staser, J.A.; Gorensek, M.B.; Weidner, J.W. Quantifying Individual Potential Contributions of the Hybrid Sulfur Electrolyzer. J. Electrochem. Soc. 2010, 157, B952–B958. [Google Scholar] [CrossRef]
- Falch, A.; Lates, V.; Kriek, R.J. Combinatorial Plasma Sputtering of PtxPdy Thin Film Electrocatalysts for Aqueous SO2 Electro-oxidation. Electrocatalysis 2015, 6, 322–330. [Google Scholar] [CrossRef]
- Cooper, J.S.; McGinn, P.J. Combinatorial screening of fuel cell cathode catalyst compositions. Appl. Surf. Sci. 2007, 254, 662–668. [Google Scholar] [CrossRef]
- Kleinke, M.; Knobel, M.; Bonugli, L.O.; Teschke, O. Amorphous alloys as anodic and cathodic materials for alkaline water electrolysis. Int. J. Hydrogen Energy 1997, 22, 759–762. [Google Scholar] [CrossRef]
- Jayaraman, S.; Hillier, A.C. Construction and Reactivity Screening of a Surface Composition Gradient for Combinatorial Discovery of Electro-Oxidation Catalysts. J. Comb. Chem. 2004, 6, 27–31. [Google Scholar] [CrossRef]
- Falch, A.; Lates, V.A.; Kotzé, H.S.; Kriek, R.J. The Effect of Rapid Thermal Annealing on Sputtered Pt and Pt3Pd2Thin Film Electrocatalysts for Aqueous SO2 Electro-Oxidation. Electrocatalysis 2016, 7, 33–41. [Google Scholar] [CrossRef]
- Falch, A.; Badets, V.A.; Labrugère, C.; Kriek, R.J. Co-sputtered PtxPdyAlzthin film electrocatalysts for the production of hydrogen via SO2(aq) electro-oxidation. Electrocatalysis 2016, 7, 376–390. [Google Scholar] [CrossRef]
- Xu, F.; Cheng, K.; Yu, Y.; Mu, S. One-pot synthesis of Pt/CeO2/C catalyst for enhancing the SO2 electrooxidation. Electrochim. Acta 2017, 229, 253–260. [Google Scholar] [CrossRef]
- Samec, Z.; Weber, J. Study of the Oxidation of SO2 Dissolved 0.5 M H2S04 on a Gold Electrode-I Stationary Electrode. Electrochim. Acta 1975, 20, 403–412. [Google Scholar] [CrossRef]
- Quijada, C.; Huerta, F.J.; Morallón, E.; Vázquez, J.L.; Berlouis, L.E.A. Electrochemical behaviour of aqueous SO2 at polycrystalline gold electrodes in acidic media: A voltammetric and in situ vibrational study. Part 1. Reduction of SO2: Deposition of monomeric and polymeric sulphur. Electrochim. Acta 2000, 45, 1847–1862. [Google Scholar] [CrossRef]
- Quijada, C.; Morallón, E.; Vázquez, J.L.; Berlouis, L.E.A. Electrochemical behaviour of aqueous SO2 at polycrystalline gold electrodes in acidic media. A voltammetric and in-situ vibrational study. Part II. Oxidation of SO2 on bare and sulphur-modified electrodes. Electrochim. Acta 2001, 46, 651–659. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Hinkley, J.T.; Donne, S.W. Electrochemical Oxidation of Aqueous Sulfur Dioxide II. Comparative Studies on Platinum and Gold Electrodes. J. Electrochem. Soc. 2012, 159, 585–593. [Google Scholar] [CrossRef]
- Santasalo-Aarnio, A.; Lokkiluoto, A.; Virtanen, J.; Gasik, M.M. Performance of electrocatalytic gold coating on bipolar plates for SO2 depolarized electrolyser. J. Power Sources 2016, 306, 1–7. [Google Scholar] [CrossRef]
- Mu, C.; Hou, M.; Xiao, Y.; Zhang, H.; Hong, S.; Shao, Z. Electrochemical oxidation of sulfur dioxide on nitrogen-doped graphite in acidic media. Electrochim. Acta 2015, 171, 29–34. [Google Scholar] [CrossRef]
- Potgieter, M.; Parrondo, J.; Ramani, V.K.; Kriek, R.J. Evaluation of Polycrystalline Platinum and Rhodium Surfaces for the Electro-Oxidation of Aqueous Sulfur Dioxide. Electrocatalysis 2016, 7, 50–59. [Google Scholar] [CrossRef]
- Tulskiy, G.; Tulskaya, A.; Skatkov, L.; Gomozov, V.; Deribo, S. Electrochemical synthesis of hydrogen with depolarization of the anodic process. Electrochem. Energy Technol. 2016, 2, 13–16. [Google Scholar] [CrossRef]
- Zhao, Q.; Hou, M.; Jiang, S.; Ai, J.; Zheng, L.; Shao, Z. Excellent Sulfur Dioxide Electrooxidation Performance and Good Stability on a Fe-N-Doped Carbon-Cladding Catalyst in H2SO4. J. Electrochem. Soc. 2017, 164, H456–H462. [Google Scholar] [CrossRef]
- Jayakumar, J.V.; Gulledge, A.; Staser, J.A.; Kim, C.-H.; Benicewicz, B.C.; Weidner, J.W. Polybenzimidazole Membranes for Hydrogen and Sulfuric Acid Production in the Hybrid Sulfur Electrolyzer. ECS Electrochem. Lett. 2012, 1, F44–F48. [Google Scholar] [CrossRef]
- Wainright, J.S.; Wang, J.-T.; Weng, D.; Savinell, R.F.; Litt, M. Acid-Doped Polybenzimidazoles: A New Polymer Electrolyte. J. Electrochem. Soc. 1995, 142, L121–L123. [Google Scholar] [CrossRef]
- Lobato, J.; Cañizares, P.; Rodrigo, M.A.; Linares, J.J.; Manjavacas, G. Synthesis and characterisation of poly[2,2-(m-phenylene)-5,5-bibenzimidazole] as polymer electrolyte membrane for high temperature PEMFCs. J. Memb. Sci. 2006, 280, 351–362. [Google Scholar] [CrossRef]
- Li, Q.; Jensen, J.O.; Savinell, R.F.; Bjerrum, N.J. High temperature proton exchange membranes based on polybenzimidazoles for fuel cells. Prog. Polym. Sci. 2009, 34, 449–477. [Google Scholar] [CrossRef]
- Lobato, J.; Cañizares, P.; Rodrigo, M.A.; Úbeda, D.; Pinar, F.J. Promising TiOSO4 Composite Polybenzimidazole-Based Membranes for High Temperature PEMFCs. ChemSusChem 2011, 4, 1489–1497. [Google Scholar] [CrossRef]
- Steimke, J.L.; Steeper, T.J.; Colón-Mercado, H.R.; Gorensek, M.B. Development and testing of a PEM SO2-depolarized electrolyzer and an operating method that prevents sulfur accumulation. Int. J. Hydrogen Energy 2015, 40, 13281–13294. [Google Scholar] [CrossRef]
- Garrick, T.R.; Wilkins, C.H.; Pingitore, A.T.; Mehlhoff, J.; Gulledge, A.; Benicewicz, B.C.; Weidner, J.W. Characterizing Voltage Losses in an SO2 Depolarized Electrolyzer Using Sulfonated Polybenzimidazole Membranes. J. Electrochem. Soc. 2017, 164, F1591–F1595. [Google Scholar] [CrossRef]
- Lobato, J.; Zamora, H.; Cañizares, P.; Plaza, J.; Rodrigo, M.A. Microporous layer based on SiC for high temperature proton exchange membrane fuel cells. J. Power Sources 2015, 288, 288–295. [Google Scholar] [CrossRef]
- Lobato, J.; Zamora, H.; Plaza, J.; Rodrigo, M.A. Composite Titanium Silicon Carbide as a Promising Catalyst Support for High-Temperature Proton-Exchange Membrane Fuel Cell Electrodes. ChemCatChem 2016, 8, 848–854. [Google Scholar] [CrossRef]
- Lobato, J.; Zamora, H.; Plaza, J.; Cañizares, P.; Rodrigo, M.A. Enhancement of high temperature PEMFC stability using catalysts based on Pt supported on SiC based materials. Appl. Catal. B Environ. 2016, 198, 516–524. [Google Scholar] [CrossRef]
- Zamora, H.; Plaza, J.; Velhac, P.; Cañizares, P.; Rodrigo, M.A.; Lobato, J. SiCTiC as catalyst support for HT-PEMFCs. Influence of Ti content. Appl. Catal. B Environ. 2017, 207, 244–254. [Google Scholar] [CrossRef]
- Millán, M.; Zamora, H.; Rodrigo, M.A.; Lobato, J. Enhancement of Electrode Stability Using Platinum–Cobalt Nanocrystals on a Novel Composite SiCTiC Support. ACS Appl. Mater. Interfaces 2017, 9, 5927–5936. [Google Scholar] [CrossRef] [PubMed]

| Intensity (A) | ||
|---|---|---|
| Before | After | |
| Pt/C Commercial | 0.31 | 0.29 |
| Pt/C handmade | 0.30 | 0.27 |
| Pt/SiC–TiC | 0.22 | 0.32 |
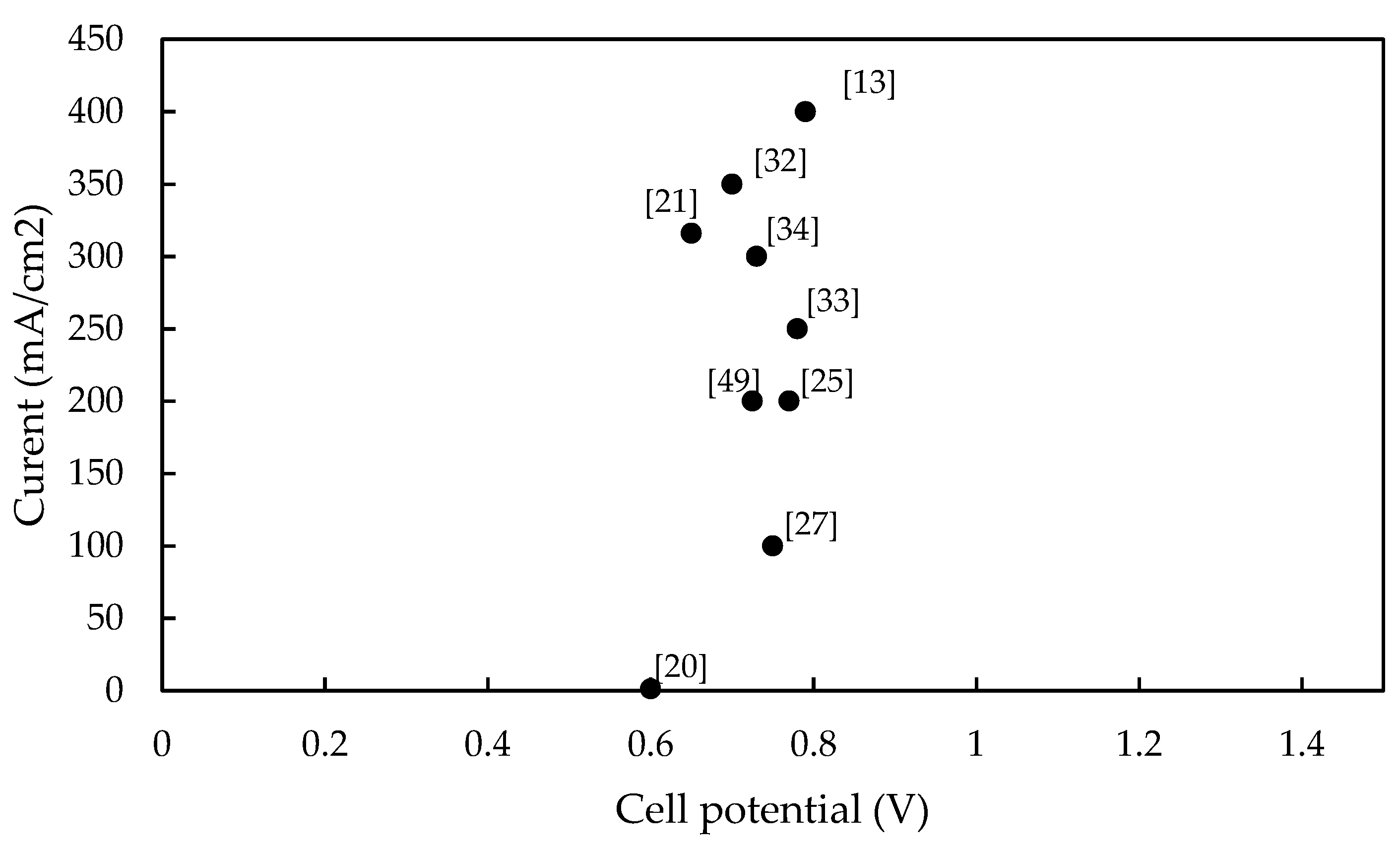
| Ref. | Year | Catalyst | Electrode | Current (A/cm2) (@ V vs. RHE) | Pressure (bar) | Temperature (°C) | [H2SO4] (wt %) | Test Array |
|---|---|---|---|---|---|---|---|---|
| [24] | 1973 | V/Al2O3 (1:3) | 6.9 mgV/Al/cm2 | 0.048 (0.69) | 1 | 100 | 3.8 (M) | half cell |
| [21] | 1980 | Platinum | 3 mgPt/cm2 | 0.316 (0.65) | 1 | 50 | 55 | half cell |
| [25] | 1982 | Pt/C | 7 mgPt/cm2 | 0.200 (0.77) | 1 | 50 | 50 | single cell |
| [26] | 1999 | Pd | Palladium electrode | 0.033 (0.76) | 1 | 25 | 0.5 (M) | half cell |
| [26] | 1999 | Graphite | Graphite electrode | 0.021 (0.76) | 1 | 70 | 0.5 (M) | half cell |
| [28] | 2005 | Pt/C | 0.88 mgPt/cm2 | 0.300 (0.73) | 4 | 70 | 30 | single cell |
| [13] | 2007 | Pt/C | 1 mgPt/cm2 | 0.400 (0.79) | 1 | 80 | (SO2 gas) | single cell |
| [45] | 2012 | Au | Au electrode | 0.080 (0.70) | 1 | 22 | 1 (M) | half cell |
| [33] | 2013 | Pt/C | 1 mgPt/cm2 | 0.100 (0.75) | 1 | 80 | 30 | single cell |
| [18] | 2014 | Pt-Cr(1:2)/C | 1 mgPt-Cr/cm2 | 0.020 (0.80) | 1 | 25 | 30 | half cell |
| [48] | 2015 | NG | 2 mg | 0.15 A (0.9) | 1 | 25 | 0.5 (M) | rotating disk |
| [47] | 2016 | Au | Au coating on 904L | 0.005 (0.90) | 1 | 25 | 15 | single cell |
| [47] | 2016 | Au | Au coating on 904L | 0.045 (0.75) | 1 | 25 | 15 | half cell |
| [49] | 2016 | Pt | Platinum electrode | 0.200 (0.73) | 1 | 25 | 0.5 (M) | rotating disk |
| [50] | 2016 | RuO2 | 3 mgRuO2/cm2 | 0.1 (0.74) | 1 | 25 | 0.5 (M) | single cell |
| [50] | 2016 | WO3 | 3.8 mgWO3/cm2 | 0.1 (0.84) | 1 | 25 | 0.5 (M) | single cell |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Abad, S.; Millán, M.; Rodrigo, M.A.; Lobato, J. Review of Anodic Catalysts for SO2 Depolarized Electrolysis for “Green Hydrogen” Production. Catalysts 2019, 9, 63. https://doi.org/10.3390/catal9010063
Díaz-Abad S, Millán M, Rodrigo MA, Lobato J. Review of Anodic Catalysts for SO2 Depolarized Electrolysis for “Green Hydrogen” Production. Catalysts. 2019; 9(1):63. https://doi.org/10.3390/catal9010063
Chicago/Turabian StyleDíaz-Abad, Sergio, María Millán, Manuel A. Rodrigo, and Justo Lobato. 2019. "Review of Anodic Catalysts for SO2 Depolarized Electrolysis for “Green Hydrogen” Production" Catalysts 9, no. 1: 63. https://doi.org/10.3390/catal9010063
APA StyleDíaz-Abad, S., Millán, M., Rodrigo, M. A., & Lobato, J. (2019). Review of Anodic Catalysts for SO2 Depolarized Electrolysis for “Green Hydrogen” Production. Catalysts, 9(1), 63. https://doi.org/10.3390/catal9010063







