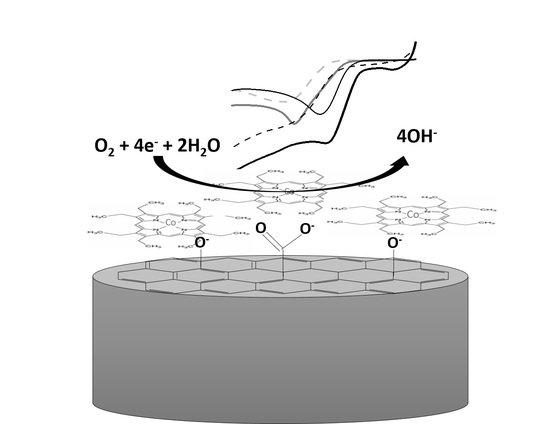
Electro-Reduction of Molecular Oxygen Mediated by a Cobalt(II)octaethylporphyrin System onto Oxidized Glassy Carbon/Oxidized Graphene Substrate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphological Studies of Modified Systems
2.2. Electrochemical Response of the Modified Systems
2.3. Electrochemical Kinetics

2.4. Electrochemical Impedance Spectroscopy
3. Materials and Methods
3.1. Equipment
3.2. Reagents
3.3. Preparation of Modified Electrodes
3.4. Instrumentation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiang, W.J.; Gu, L.; Li, L.; Zhang, Y.; Zhang, X.; Zhang, L.J.; Wang, J.Q.; Hu, J.S.; Wei, Z.; Wan, L.J. Understanding the high activity of Fe-N-C electrocatalysts in oxygen reduction: Fe/Fe3C nanoparticles boost the activity of Fe-N(x). J. Am. Chem. Soc. 2016, 138, 3570–3578. [Google Scholar] [CrossRef] [PubMed]
- Geng, D.; Chen, Y.; Chen, Y.; Li, Y.; Li, R.; Sun, X.; Ye, S.; Knights, S. High oxygen-reduction activity and durability of nitrogen-doped graphene. Energy Environ. Sci. 2011, 4, 760–764. [Google Scholar] [CrossRef]
- Steele, B.C.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.W.; Zhuang, X.; Bruller, S.; Feng, X.; Mullen, K. Hierarchically porous carbons with optimized nitrogen doping as highly active electrocatalysts for oxygen reduction. Nat. Commun. 2014, 5, 4973. [Google Scholar] [CrossRef] [Green Version]
- Van der Vliet, D.F.; Wang, C.; Tripkovic, D.; Strmcnik, D.; Zhang, X.F.; Debe, M.K.; Atanasoski, R.T.; Markovic, N.M.; Stamenkovic, V.R. Mesostructured thin films as electrocatalysts with tunable composition and surface morphology. Nat. Mater. 2012, 11, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Ma, R.; Zhou, Y.; Xing, R.; Liu, Q.; Zhu, Y.; Wang, J. Efficient N-doping of hollow core-mesoporous shelled carbon spheres via hydrothermal treatment in ammonia solution for the electrocatalytic oxygen reduction reaction. Microporous Mesoporous Mater. 2018, 261, 88–97. [Google Scholar] [CrossRef]
- Alia, S.M.; Jensen, K.; Contreras, C.; Garzon, F.; Pivovar, B.; Yan, Y. Platinum coated copper nanowires and platinum nanotubes as oxygen reduction electrocatalysts. ACS Catal. 2013, 3, 358–362. [Google Scholar] [CrossRef]
- Narayanamoorthy, B.; Datta, K.K.R.; Eswaramoorthy, M.; Balaji, S. Improved oxygen reduction reaction catalyzed by Pt/Clay/nafion nanocomposite for PEM fuel cells. ACS Appl. Mater. Interfaces 2012, 4, 3620–3626. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, T.; Fang, J.; Xu, P.; Li, X.; Xu, J.; Liu, C.-C. Integrated Pt2Ni alloy@Pt core-shell nanoarchitectures with high electrocatalytic activity for oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 11400–11407. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, Y.; Liu, C.; Xu, G.; Chen, Y.; Tang, Y.; Lu, T. Pd@Pt core-shell tetrapods as highly active and stable electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 20855–20860. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Kocha, S.S.; Sompalli, B.; Wagner, F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B Environ. 2005, 56, 9–35. [Google Scholar] [CrossRef]
- Chen, A.; Holt-Hindle, P. Platinum-based nanostructured materials: Synthesis, properties, and applications. Chem. Rev. 2010, 110, 3767–3804. [Google Scholar] [CrossRef] [PubMed]
- You, P.Y.; Kamarudin, S.K. Recent progress of carbonaceous materials in fuel cell applications: An overview. Chem. Eng. J. 2017, 309, 489–502. [Google Scholar] [CrossRef]
- Chabot, V.; Higgins, D.; Yu, A.; Xiao, X.; Chena, Z.; Zhang, J. A review of graphene and graphene oxide sponge: Material synthesis and applications to energy and the environment. Energy Environ. Sci. 2014, 7, 1564–1596. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Ge, L.; Chen, Z.-G.; Liang, F.; Xu, H.-Y.; Motuzas, J.; Julbe, A.; Zhu, Z. Amorphous iron oxide decorated 3D heterostructured electrode for highly efficient oxygen reduction. Chem. Mater. 2011, 23, 4193–4198. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, D.; Wang, Y.; Wan, Y.; Hou, B. Catalytic activity of graphene–cobalt hydroxide composite for oxygen reduction reaction in alkaline media. J. Power Sources 2012, 198, 122–126. [Google Scholar] [CrossRef]
- Bai, H.; Li, C.; Shi, G. Functional composite materials based on chemically converted graphene. Adv. Mater. 2011, 23.9, 1089–1115. [Google Scholar] [CrossRef]
- Alenezi, K. Electrocatalytic Hydrogen Evolution Reaction Using mesotetrakis-(pentafluorophenyl)porphyrin iron(III) chloride. Int. J. Electrochem. Sci. 2017, 12, 812–818. [Google Scholar] [CrossRef]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef]
- Belova, A.I.; Kwabi, D.G.; Yashina, L.V.; Shao-Horn, Y.; Itkis, D.M. Mechanism of Oxygen Reduction in Aprotic Li–Air Batteries: The Role of Carbon Electrode Surface Structure. J. Phys. Chem. C 2017, 121, 1569–1577. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, H.-L.; Guo, S. Towards high-efficiency nanoelectrocatalysts for oxygen reduction through engineering advanced carbon nanomaterials. Chem. Soc. Rev. 2016, 45, 1273–1307. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Min, S.; Wang, Q.; Li, D.; Casillas, G.; Ma, C.; Li, Y.; Liu, Z.; Li, L.-J.; Yuan, J.; et al. Nitrogen-Doped Nanoporous Carbon Membranes with Co/CoP Janus-Type Nanocrystals as Hydrogen Evolution Electrode in Both Acidic and Alkaline Environments. ACS Nano 2017, 11, 4358–4364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zagal, J.H.; Bedioui, F. Electrochemistry of N4 Macrocyclic Metal Complexes: Volume 2: Biomimesis, Electroanalysis and Electrosynthesis of MN4 Metal Complexes; Springer: Berlin, Germany, 2016. [Google Scholar]
- Manbeck, G.F.; Fujita, E. A review of iron and cobalt porphyrins, phthalocyanines and related complexes for electrochemical and photochemical reduction of carbon dioxide. J. Porphyr. Phthalocyanines 2015, 19, 45–46. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Chen, L.; Liu, J.; Parvez, K.; Liang, H.; Shu, J.; Sachdev, H.; Graf, R.; Feng, X.; Müllen, K. High-Performance Electrocatalysts for Oxygen Reduction Derived from Cobalt Porphyrin-Based Conjugated Mesoporous Polymers. Adv. Mater. 2014, 26, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Sengupta, K.; Mondal, B.; Dey, S.; Dey, A. Factors Determining the Rate and Selectivity of 4e−/4H+ Electrocatalytic Reduction of Dioxygen by Iron Porphyrin Complexes. Acc. Chem. Res. 2017, 50, 1744–1753. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-M.; Salmi, Z.; Lillethorup, M.; Pedersen, E.B.; Robert, M.; Pedersen, S.U.; Skrydstrup, T.; Daasbjerg, K. Controlled electropolymerisation of a carbazole-functionalised iron porphyrin electrocatalyst for CO2 reduction. Chem. Commun. 2016, 52, 5864–5867. [Google Scholar] [CrossRef]
- Hod, I.; Farha, O.K.; Hupp, J.T. Electrocatalysis: Powered by porphyrin packing. Nat. Mater. 2015, 14, 1192–1193. [Google Scholar] [CrossRef]
- Canales, C.; Ramírez, G. Glassy carbon electrodes modified with supramolecular assemblies generated by p-stacking of Cobalt (II) octaethylporphyrins. A 4 electrons-dioxygen reduction reaction occurring at positive potentials. Electrochim. Acta 2015, 173, 636–641. [Google Scholar] [CrossRef]
- Canales, C.; Varas-Concha, F.; Mallouk, T.E.; Ramírez, G. Enhanced electrocatalytic hydrogen evolution reaction: Supramolecular assemblies of metalloporphyrins on glassy carbon electrodes. Appl. Catal. B Environ. 2016, 188, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Canales, C.; Olea, A.F.; Gidi, L.; Arce, R.; Ramírez, G. Enhanced light-induced hydrogen evolution reaction by supramolecular systems of cobalt(II) and copper(II) octaethylporphyrins on glassy carbon electrodes. Electrochim. Acta 2017, 258, 850–857. [Google Scholar] [CrossRef]
- Tang, H.; Yin, H.; Wang, J.; Yang, N.; Wang, D.; Tan, Z. Molecular Architecture of Cobalt Porphyrin Multilayers on Reduced Graphene Oxide Sheets for High-Performance Oxygen Reduction Reaction. Angew. Chem. 2013, 125, 5695–5699. [Google Scholar] [CrossRef]
- Jiang, R.; Chu, D. Comparative study of CoFeNx/C catalyst obtained by pyrolysis of hemin and cobalt porphyrin for catalytic oxygen reduction in alkaline and acidic electrolytes. J. Power Sources 2014, 245, 352–361. [Google Scholar] [CrossRef]
- Oldacre, A.N.; Friedman, A.E.; Cook, T.R. A Self-Assembled Cofacial Cobalt Porphyrin Prism for Oxygen Reduction Catalysis. J. Am. Chem. Soc. 2017, 139, 1424–1427. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.Y.; Dai, S.; Jaroniec, M.; Qiao, S.Z. Metal−Organic Framework Derived Hybrid Co3O4-Carbon Porous Nanowire Arrays as Reversible Oxygen Evolution Electrodes. J. Am. Chem. Soc. 2014, 136, 13925–13931. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Sheng, W.; Yan, Y. Synthesis of Monodispere Au@Co3O4 Core-Shell Nanocrystals and Their Enhanced Catalytic Activity for Oxygen Evolution Reaction. Adv. Mater. 2014, 26, 3950–3955. [Google Scholar] [CrossRef] [PubMed]
- Scholz, F. (Ed.) Electroanalytical Methods: Guide to Experiments and Applications; Springer: Berlin, Germany, 2013; ISBN 3662047578, 9783662047576. [Google Scholar]
- Marín, A.; Aguirre, M.J.; Muena, J.P.; Dehaen, W.; Maes, W.; Ngo, T.H.; Ramírez, G.; Arévalo, M.C. Electro-reduction of oxygen to water mediated by stable glassy carbon electrodes modified by Co(II)-porphyrins with voluminous meso-substituent. Int. J. Electrochem. Sci. 2015, 10, 3949. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA, 2000; ISBN 978-0-471-04372-0. [Google Scholar]
- Ríos, R.; Marín, A.; Ramírez, G. Nitrite electro-oxidation mediated by Co(II)-[tetra(4-aminophenyl) porphyrin]-modified electrodes: Behavior as an amperometric sensor. J. Coord. Chem. 2010, 63, 1283–1294. [Google Scholar] [CrossRef]
- Tham, M.K.; Walker, R.D.; Gubbins, K.E. Diffusion of Oxygen and Hydrogen in Aqueous Potassium Hydroxide Solutions. J. Phys. Chem. 1970, 74, 1747–1751. [Google Scholar] [CrossRef]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater, 22nd ed.; United Book Press Inc.: Baltimore, MD, USA, 2012. [Google Scholar]
- Andrieux, C.P.; Seavant, J.M. Heterogeneous versus homogeneous catalysis of electrochemical reactions. J. Electroanal. Chem. 1978, 93, 163–168. [Google Scholar] [CrossRef]
- Srejic, I.; Rakocevic, Z.; Nenadovic, M.; Strbac, S. Oxygen reduction on polycrystalline palladium in acid and alkaline solutions: Topographical and chemical Pd surface changes. Electrochim. Acta 2015, 169, 22–31. [Google Scholar] [CrossRef]
- Blizanac, B.; Ross, P.; Markovic, N. Oxygen Reduction on Silver Low-Index Single-Crystal Surfaces in Alkaline Solution: Rotating Ring DiskAg (h kl) Studies. J. Phys. Chem. B 2006, 110, 4735–4741. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, V.; Schmidt, T.; Ross, P.; Markovic, N. Surface composition effects in electrocatalysis: Kinetics of oxygen reduction on well-defined Pt3Ni and Pt3Co alloy surfaces. J. Phys. Chem. B 2002, 106, 11970–11979. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Zhao, Y.; Wang, F. Composition-Controlled Synthesis of Carbon-Supported Pt–Co Alloy Nanoparticles and Their Origin of the Activity Enhancement for Oxygen Reduction Reaction. Phys. Chem. Chem. Phys. 2014, 16, 19298–19306. [Google Scholar] [CrossRef] [PubMed]
- Córdoba-Torres, P.; Mesquita, T.J.; Nogueira, R.P. Relationship between the origin of constant-phase element behavior in electrochemical impedance spectroscopy and electrode surface structure. J. Phys. Chem. C 2015, 119, 4136–4147. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Xia, Z.; Roy, A.; Chang, D.W.; Baek, J.B.; Dai, L. BCN Graphene as Efficient Metal-Free Electrocatalyst for the Oxygen Reduction Reaction. Angew. Chem. 2012, 124, 4285–4288. [Google Scholar] [CrossRef]
- Luo, Z.; Lim, S.; Tian, Z.; Shang, J.; Lai, L.; MacDonald, B.; Fu, C.; Shen, Z.; Yu, T.; Lin, J. Pyridinic N doped graphene: Synthesis, electronic structure, and electrocatalytic property. J. Mater. Chem. 2011, 21, 8038–8044. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Cheng, H.; Hu, Y.; Shi, G.; Dai, L.; Qu, L. Nitrogen-Doped Graphene Quantum Dots with Oxygen-Rich Functional Groups. J. Am. Chem. Soc. 2012, 134, 15–18. [Google Scholar] [CrossRef]
- Jiang, L.; Cui, L.; He, X. Cobalt-porphyrin noncovalently functionalized graphene as nonprecious-metal electrocatalyst for oxygen reduction reaction in an alkaline medium. J. Solid State Electrochem. 2015, 19, 497–506. [Google Scholar] [CrossRef]
- Gidi, L.; Canales, C.; Aguirre, M.J.; Armijo, F.; Ramírez, G. Four-Electron Reduction of Oxygen Electrocatalyzed by a Mixture of Porphyrin Complexes onto Glassy Carbon Electrode. Int. J. Electrochem. Sci. 2018, 13, 1666–1682. [Google Scholar] [CrossRef]







| System | Rq (nm) |
|---|---|
| GC | 6.0 |
| GC + GPH | 6.0 |
| GC ox + GPH ox | 60.0 |
| GC ox + GPH ox + Co(II)OEP | 355.0 |
| System | EO (V) | Rs (Ω) | Rct (Ω) | CPE (T, P) (s Ω−1) |
|---|---|---|---|---|
| GC | −0.34 | 742.1 | 27,244 | 3.53 × 10−6, 0.88 |
| GC ox + GPH ox | −0.25 | 810.9 | 12,999 | 5.20 × 10−6, 0.88 |
| GC ox + GPH ox + Co(II)OEP | −0.08 | 796.1 | 11,905 | 5.22 × 10−6, 0.88 |
| Material | Medium | ORR Onset (V) | Number Electrons ORR | Reference |
|---|---|---|---|---|
| GC/GPH | 0.1 M KOH | −0.18 vs. SCE | ≈4 | [50] |
| Cu foil/GPH | 0.1 M KOH | −0.3 vs. Ag/AgCl | ≈2 | [51] |
| GC/GPH Quantum Dots | 0.1 M KOH | −0.16 vs. Ag/AgCl | 3.6–4.4 | [52] |
| CoTPyP/PSS-rGO * | 0.1 M KOH | −0.15 vs. Ag/AgCl | 3.61–3.67 | [53] |
| CoP-CMP800 ** | 0.1 M KOH | −0.1 vs. Ag/AgCl | 3.83–3.86 | [26] |
| GC Co(II)OEP | 0.1 M NaOH | −0.2 vs. Ag/AgCl | - | [54] |
| GC Co(II)OEP-Fe(III)OEP | 0.1 M NaOH | −0.13 vs. Ag/AgCl | ≈4 | [54] |
| GC ox/Co(II)OEP | 0.1 M NaOH | +0.05 vs. Ag/AgCl | ≈4 | [30] |
| GC ox + GPH ox + Co(II)OEP | 0.1 M NaOH | −0.08 vs. AgAgCl | ≈4 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canales, C.; Gidi, L.; Arce, R.; Armijo, F.; Aguirre, M.J.; Ramírez, G. Electro-Reduction of Molecular Oxygen Mediated by a Cobalt(II)octaethylporphyrin System onto Oxidized Glassy Carbon/Oxidized Graphene Substrate. Catalysts 2018, 8, 629. https://doi.org/10.3390/catal8120629
Canales C, Gidi L, Arce R, Armijo F, Aguirre MJ, Ramírez G. Electro-Reduction of Molecular Oxygen Mediated by a Cobalt(II)octaethylporphyrin System onto Oxidized Glassy Carbon/Oxidized Graphene Substrate. Catalysts. 2018; 8(12):629. https://doi.org/10.3390/catal8120629
Chicago/Turabian StyleCanales, Camila, Leyla Gidi, Roxana Arce, Francisco Armijo, María J. Aguirre, and Galo Ramírez. 2018. "Electro-Reduction of Molecular Oxygen Mediated by a Cobalt(II)octaethylporphyrin System onto Oxidized Glassy Carbon/Oxidized Graphene Substrate" Catalysts 8, no. 12: 629. https://doi.org/10.3390/catal8120629