The Use of Zeolites for VOCs Abatement by Combining Non-Thermal Plasma, Adsorption, and/or Catalysis: A Review
Abstract
:1. Introduction
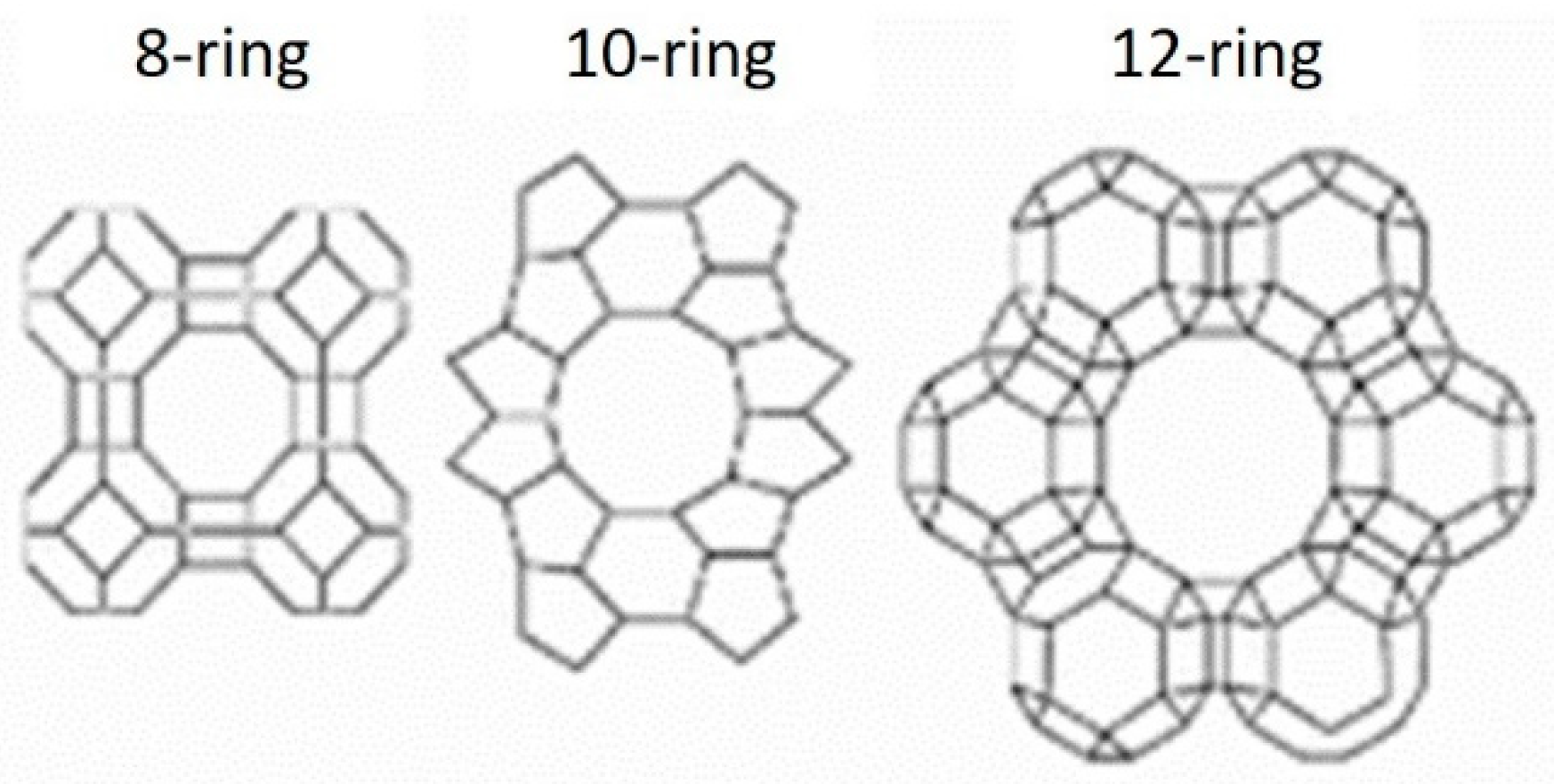
2. Zeolites
3. Adsorption Plasma Catalysis for VOC Abatement
3.1. Adsorption of VOCs on Zeolite
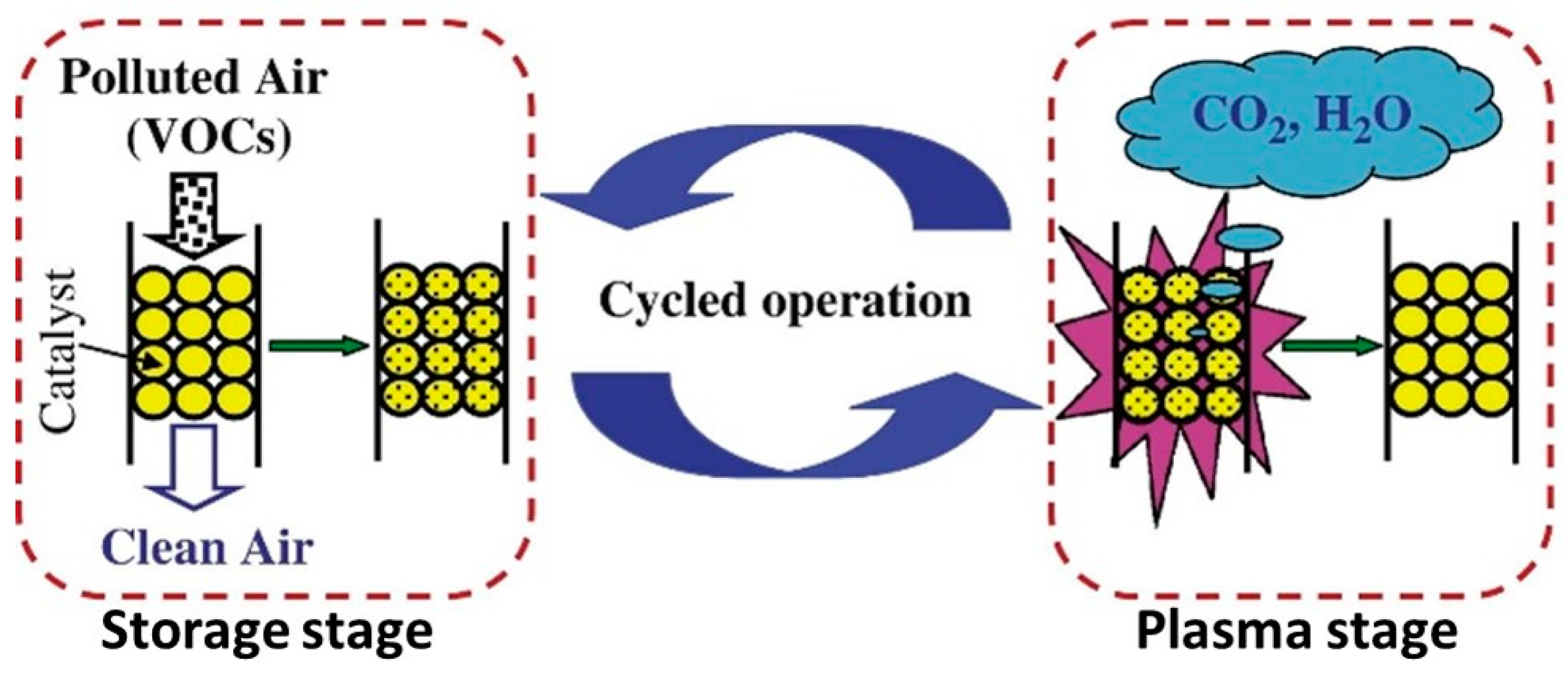
3.2. Plasma Catalysis of Adsorbed VOCs on Zeolite
3.3. Stability of Zeolite in Adsorption-Plasma Catalysis
4. Effect of Process Parameters
4.1. Effect of Humidity
4.2. Effect of Initial Concentration
4.3. Effect of Discharge Gas
4.4. Effect of Gas Flow Rate
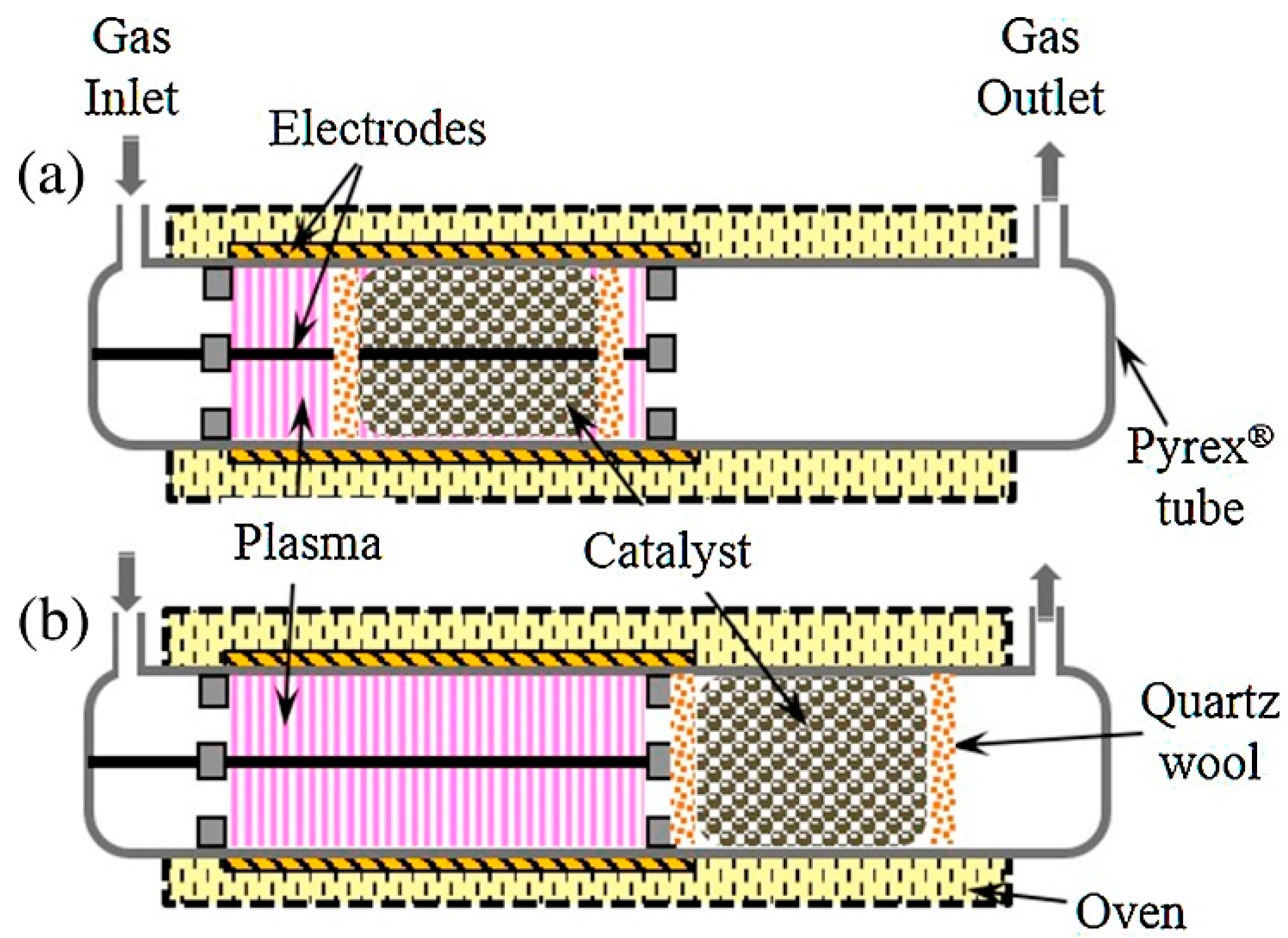
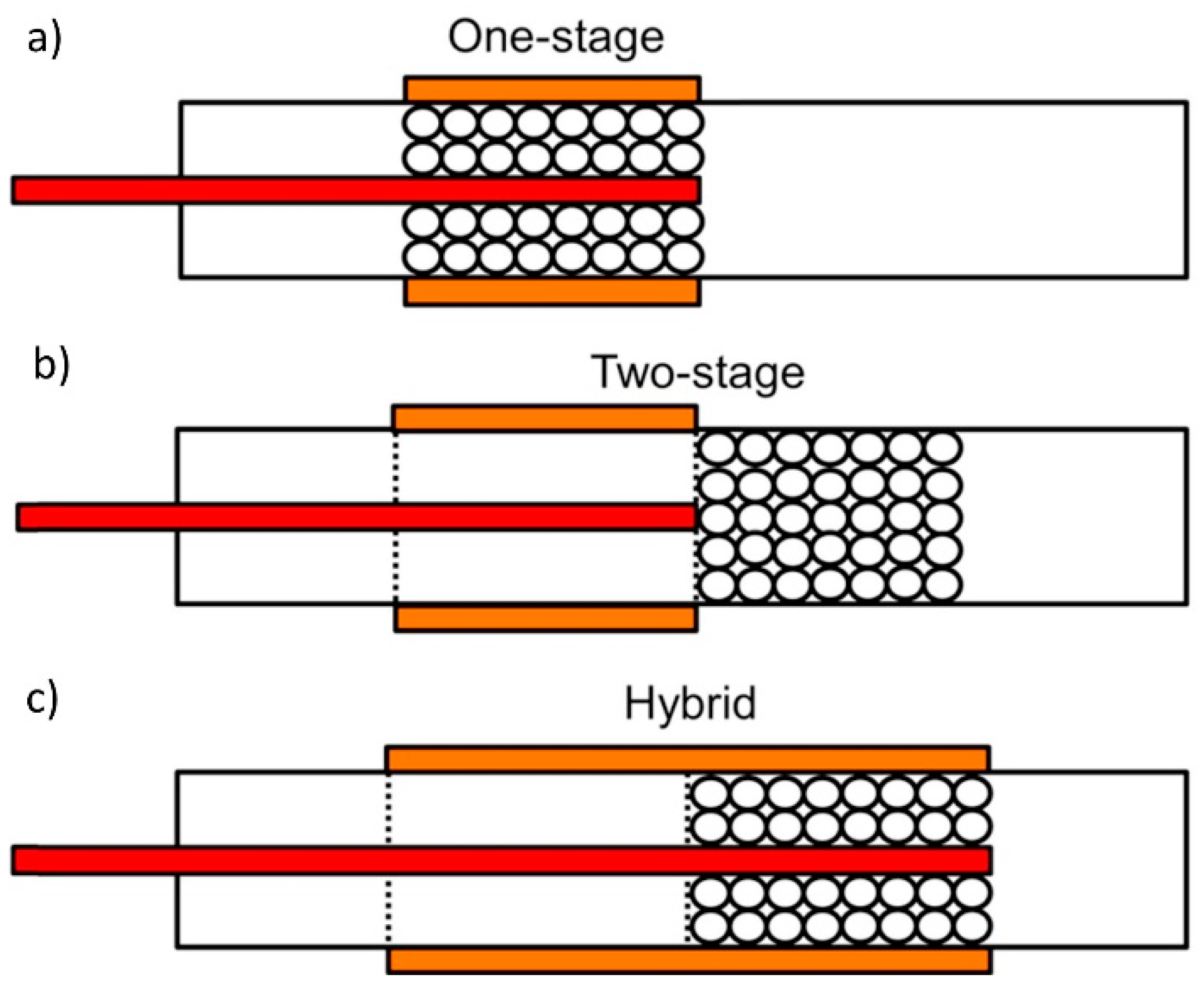
4.5. Reactor Configuration
4.5.1. Desorption Method
4.5.2. Position of Catalysts
4.6. Energy Cost
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- EUR-Lex—31999L0013—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A31999L0013 (accessed on 3 September 2018).
- Liu, Y.; Shao, M.; Fu, L.; Lu, S.; Zeng, L.; Tang, D. Source profiles of volatile organic compounds (VOCs) measured in China: Part I. Atmos. Environ. 2008, 42, 6247–6260. [Google Scholar] [CrossRef]
- Kim, Y.M.; Harrad, S.; Harrison, R.M. Concentrations and sources of VOCs in urban domestic and public microenvironments. Environ. Sci. Technol. 2001, 35, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
- Finlayson-Pitts, B.J.; Pitts, J.N., Jr. Tropospheric air pollution: Ozone, airborne toxics, polycyclic aromatic hydrocarbons, and particles. Science 1997, 276, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lee, S.C.; Chan, L.Y.; Li, W.M. Risk assessment of exposure to volatile organic compounds in different indoor environments. Environ. Res. 2004, 94, 57–66. [Google Scholar] [CrossRef]
- Pitten, F.A.; Bremer, J.; Kramer, A. Air pollution by volatile organic compounds (VOC) and health complaints. Dtsch. Med. Wochenschr. 2000, 125, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Ogata, A.; Futamura, S. Oxygen partial pressure-dependent behavior of various catalysts for the total oxidation of VOCs using cycled system of adsorption and oxygen plasma. Appl. Catal. B Environ. 2008, 79, 356–367. [Google Scholar] [CrossRef]
- Jones, A.P. Indoor air quality and health. Atmos. Environ. 1999, 33, 4535–4564. [Google Scholar] [CrossRef]
- Wolkoff, P.; Nielsen, G.D. Organic compounds in indoor air-their relevance for perceived indoor air quality? Atmos. Environ. 2001, 35, 4407–4417. [Google Scholar] [CrossRef]
- Luengas, A.; Barona, A.; Hort, C.; Gallastegui, G.; Platel, V.; Elias, A. A review of indoor air treatment technologies. Rev. Environ. Sci. Biotechnol. 2015, 14, 499–522. [Google Scholar] [CrossRef]
- Katari, V.S.; Vatavuk, W.M.; Wehe, A.H. Incineration techniques for control of volatile organic compound emissions Part I. fundamentals and process design considerations. JAPCA 1987, 37, 91–99. [Google Scholar] [CrossRef]
- Van der Vaart, D.R.; Vatvuk, W.M.; Wehe, A.H. Thermal and catalytic incinerators for the control of VOCs. J. Air Waste Manag. Assoc. 1991, 41, 92–98. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Z.; Shangguan, W. Low-temperature catalysis for VOCs removal in technology and application: A state-of-the-art review. Catal. Today 2016, 264, 270–278. [Google Scholar] [CrossRef]
- Iranpour, R.; Cox, H.H.J.; Deshusses, M.A.; Schroeder, E.D. Literature review of air pollution control biofilters and biotrickling filters for odor and volatile organic compound removal. Environ. Prog. 2005, 24, 254–267. [Google Scholar] [CrossRef]
- Guieysse, B.; Hort, C.; Platel, V.; Munoz, R.; Ondarts, M.; Revah, S. Biological treatment of indoor air for VOC removal: Potential and challenges. Biotechnol. Adv. 2008, 26, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Qian, H.; Li, X.; Cheng, Y.; He, H.; Zeng, G.; Xi, J. Simultaneous removal of multicomponent VOCs in biofilters. Trends Biotechnol. 2018, 36, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef]
- Vandenbroucke, A.M.; Morent, R.; De Geyter, N.; Leys, C. Non-thermal plasmas for non-catalytic and catalytic VOC abatement. J. Hazard. Mater. 2011, 195, 30–54. [Google Scholar] [CrossRef]
- Vandenbroucke, A.M.; Morent, R.; De Geyter, N.; Leys, C. Decomposition of toluene with plasma-catalysis: A review. J. Adv. Oxid. Technol. 2012, 15, 232–241. [Google Scholar] [CrossRef]
- Schmid, S.; Jecklin, M.C.; Zenobi, R. Degradation of volatile organic compounds in a non-thermal plasma air purifier. Chemosphere 2010, 79, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Zhang, Y.; Xu, Q.; Lamson, J.J.; Zhao, R. Photocatalytic purification of volatile organic compounds in indoor air: A literature review. Atmos. Environ. 2009, 43, 2229–2246. [Google Scholar] [CrossRef]
- Huang, Y.; Ho, S.S.H.; Lu, Y.; Niu, R.; Xu, L.; Cao, J.; Lee, S. Removal of indoor volatile organic compounds via photocatalytic oxidation: A short review and prospect. Molecules 2016, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Kaliya Perumal Veerapandian, S.; Leys, C.; De Geyter, N.; Morent, R. Abatement of VOCs using packed bed non-thermal plasma reactors: A review. Catalysts 2017, 7, 113. [Google Scholar] [CrossRef]
- Thevenet, F.; Sivachandiran, L.; Guaitella, O.; Barakat, C.; Rousseau, A. Plasma-catalyst coupling for volatile organic compound removal and indoor air treatment: A review. J. Phys. D Appl. Phys. 2014, 47, 224011. [Google Scholar] [CrossRef]
- Zhu, T.; Wan, Y.D.; Li, J.; He, X.W.; Xu, D.Y.; Shu, X.Q.; Liang, W.J.; Jin, Y.Q. Volatile organic compounds decomposition using nonthermal plasma coupled with a combination of catalysts. Int. J. Environ. Sci. Technol. 2011, 8, 621–630. [Google Scholar] [CrossRef] [Green Version]
- Kuroki, T.; Fujioka, T.; Kawabata, R.; Okubo, M.; Yamamoto, T. Regeneration of honeycomb zeolite by nonthermal plasma desorption of toluene. IEEE Trans. Ind. Appl. 2009, 45, 10–15. [Google Scholar] [CrossRef]
- Kuroki, T.; Fujioka, T.; Okubo, M.; Yamamoto, T. Toluene concentration using honeycomb nonthermal plasma desorption. Thin Solid Films 2007, 515, 4272–4277. [Google Scholar] [CrossRef]
- Karuppiah, J.; Sivachandiran, L.; Karvembu, R.; Subrahmanyam, C. Catalytic nonthermal plasma reactor for the abatement of low concentrations of isopropanol. Chem. Eng. J. 2010, 165, 194–199. [Google Scholar] [CrossRef]
- Tang, X.; Feng, F.; Ye, L.; Zhang, X.; Huang, Y.; Liu, Z.; Yan, K. Removal of dilute VOCs in air by post-plasma catalysis over Ag-based composite oxide catalysts. Catal. Today 2013, 211, 39–43. [Google Scholar] [CrossRef]
- Kim, H.-H.; Ogata, A. Nonthermal plasma activates catalyst: From current understanding and future prospects. Eur. Phys. J. Appl. Phys. 2011, 55, 13806. [Google Scholar] [CrossRef]
- Yamagata, Y.; Niho, K.; Inoue, K.; Okano, H.; Muraoka, K. Decomposition of volatile organic compounds at low concentrations using combination of densification by zeolite adsorption and dielectric barrier Discharge. Jpn. J. Appl. Phys. 2006, 45, 8251–8254. [Google Scholar] [CrossRef]
- Mok, Y.S.; Kim, D.H. Treatment of toluene by using adsorption and nonthermal plasma oxidation process. Curr. Appl. Phys. 2011, 11, S58–S62. [Google Scholar] [CrossRef]
- Lin, B.Y.; Chang, M.B.; Chen, H.L.; Lee, H.M.; Yu, S.J.; Li, S.N. Removal of C3F8 via the combination of non-thermal plasma, adsorption and catalysis. Plasma Chem. Plasma Process. 2011, 31, 585–594. [Google Scholar] [CrossRef]
- Oh, S.M.; Kim, H.H.; Einaga, H.; Ogata, A.; Futamura, S.; Park, D.W. Zeolite-combined plasma reactor for decomposition of toluene. Thin Solid Films 2006, 506–507, 418–422. [Google Scholar] [CrossRef]
- Inoue, K.; Okano, H.; Yamagata, Y.; Muraoka, K.; Teraoka, Y. Performance tests of newly developed adsorption/plasma combined system for decomposition of volatile organic compounds under continuous flow condition. J. Environ. Sci. 2011, 23, 139–144. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Zhu, T.; Fan, X. Removal of gas phase low-concentration toluene over Mn, Ag and Ce modified HZSM-5 catalysts by periodical operation of adsorption and non-thermal plasma regeneration. J. Hazard. Mater. 2015, 292, 70–78. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Liu, J.; Wu, J.; Zhu, A. Cycled storage-discharge (CSD) plasma catalytic removal of benzene over AgMn/HZSM-5 using air as discharge gas. Catal. Sci. Technol. 2016, 6, 3788–3796. [Google Scholar] [CrossRef]
- Pham Huu, T.; Gil, S.; Da Costa, P.; Giroir-Fendler, A.; Khacef, A. Plasma-catalytic hybrid reactor: Application to methane removal. Catal. Today 2015, 257, 86–92. [Google Scholar] [CrossRef]
- Kim, H.H.; Kim, J.H.; Ogata, A. Adsorption and oxygen plasma-driven catalysis for total oxidation of VOCs. Int. J. Plasma Environ. Sci. Technol. 2008, 2, 106–112. [Google Scholar]
- Ohshima, T.; Kondo, T.; Kitajima, N.; Sato, M. Adsorption and plasma decomposition of gaseous acetaldehyde on fibrous activated carbon. IEEE Trans. Ind. Appl. 2010, 46, 23–28. [Google Scholar] [CrossRef]
- Sivachandiran, L.; Thevenet, F.; Rousseau, A. Non-thermal plasma assisted regeneration of acetone adsorbed TiO2 surface. Plasma Chem. Plasma Process. 2013, 33, 855–871. [Google Scholar] [CrossRef]
- Zhao, D.-Z.; Li, X.-S.; Shi, C.; Fan, H.-Y.; Zhu, A.-M. Low-concentration formaldehyde removal from air using a cycled storage-discharge (CSD) plasma catalytic process. Chem. Eng. Sci. 2011, 66, 3922–3929. [Google Scholar] [CrossRef]
- Rajanikanth, B.S.; Srinivasan, A.D.; Nandiny, B.A. A cascaded discharge plasma-adsorbent technique for engine exhaust treatment. Plasma Sci. Technol. 2003, 5, 1825–1833. [Google Scholar] [CrossRef]
- Nishimura, J.; Kawamura, T.; Takahashi, K.; Teramoto, Y.; Takaki, K.; Koide, S.; Suga, M.; Orikasa, T.; Uchino, T. Removal of ethylene and by-products using packed bed dielectric barrier discharge with Ag nanoparticle-loaded zeolite. Electron. Commun. Jpn. 2017, 100, 320–327. [Google Scholar] [CrossRef]
- Kuroki, T.; Hirai, K.; Kawabata, R.; Okubo, M.; Yamamoto, T. Decomposition of adsorbed xylene on adsorbents using nonthermal plasma with gas circulation. IEEE Trans. Ind. Appl. 2010, 46, 672–679. [Google Scholar] [CrossRef]
- Bagreev, A.; Rahman, H.; Bandosz, T.J. Thermal regeneration of a spent activated carbon previously used as hydrogen sulfide adsorbent. Carbon 2001, 39, 1319–1326. [Google Scholar] [CrossRef]
- Yun, J.-H.; Yun, J.-H.; Choi, D.-K.; Moon, H. Benzene adsorption and hot purge regeneration in activated carbon beds. Chem. Eng. J. 2000, 55, 5857–5872. [Google Scholar] [CrossRef]
- Lee, D.-G.; Kim, J.-H.; Lee, C.-H. Adsorption and thermal regeneration of acetone and toluene vapors in dealuminated Y-zeolite bed. Sep. Purif. Technol. 2011, 77, 312–324. [Google Scholar] [CrossRef]
- Kim, K.-J.; Ahn, H.-G. The effect of pore structure of zeolite on the adsorption of VOCs and their desorption properties by microwave heating. Microporous Mesoporous Mater. 2012, 152, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Ghoshal, A.K.; Manjare, S.D. Selection of appropriate adsorption technique for recovery of VOCs: An analysis. J. Loss Prev. Process Ind. 2002, 15, 413–421. [Google Scholar] [CrossRef]
- Fan, Y.; Cai, Y.; Li, X.; Yin, H.; Chen, L.; Liu, S. Regeneration of the HZSM-5 zeolite deactivated in the upgrading of bio-oil via non-thermal plasma injection (NTPI) technology. J. Anal. Appl. Pyrolysis 2015, 111, 209–215. [Google Scholar] [CrossRef]
- Shiau, C.H.; Pan, K.L.; Yu, S.J.; Yan, S.Y.; Chang, M.B. Desorption of isopropyl alcohol from adsorbent with non-thermal plasma. Environ. Technol. 2017, 38, 2314–2323. [Google Scholar] [CrossRef] [PubMed]
- Ogata, A.; Einaga, H.; Kabashima, H.; Futamura, S.; Kushiyama, S.; Kim, H.-H. Effective combination of nonthermal plasma and catalysts for decomposition of benzene in air. Appl. Catal. B Environ. 2003, 46, 87–95. [Google Scholar] [CrossRef]
- Bahri, M.; Haghighat, F.; Rohani, S.; Kazemian, H. Metal organic frameworks for gas-phase VOCs removal in a NTP-catalytic reactor. Chem. Eng. J. 2017, 320, 308–318. [Google Scholar] [CrossRef]
- Delkash, M.; Bakhshayesh, B.E.; Kazemian, H. Using zeolitic adsorbents to cleanup special wastewater streams: A review. Microporous Mesoporous Mater. 2015, 214, 224–241. [Google Scholar] [CrossRef]
- Zaitan, H.; Manero, M.H.; Valdés, H. Application of high silica zeolite ZSM-5 in a hybrid treatment process based on sequential adsorption and ozonation for VOCs elimination. J. Environ. Sci. 2016, 41, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zou, J.; Yu, K.; Cheng, D.; Han, Y.; Zhan, J.; Ratanatawanate, C.; Jang, B.W.-L. Plasma application for more environmentally friendly catalyst preparation. Pure Appl. Chem. 2006, 78, 1227–1238. [Google Scholar] [CrossRef]
- Rhodes, C.J. Electric fields in zeolites: Fundamental features and environmental implications. Chem. Pap. 2016, 70, 4–21. [Google Scholar] [CrossRef]
- Yi, H.; Yang, X.; Tang, X.; Zhao, S.; Xie, X.; Feng, T.; Ma, Y.; Cui, X. Performance and pathways of toluene degradation over Co/13X by different processes based on nonthermal plasma. Energy Fuels 2017, 31, 11217–11224. [Google Scholar] [CrossRef]
- Weitkamp, J. Zeolites and Catalysis. Solid State Ion. 2000, 131, 175–188. [Google Scholar] [CrossRef]
- Moshoeshoe, M.; Nadiye-tabbiruka, M.S.; Obuseng, V. A Review of the Chemistry, Structure, Properties and Applications of Zeolites. Am. J. Mater. Sci. 2017, 7, 196–221. [Google Scholar]
- Zhang, L.; Peng, Y.; Zhang, J.; Chen, L.; Meng, X.; Xiao, F.-S. Adsorptive and catalytic properties in the removal of volatile organic compounds over zeolite-based materials. Chin. J. Catal. 2016, 37, 800–809. [Google Scholar] [CrossRef]
- Khodayar, M.; Franzson, H. Fracture pattern of Thjórsárdalur central volcano with respect to rift-jump and a migrating transform zone in South Iceland. J. Struct. Geol. 2007, 29, 898–912. [Google Scholar] [CrossRef]
- Wang, H.; Cao, Y.; Chen, Z.; Yu, Q.; Wu, S. High-efficiency removal of NOx over natural mordenite using an enhanced plasma-catalytic process at ambient temperature. Fuel 2018, 224, 323–330. [Google Scholar] [CrossRef]
- Barrer, R.M. Zeolites and their synthesis. Zeolites 1981, 1, 130–140. [Google Scholar] [CrossRef]
- Barrer, R.M. Synthesis of a zeolitic mineral with chabazite-like sorptive properties. J. Chem. Soc. 1948, 127–132. [Google Scholar] [CrossRef]
- Breck, D.W.; Eversole, W.G.; Milton, R.M. New synthetic crystalline zeolites. J. Am. Chem. Soc. 1956, 78, 2338–2339. [Google Scholar] [CrossRef]
- Jha, B.; Singh, D.N. A review on synthesis, characterization and industrial applications of flyash zeolites. J. Mater. Educ. 2011, 33, 65–132. [Google Scholar]
- Valdés, M.G.; Pérez-Cordoves, A.I.; Díaz-García, M.E. Zeolites and zeolite-based materials in analytical chemistry. Trends Anal. Chem. 2006, 25, 24–30. [Google Scholar] [CrossRef]
- Hashimoto, S. Zeolite photochemistry: Impact of zeolites on photochemistry and feedback from photochemistry to zeolite science. J. Photochem. Photobiol. C Photochem. Rev. 2003, 4, 19–49. [Google Scholar] [CrossRef]
- Corma, A.; Rey, F.; Valencia, S.; Jordá, J.L.; Rius, J. A zeolite with interconnected 8-, 10- and 12-ring pores and its unique catalytic selectivity. Nat. Mater. 2003, 2, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, Y.; Kim, H.-H.; Negishi, N.; Ogata, A. The role of ozone in the reaction mechanism of a bare zeolite-plasma hybrid system. Catalysts 2015, 5, 838–850. [Google Scholar] [CrossRef]
- Xu, M.; Mukarakate, C.; Robichaud, D.J.; Nimlos, M.R.; Richards, R.M.; Trewyn, B.G. Elucidating zeolite deactivation mechanisms during biomass catalytic fast pyrolysis from model reactions and zeolite syntheses. Top. Catal. 2016, 59, 73–85. [Google Scholar] [CrossRef]
- Lutz, W.; Zeolite, Y. Synthesis, modification, and properties—A case revisited. Adv. Mater. Sci. Eng. 2014, 36, 1389–1404. [Google Scholar]
- Guo, Y.; Zhang, H.; Liu, Y. Desorption characteristics and kinetic parameters determination of molecular sieve by thermogravimetric analysis/differential thermogravimetric analysis technique. Adsorpt. Sci. Technol. 2018, 36, 1389–1404. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.A.; Yoo, D.K.; Bhadra, B.N.; Jun, J.W.; Kim, T.-W.; Kim, C.-U.; Jhung, S.H. Preparation of SSZ-13 zeolites from beta zeolite and their application in the conversion of ethylene to propylene. Chem. Eng. J. 2018. [Google Scholar] [CrossRef]
- Al-Dughaither, A.S.; De Lasa, H. HZSM-5 zeolites with different SiO2/Al2O3 ratios. Characterization and NH3 desorption kinetics. Ind. Eng. Chem. Res. 2014, 53, 15303–15316. [Google Scholar] [CrossRef]
- Datka, J.; Tuznik, E. Infrared Spectroscopic Studies of Acid Properties of NaHZSM-5 Zeolites. J. Catal. 1986, 102, 43–51. [Google Scholar] [CrossRef]
- Reitmeier, S.J.; Gobin, O.C.; Jentys, A.; Lercher, J.A. Enhancement of sorption processes in the zeolite H-ZSM5 by postsynthetic surface modification. Angew. Chem. Int. Ed. 2009, 48, 533–538. [Google Scholar] [CrossRef]
- Weber, R.W.; Fletcher, J.C.Q.; Möller, K.P.; O’Connor, C.T. The characterization and elimination of the external acidity of ZSM-5. Microporous Mater. 1996, 7, 15–25. [Google Scholar] [CrossRef]
- Armaroli, T.; Simon, L.J.; Digne, M.; Montanari, T.; Bevilacqua, M.; Valtchev, V.; Patarin, J.; Busca, G. Effects of crystal size and Si/Al ratio on the surface properties of H-ZSM-5 zeolites. Appl. Catal. A Gen. 2006, 306, 78–84. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; Kalantarifard, A.; Yang, G.S.; Kim, C.S. In-situ immobilization of silver nanoparticles on ZSM-5 type zeolite by catechol redox chemistry, a green catalyst for A3-coupling reaction. Microporous Mesoporous Mater. 2016, 225, 296–302. [Google Scholar] [CrossRef]
- Dou, B.; Liu, D.; Zhang, Q.; Zhao, R.; Hao, Q.; Bin, F.; Cao, J. Enhanced removal of toluene by dielectric barrier discharge coupling with Cu-Ce-Zr supported ZSM-5/TiO2/Al2O3. Catal. Commun. 2017, 92, 15–18. [Google Scholar] [CrossRef]
- Ogata, A.; Ito, D.; Mizuno, K.; Kushiyama, S.; Yamamoto, T. Removal of dilute benzene using a zeolite-hybrid plasma reactor. IEEE Trans. Ind. Appl. 2001, 37, 959–964. [Google Scholar] [CrossRef]
- Makowski, W.; Ogorzałek, Ł. Determination of the adsorption heat of n-hexane and n-heptane on zeolites beta, L, 5A, 13X, Y and ZSM-5 by means of quasi-equilibrated temperature-programmed desorption and adsorption (QE-TPDA). Thermochim. Acta 2007, 465, 30–39. [Google Scholar] [CrossRef]
- Yi, H.; Yang, X.; Tang, X.; Zhao, S.; Wang, J.; Cui, X.; Feng, T.; Ma, Y. Removal of toluene from industrial gas over 13X zeolite supported catalysts by adsorption-plasma catalytic process. J. Chem. Technol. Biotechnol. 2017, 92, 2276–2286. [Google Scholar] [CrossRef]
- Huang, R.; Lu, M.; Wang, P.; Chen, Y.; Wu, J.; Fu, M.; Chen, L.; Ye, D. Enhancement of the non-thermal plasma-catalytic system with different zeolites for toluene removal. RSC Adv. 2015, 5, 72113–72120. [Google Scholar] [CrossRef]
- Díaz, E.; Ordóñez, S.; Vega, A.; Coca, J. Adsorption characterisation of different volatile organic compounds over alumina, zeolites and activated carbon using inverse gas chromatography. J. Chromatogr. A 2004, 1049, 139–146. [Google Scholar] [CrossRef]
- Yu, Y.; Zheng, L.; Wang, J. Adsorption behavior of toluene on modified 1X molecular sieves. J. Air Waste Manag. Assoc. 2012, 62, 1227–1232. [Google Scholar] [CrossRef]
- Triebe, R.W.; Tezel, F.H.; Khulbe, K.C. Adsorption of methane, ethane and ethylene on molecular sieve zeolites. Gas Sep. Purif. 1996, 10, 81–84. [Google Scholar] [CrossRef]
- Trinh, Q.H.; Mok, Y.S. Effect of the adsorbent/catalyst preparation method and plasma reactor configuration on the removal of dilute ethylene from air stream. Catal. Today 2015, 256, 170–177. [Google Scholar] [CrossRef]
- Van Mao, R.L.; Mclaughlin, G.P. Ethylene recovery from low grade gas stream by adsorption on zeolites and controlled desorption. Can. J. Chem. Eng. 1988, 66, 686–690. [Google Scholar]
- Trinh, Q.H.; Lee, S.B.; Mok, Y.S. Removal of ethylene from air stream by adsorption and plasma-catalytic oxidation using silver-based bimetallic catalysts supported on zeolite. J. Hazard. Mater. 2015, 285, 525–534. [Google Scholar] [CrossRef]
- Takahashi, A.; Yang, F.H.; Yang, R.T. Aromatics/aliphatics separation by adsorption: New sorbents for selective aromatics adsorption by π-complexation. Ind. Eng. Chem. Res. 2000, 39, 3856–3867. [Google Scholar] [CrossRef]
- Zhu, R.; Mao, Y.; Jiang, L.; Chen, J. Performance of chlorobenzene removal in a nonthermal plasma catalysis reactor and evaluation of its byproducts. Chem. Eng. J. 2015, 279, 463–471. [Google Scholar] [CrossRef]
- Kim, H.; Park, J.; Jung, Y. The binding nature of light hydrocarbons on Fe/MOF-74 for gas separation. Phys. Chem. Chem. Phys 2013, 15, 19644–19650. [Google Scholar] [CrossRef]
- Shen, B.; Fan, K.; Wang, W.; Deng, J. Ab initio study on the adsorption and oxidation of HCHO with Ag2 cluster. J. Mol. Struct. 1999, 469, 157–161. [Google Scholar] [CrossRef]
- Qin, C.; Huang, X.; Zhao, J.; Huang, J.; Kang, Z.; Dang, X. Removal of toluene by sequential adsorption-plasma oxidation: Mixed support and catalyst deactivation. J. Hazard. Mater. 2017, 334, 29–38. [Google Scholar] [CrossRef]
- Fan, H.-Y.; Shi, C.; Li, X.-S.; Zhao, D.-Z.; Xu, Y.; Zhu, A.-M. High-efficiency plasma catalytic removal of dilute benzene from air. J. Phys. D Appl. Phys. 2009, 42, 225105. [Google Scholar] [CrossRef]
- Fan, H.-Y.; Li, X.-S.; Shi, C.; Zhao, D.-Z.; Liu, J.-L.; Liu, Y.-X.; Zhu, A.-M. Plasma catalytic oxidation of stored benzene in a cycled storage-discharge (CSD) process: Catalysts, reactors and operation conditions. Plasma Chem. Plasma Process. 2011, 31, 799–810. [Google Scholar] [CrossRef]
- Einaga, H.; Futamura, S. Catalytic oxidation of benzene with ozone over Mn ion-exchanged zeolites. Catal. Commun. 2007, 8, 557–560. [Google Scholar] [CrossRef]
- Xia, J.F.; Gao, X.X.; Kong, J.Y.; Hui, H.X.; Cui, M.; Yan, K.P. By-products NOx control and performance improvement of a packed-bed nonthermal plasma reactor. Plasma Chem. Plasma Process. 2000, 20, 225–233. [Google Scholar] [CrossRef]
- Xu, X.; Wang, P.; Xu, W.; Wu, J.; Chen, L.; Fu, M.; Ye, D. Plasma-catalysis of metal loaded SBA-15 for toluene removal: Comparison of continuously introduced and adsorption-discharge plasma system. Chem. Eng. J. 2016, 283, 276–284. [Google Scholar] [CrossRef]
- Bahri, M.; Haghighat, F. Plasma-Based Indoor Air Cleaning Technologies: The State of the Art-Review. Clean-Soil Air Water 2014, 42, 1667–1680. [Google Scholar] [CrossRef]
- Oda, T.; Takahashi, T.; Kohzuma, S. Decomposition of dilute trichloroethylene by using non-thermal plasma processing—Frequency and catalyst effect. In Proceedings of the Thirty-Third IAS Annual Meeting Conference Record of 1998 IEEE Industry Applications Conference, St. Louis, MO, USA, 12–15 October 1998; pp. 1871–1876. [Google Scholar]
- Oda, T. Non-thermal plasma processing for environmental proection: Decomposition of dilute VOCs in air. J. Electrost. 2003, 57, 293–311. [Google Scholar] [CrossRef]
- Trinh, Q.H.; Gandhi, M.S.; Mok, Y.S. Adsorption and plasma-catalytic oxidation of acetone over zeolite-supported silver catalyst. Jpn. J. Appl. Phys. 2015, 54, 01AG04. [Google Scholar] [CrossRef]
- Kim, H.H.; Ogata, A.; Futamura, S. Effect of different catalysts on the decomposition of VOCs using flow-type plasma-driven catalysis. IEEE Trans. Plasma Sci. 2006, 34, 984–995. [Google Scholar] [CrossRef]
- Hu, J.; Jiang, N.; Li, J.; Shang, K.; Lu, N.; Wu, Y. Degradation of benzene by bipolar pulsed series surface/packed-bed discharge reactor over MnO2-TiO2/zeolite catalyst. Chem. Eng. J. 2016, 293, 216–224. [Google Scholar] [CrossRef]
- Li, W.; Gibbs, G.V.; Oyama, S.T. Mechanism of ozone decomposition on a manganese oxide catalyst. 1. In situ Raman spectroscopy and ab initio molecular orbital calculations. J. Am. Chem. Soc. 1998, 120, 9041–9046. [Google Scholar] [CrossRef]
- Li, W.; Oyama, S.T. Mechanism of ozone decomposition on a manganese oxide catalyst. 2. Steady-state and transient kinetic studies. J. Am. Chem. Soc. 1998, 120, 9047–9052. [Google Scholar] [CrossRef]
- Reed, C.; Xi, Y.; Oyama, S.T. Distinguishing between reaction intermediates and spectators: A kinetic study of acetone oxidation using ozone on a silica-supported manganese oxide catalyst. J. Catal. 2005, 235, 378–392. [Google Scholar] [CrossRef]
- Reed, C.; Lee, Y.-K.; Oyama, S.T. Structure and oxidation state of silica-supported manganese oxide catalysts and reactivity for acetone oxidation with ozone. J. Phys. Chem. B 2006, 110, 4207–4216. [Google Scholar] [CrossRef] [PubMed]
- Garcia, T.; Agouram, S.; Sánchez-Royo, J.F.; Murillo, R.; Mastral, A.M.; Aranda, A.; Vázquez, I.; Dejoz, A.; Solsona, B. Deep oxidation of volatile organic compounds using ordered cobalt oxides prepared by a nanocasting route. Appl. Catal. A Gen. 2010, 386, 16–27. [Google Scholar] [CrossRef]
- Youn, J.S.; Bae, J.; Park, S.; Park, Y.K. Plasma-assisted oxidation of toluene over Fe/zeolite catalyst in DBD reactor using adsorption/desorption system. Catal. Commun. 2018, 113, 36–40. [Google Scholar] [CrossRef]
- Qin, C.; Guo, H.; Liu, P.; Bai, W.; Huang, J.; Huang, X.; Dang, X.; Yan, D. Toluene abatement through adsorption and plasma oxidation using ZSM-5 mixed with γ-Al2O3, TiO2or BaTiO3. J. Ind. Eng. Chem. 2018, 63, 449–455. [Google Scholar] [CrossRef]
- Kim, H.-H.; Teramoto, Y.; Negishi, N.; Ogata, A. A multidisciplinary approach to understand the interactions of nonthermal plasma and catalyst: A review. Catal. Today 2015, 256, 13–22. [Google Scholar] [CrossRef]
- Patil, B.S.; Cherkasov, N.; Lang, J.; Ibhadon, A.O.; Hessel, V.; Wang, Q. Low temperature plasma-catalytic NOx synthesis in a packed DBD reactor: Effect of support materials and supported active metal oxides. Appl. Catal. B Environ. 2016, 194, 123–133. [Google Scholar] [CrossRef]
- Kim, H.-H.; Teramoto, Y.; Sano, T.; Negishi, N.; Ogata, A. Effects of Si/Al ratio on the interaction of nonthermal plasma and Ag/HY catalysts. Appl. Catal. B Environ. 2015, 166–167, 9–17. [Google Scholar] [CrossRef]
- Hamada, S.; Hojo, H.; Einaga, H. Effect of catalyst composition and reactor configuration on benzene oxidation with a nonthermal plasma-catalyst combined reactor. Catal. Today 2018. [Google Scholar] [CrossRef]
- Wallis, A.E.; Whitehead, J.C.; Zhang, K. The removal of dichloromethane from atmospheric pressure nitrogen gas streams using plasma-assisted catalysis. Appl. Catal. B Environ. 2007, 72, 282–288. [Google Scholar] [CrossRef]
- Fitzsimmons, C.; Ismail, F.; Whitehead, J.C.; Wilman, J.J. The chemistry of dichloromethane destruction in atmospheric-pressure gas streams by a dielectric packed-bed plasma reactor. J. Phys. Chem. A 2000, 104, 6032–6038. [Google Scholar] [CrossRef]
- Jiang, L.; Nie, G.; Zhu, R.; Wang, J.; Chen, J.; Mao, Y.; Cheng, Z.; Anderson, W.A. Efficient degradation of chlorobenzene in a non-thermal plasma catalytic reactor supported on CeO2/HZSM-5 catalysts. J. Environ. Sci. 2017, 55, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.F.; Fu, X.; Liu, Y.; Abbas, Y.; Wang, H.; Lu, W. Volatile organic compounds (VOCs) removal in non-thermal plasma double dielectric barrier discharge reactor. J. Hazard. Mater. 2018, 347, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Formisano, B.; Bonten, C. Extruded zeolitic honeycombs for sorptive heat storage. AIP Conf. Proc. 2016, 1779, 030003. [Google Scholar] [Green Version]
- Chang, F.-T.; Lin, Y.-C.; Bai, H.; Pei, B.-S. Adsorption and desorption characteristics of semiconductor volatile organic compounds on the thermal swing honeycomb zeolite concentrator. J. Air Waste Manag. Assoc. 2003, 53, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-H.; Oh, S.-M.; Ogata, A.; Futamura, S. Decomposition of gas-phase benzene using plasma—Driven catalyst reactor: Complete oxidation of adsorbed benzene using oxygen plasma. J. Adv. Oxid. Technol. 2005, 8, 226–233. [Google Scholar] [CrossRef]
- Dang, X.; Huang, J.; Cao, L.; Zhou, Y. Plasma-catalytic oxidation of adsorbed toluene with gas circulation. Catal. Commun. 2013, 40, 116–119. [Google Scholar] [CrossRef]
- Yi, H.; Yang, X.; Tang, X.; Zhao, S. Removal of toluene from industrial gas by adsorption–plasma catalytic process: Comparison of closed discharge and ventilated discharge. Plasma Chem. Plasma Process. 2018, 38, 331–345. [Google Scholar] [CrossRef]
- Oh, S.M.; Kim, H.H.; Ogata, A.; Einaga, H.; Futamura, S.; Park, D.W. Effect of zeolite in surface discharge plasma on the decomposition of toluene. Catal. Lett. 2005, 99, 101–104. [Google Scholar] [CrossRef]
- Urashima, K.; Kostov, K.G.; Chang, J.S.; Okayasu, Y.; Iwaizumi, T.; Yoshimura, K.; Kato, T. Removal of C2F6 from a semiconductor process flue gas by a ferroelectric packed-bed barrier discharge reactor with an adsorber. IEEE Trans. Ind. Appl. 2001, 37, 1456–1463. [Google Scholar] [CrossRef]










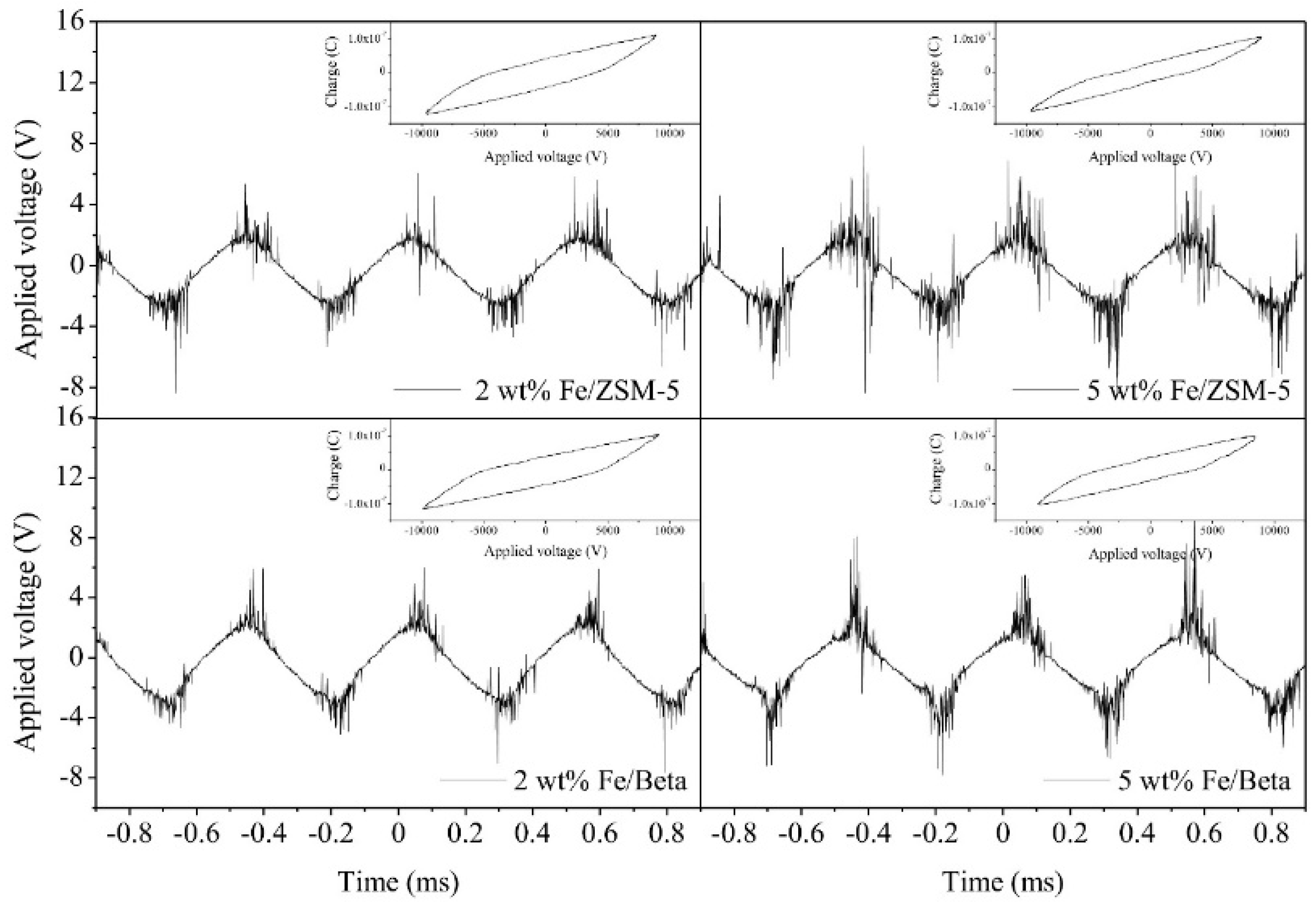





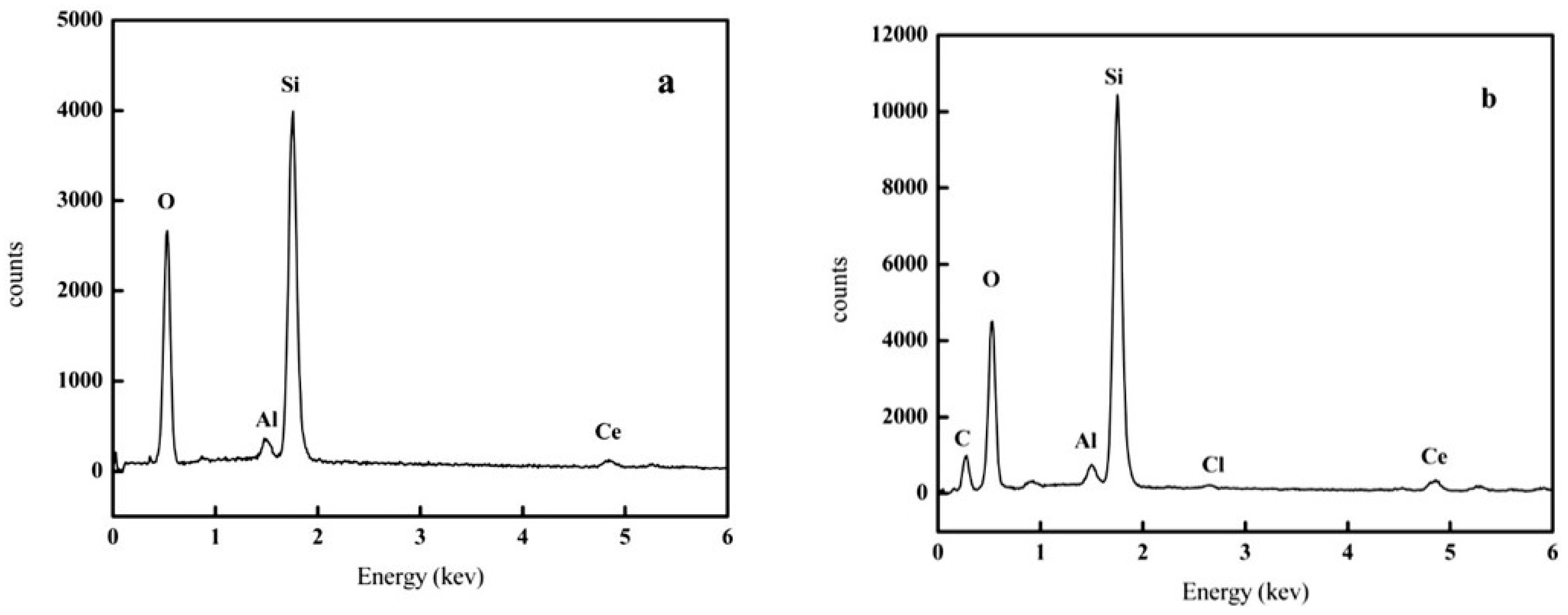



| Zeolite | Specific Surface Area (m2/g) | Pore Diameter (Å) | Adsorption Capacity (mg/g) |
|---|---|---|---|
| MS-3A | 224.95 | 3.52 | 17.75 |
| MS-4A | 290.54 | 4.79 | 19.21 |
| MS-5A | 391.94 | 5.33 | 21.27 |
| MS-10A | 439.31 | 9.66 | 126.61 |
| Catalyst | SBET (m2g−1) | Toluene Adsorption Amount (mmol) |
|---|---|---|
| Ag/HZSM-5 | 367.6 | 0.29 |
| Mn/HZSM-5 | 398.5 | 0.22 |
| Ce/HZSM-5 | 380.6 | 0.21 |
| Ag–Mn/HZSM-5 | 350.0 | 0.27 |
| Ce–Mn/HZSM-5 | 344.0 | 0.21 |
| HZSM-5 | 486.6 | 0.26 |
| Sample | Breakthrough Capacity (μmol/mL-cat) |
|---|---|
| HZSM-5 | 8.5 |
| Ag/HZSM-5 | 18.5 |
| Cu/HZSM-5 | 11.8 |
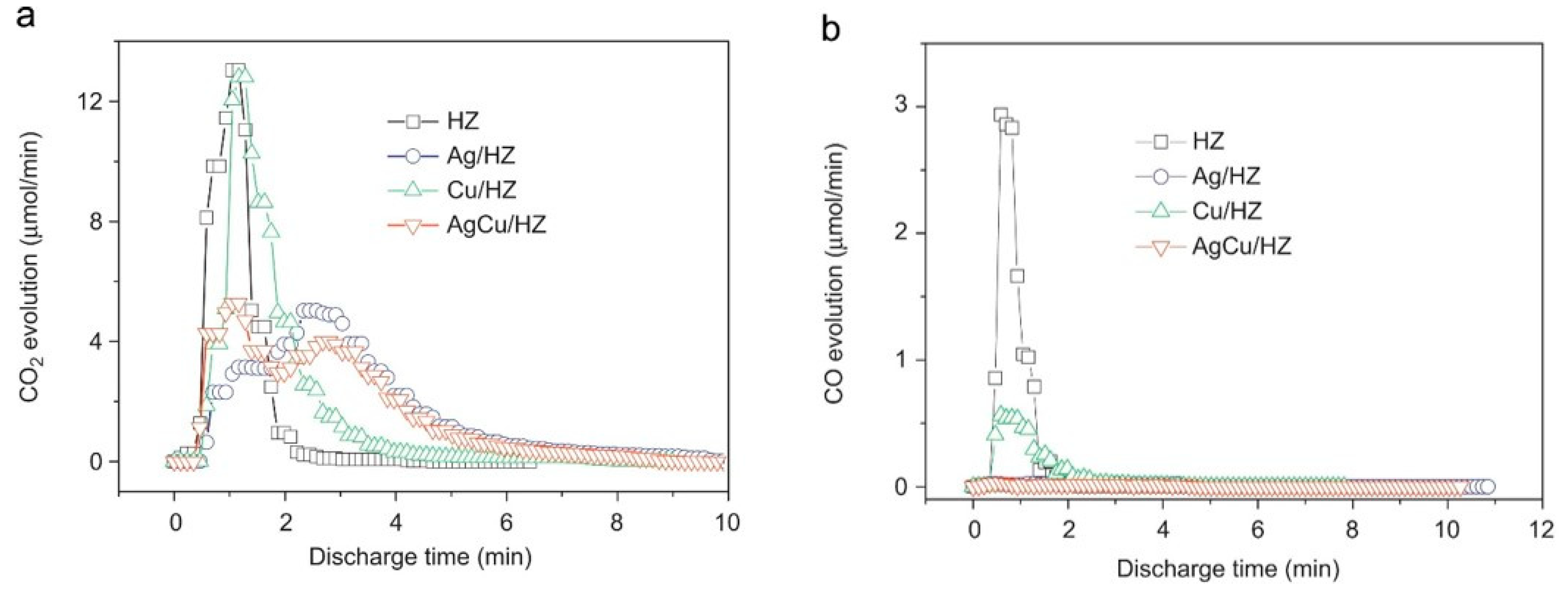
| AgCu/HZSM-5 | 38.9 |
| VOCs | Zeolite | SBET (m2/g) * | Pore Size (Å) * | Type of Discharge | Carrier Gas | Flow Rate (L/min) * | Initial VOC Concentration (ppm) * | Amount of Adsorption/Removal Efficiency | Energy Density and Frequency | By-Products | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetone | 3 wt % Ag/β-zeolite (15 g) | -- | -- | PBDBD (IPC) t1 = 100 min | N2 | 2 | 300 | 2.45 mmol | -- | -- | [109] |
| PBDBD (IPC) t2 = 15 min | O2 | 2 | -- | 97% | 28 W (400 Hz) | CH4 (less), HCHO, CH3CHO | |||||
| Benzene | Ferrierite | 270 | -- | SD (IPC) adsorption | N2 | 4–5 | 200 | -- | -- | -- | [8] |
| SD (IPC) Closed discharge | 50% O2/N2 | 5–8 | -- | 57% | 130 J/L | ||||||
| MS-13X | 540 | -- | SD (IPC) adsorption | N2 | 4–5 | 200 | -- | -- | -- | ||
| SD (IPC) Closed discharge | 50% O2/N2 | 5–8 | -- | 43% | 89 J/L | ||||||
| H-Y zeolite | -- | -- | SD (IPC) adsorption | N2 | 10 | 200 | -- | -- | -- | ||
| SD (IPC) Closed discharge | 50% O2/N2 | 5–8 | -- | 84% | 140 J/L | ||||||
| 2% Ag/H-Y zeolite | 520 | -- | SD (IPC) adsorption | N2 | 10 | 200 | -- | -- | -- | ||
| SD (IPC) Closed discharge | 50% O2/N2 | 5–8 | -- | 89% | 160 J/L | ||||||
| Benzene | TiO2-MnO2/zeolite | -- | -- | SSPBD (IPC) Continuous | Air | 0.5 | 400 | 81% | 10.33 W (50 Hz; 1 nF) | CO | [111] |
| Benzene | Mn/USY zeolite | 715 | -- | SD (IPC) Continuous | 20% O2/N2 | 1 | 200 | 95% | 12 W (60 Hz) | CO | [122] |
| Benzene | Ferrierite | -- | -- | SD (IPC) Continuous | 50% O2/N2 | 5–8 | 200 | 58% | 130 J/L (500 Hz) | -- | [41] |
| 10% Ag/Mordenite | -- | -- | 50% O2/N2 | 97% | 130 J/L (500 Hz) | -- | |||||
| 10% Ag/HY | -- | -- | 60% O2/N2 | 96% | 154 J/L (500 Hz) | -- | |||||
| 10% Ag/MS-13X | -- | -- | 60% O2/N2 | 88% | 154 J/L (500 Hz) | -- | |||||
| Benzene | Ferrierite | 270 | 4.3–5.3 | SD (IPC) Continuous | Synthetic air | 4 | 200 | 78% | 200 J/L (500 Hz) | -- | [110] |
| 2 wt % Ag/H-Y | 520 | 7.4 | 10 | 75% | |||||||
| 2 wt % Ag/H-Y & 0.5 wt % Pt/γ-Al2O3 | -- | -- | 10 | 86% | |||||||
| Benzene | Ag/HZSM-5 | 334 | -- | DBD (IPC) t1 = 60 min | Humid air (RH = 50%) | 0.6 | 4.7 | 7.6 μmol | -- | -- | [101] |
| DBD (IPC) t2 = 13 min | O2 | 0.06 | -- | 100% | 4.7 W (2 kHz) | CO | |||||
| Benzene | HZSM-5 (0.05 g) | 341 | -- | DBD (IPC) adsorption up to 5% of initial benzene | Humid air (1.5 vol % H2O) | 100 SSCM | 20 | 45 μmol/cat | -- | -- | [39] |
| 0.7 wt % Ag/HZSM-5 (0.05 g) | 341 | -- | 78 μmol/cat | ||||||||
| 2.4 wt % Mn/HZSM-5 (0.05 g) | 334 | -- | 94 μmol/cat | ||||||||
| 0.8 wt % Ag 2.4 wt % Mn/HZSM-5 (0.05 g) | 336 | -- | 131 μmol/cat | ||||||||
| Chloro-benzene | 12 wt % CeO2/HZSM-5 (0.3 g) | -- | -- | DBD (IPC) Continuous | Dry air | -- | 1250 mg/m3 | 96% | 7 kV (10 kHz) | -- | [125] |
| Chloro-benzene | 12 wt % CeO2/HZSM-5 (0.3 g) | 315.8 | -- | DBD (IPC) Continuous | Air | 1 | 1250 mg/m3 | 72.6% | 5 kV (10 kHz) | CO | [97] |
| Dichloro-methane | HZSM-5 (140 °C) | 338 | 5.4 | PBDBD (PPC) Continuous | Air | 1 | 500 | 36% | 0.9 W (10.25–13.25 kHz) | CO, NOx, HCOCl | [123] |
| Calcined HZSM-5 (140 °C) | 338 | 5.4 | 30% | CO, NOx, HCOCl | |||||||
| NaZSM-5 (140 °C) | 338 | 5.4 | 32% | <NOx, HCOCl, CO | |||||||
| NaA (140 °C) | -- | 4 | 32% | <HCOCl, CO <NOx | |||||||
| NaX (140 °C) | -- | 10 | 32% | <NOx, HCOCl, CO | |||||||
| Ethylene | Ag-Fe (1.5–0.5%)/13X (45 g) | -- | -- | PBDBD (IPC) t1 = 60 min | 21% O2 + 79% N2 | 1 | 270 | 662.2 μmol | -- | -- | [95] |
| PBDBD (IPC) t2 = 30 min | -- | 100% | 15 kV (400 Hz) | -- | |||||||
| Ethylene | Ag/13X (30 g) | 699.6 | -- | PBDBD (IPC) t1 = 100 min | 21% O2 + 79% N2 | 1 | 200 | 817.5 μmol | -- | -- | [93] |
| PBDBD (IPC) dist2 = 20 min | -- | 42% | 20 kV (400 Hz) | CO | |||||||
| Ethylene | 10 wt % Ag/MS-13X (10 g) | -- | -- | PBDBD (IPC) Continuous | 10% O2, 10% CO2/N2 | 5 | 200 | 100% | 0–90 J/L (10 kHz) | CO | [46] |
| Formaldehyde | 3.6 wt % Ag 2.1 wt % Cu/HZSM-5 | 299 | -- | PBDBD (IPC) t1 = 40 min | Humid air (RH = 50%) | 0.3 | 26.6 | 14 μmol | -- | -- | [44] |
| PBDBD (IPC) Open discharge | O2 | 0.06 | -- | 100% | 2.3 W (2 kHz) | -- | |||||
| Hexafluro-ethane | BaTiO3/zeolite | -- | -- | PBDBD (PPC) Continuous | N2 | 0.025 | 3000 | 100% | 8 kV (60 Hz) | -- | [133] |
| Iso-propyl alcohol | MS-10A | 439.31 | 9.66 | PBDBD (IPC) t1 = 60 min | N2 | 1 | 400 | -- | -- | -- | [54] |
| PBDBD (IPC) Open discharge | N2 | 2 | -- | -- | 9.66 W (300 Hz) | -- | |||||
| Methane | 13X | -- | -- | PBDBD (IPC) Continuous | -- | -- | <1000 | 26% | 173–200 Wh/m3 (20 kHz) | NOx | [104] |
| Trichloro-ethylene | Cu-ZSM-5 | -- | - | DBD (IPC) Continuous | Dry air | 0.4 | 1000 | >95% | 1 W (50 Hz) | -- | [107] |
| Toluene | ZSM-5-BaTiO3 | 165 | -- | PBDBD (IPC) Adsorption till breakthrough | Humid air (19%) | 0.6 | 1632 mg/m3 | 6.75 mg/g | -- | [118] | |
| PBDBD (IPC) t2 = 120 min | Air | -- | 100% | 22 kV (50 Hz) | N2O | ||||||
| AgMn-ZSM-5-BaTiO3 | 139 | -- | PBDBD (IPC) Adsorption till breakthrough | Humid air (19%) | 1632 mg/m3 | 12.5 mg/g | -- | ||||
| PBDBD (IPC) t2 = 120 min | Air | -- | 100% | 22 kV (50 Hz) | <N2O | ||||||
| Toluene | Na-Y | 750 | 7.4 | DBD (PPC) ttotal = 160 min t1 = 30 min t2 = 10 min | Humid air (0.5% H2O) | 500 cm3/min | 200 | 52 | 5 W (24 kHz) | CO, HCOOH | [74] |
| H-Y (1) | 650 | 7.4 | 55 | ||||||||
| H-Y (2) | 520 | 7.4 | 50 | ||||||||
| Mordenite | 460 | 6.7 × 7.0 | 35 | ||||||||
| Ferrierite | 270 | 4.3 × 5.5 | 20 | ||||||||
| Toluene | NaY | -- | -- | SD (IPC) t1 = 160 min | Synthetic humid air (0.5% H2O) | 0.5 | 200 | 6.5 × 10−4 mol/g | -- | -- | [36] |
| SD (IPC) Open discharge | -- | 50% | 5 W (50 Hz) | -- | |||||||
| Toluene | 13X | -- | -- | DBD (IPC) t1 = 282 mins | Air | 0.4 | 150 | 0.57 mmol | -- | -- | [131] |
| DBD (IPC) closed discharge (t2 = 1 h) | -- | -- | -- | 95% | 20 W | -- | |||||
| DBD (IPC) ventilated discharge (t2 = 1 h) | Synthetic air (80% N2 + 20% O2) | 0.1 | -- | 95% | 20 W | -- | |||||
| Co/13X | 386.214 | -- | DBD (IPC) ventilated discharge (t2 = 1 h) | O2 | 0.02 | -- | 93.9% | 20 W | -- | ||
| 0.03 | -- | 93.5% | 20 W | -- | |||||||
| Air (80% N2 + 20% O2) | 0.1 | -- | 92.7% | 20 W | -- | ||||||
| 0.15 | -- | 89.8% | 20 W | -- | |||||||
| MS 5A | -- | -- | DBD (IPC) t1 = 30 min | Air | 0.4 | 150 | 0.021 mmol | -- | -- | ||
| DBD (IPC) closed discharge (t2 = 1 h) | -- | -- | 95% | 20 W | -- | ||||||
| DBD (IPC) ventilated discharge (t2 = 1 h) | Synthetic air (80% N2 + 20% O2) | 0.1 | -- | 91% | 20 W | -- | |||||
| Toluene | HZSM-5 (1.6 g) | 486.587 | -- | DBD (IPC) t1 = 8 h | Humid air (RH = 40%) | 3 | 3 | 0.25 mmol | -- | -- | [38] |
| DBD (IPC) Open discharge | Synthetic air | 0.8 | -- | 100% | 2.4 W | -- | |||||
| Ag-Mn/HZSM-5 (1.6 g) | 350.051 | -- | DBD (IPC) t1 = 8 h | Humid air (RH = 40%) | 3 | 3 | 0.27 mmol | -- | -- | ||
| DBD (IPC) Open discharge | Synthetic air | 0.8 | -- | 100% | 2.4 W | -- | |||||
| Toluene | Honeycomb zeolite | -- | -- | DBD (IPC) t1 = 60 min | Dry air | 2 | 30 | 3.5 mL | -- | -- | [29] |
| DBD (IPC) Open discharge | Dry air–forward flow | 1 | -- | 83% | 20 W (420 Hz) | -- | |||||
| Dry air–reverse flow | 51% | 20 W (420 Hz) | -- | ||||||||
| N2–forward flow | 68% | 20 W (420 Hz) | -- | ||||||||
| N2–reverse flow | 37% | 20 W (420 Hz) | -- | ||||||||
| Toluene | MS-13X | 626.439 | 26.6 | DBD (IPC) Adsorption till breakthrough | Air | 0.4 | 150 | 0.57 mmol | -- | -- | [88] |
| DBD (IPC) Open discharge | Synthetic air | 0.1 | -- | 94% | 20 W | -- | |||||
| 5% Co/13X | 386.214 | DBD (IPC) Adsorption till breakthrough | Air | 0.4 | 150 | 0.51 mmol | -- | -- | |||
| DBD (IPC) Open discharge | Synthetic air | 0.1 | -- | 92.7% | 20 W | -- | |||||
| Toluene | Honeycomb zeolite (5.2 g) | -- | -- | DBD (IPC) t1 = 60 min | Air | 2 | 30 | 3.6 mL | -- | -- | [28] |
| DBD (IPC) t2 = 5 mins (with 1 min closing time) Reverse flow | 1 | -- | Reg Eff = 82.3% | 20 W Pulsed hv (420 Hz) | NO, NO2 | ||||||
| DBD (IPC)t2 = 4 mins Reverse flow | Reg Eff = 78.3% | ||||||||||
| Toluene | Co/13X | 386.214 | 37.78 | DBD (IPC) t1 = till equilibrium | Synthetic air | 0.4 | 150 | 0.51 mmol | -- | -- | [61] |
| DBD (IPC) t2 = 1 h | 0.1 | -- | 92.7% | 20 W | -- | ||||||
| Toluene | 5A | -- | 5 | PBDBD (IPC) Continuous | Dry air | 0.3 | 100 | 79.8% | 3.3–3.7 W (2 kHz) | -- | [89] |
| HZSM-5 | -- | 5.5 | 80.8% | ||||||||
| Hβ | -- | 6.6 | 98.4% | ||||||||
| H-Y | -- | 7.4 | 85.2% | ||||||||
| Ag/H-Y | -- | -- | 97% | ||||||||
| Toluene | CuCeZr/ZSM-5 | 295 | 3.7 | PBDBD (IPC) Continuous | Dry air | 1 | 1400 | 77% | 43 W | -- | [85] |
| Toluene | Honeycomb zeolite | -- | -- | DBD (IPC) Continuous | - | 150 | 30 | 79% | 89 W (60 Hz) | - | [33] |
| DBD (IPC) APC-closed discharge | 25 | 70% to 93% | |||||||||
| p-xylene | HiSiv3000 (4.8 g) | -- | -- | DBD (IPC) t1 = 130 min | Dry air | 2 | 20 | 5.2 mL | -- | -- | [47] |
| DBD (IPC) Open discharge | Gas circulation | 0.5 | -- | 43% | 38 W (60 Hz) | O3 | |||||
| Xylene mixture (p-, o-, and m- xylene) | HiSiv3000 (2.5 g) and HiSiv1000 (5.5 g) | -- | -- | DBD (IPC) t1 = 130 min | Dry air | 2 | 20 | 5.2 mL | -- | -- | |
| DBD (IPC) Open discharge | Gas circulation | 0.5 | -- | 69% | 38 W (60 Hz) | O3 | |||||
| VOC mixture (C2Cl4, C7H8, C2HCl3, C6H6, C4H8O2, CS2) | HZSM-5 (2 g) | 202.82 | -- | DDBD (IPC) Continuous | N2 | 4 | 100 | 100% | 65.8 W (900 Hz) | SO2, C2H5Cl, C2H4O, C6H8N2, CH4, C2H3NO | [126] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
K. P. Veerapandian, S.; De Geyter, N.; Giraudon, J.-M.; Lamonier, J.-F.; Morent, R. The Use of Zeolites for VOCs Abatement by Combining Non-Thermal Plasma, Adsorption, and/or Catalysis: A Review. Catalysts 2019, 9, 98. https://doi.org/10.3390/catal9010098
K. P. Veerapandian S, De Geyter N, Giraudon J-M, Lamonier J-F, Morent R. The Use of Zeolites for VOCs Abatement by Combining Non-Thermal Plasma, Adsorption, and/or Catalysis: A Review. Catalysts. 2019; 9(1):98. https://doi.org/10.3390/catal9010098
Chicago/Turabian StyleK. P. Veerapandian, Savita, Nathalie De Geyter, Jean-Marc Giraudon, Jean-François Lamonier, and Rino Morent. 2019. "The Use of Zeolites for VOCs Abatement by Combining Non-Thermal Plasma, Adsorption, and/or Catalysis: A Review" Catalysts 9, no. 1: 98. https://doi.org/10.3390/catal9010098






