Eucalyptol as a Bio-Based Solvent for Buchwald-Hartwig Reaction on O,S,N-Heterocycles
Abstract
:1. Introduction
2. Results and Discussion
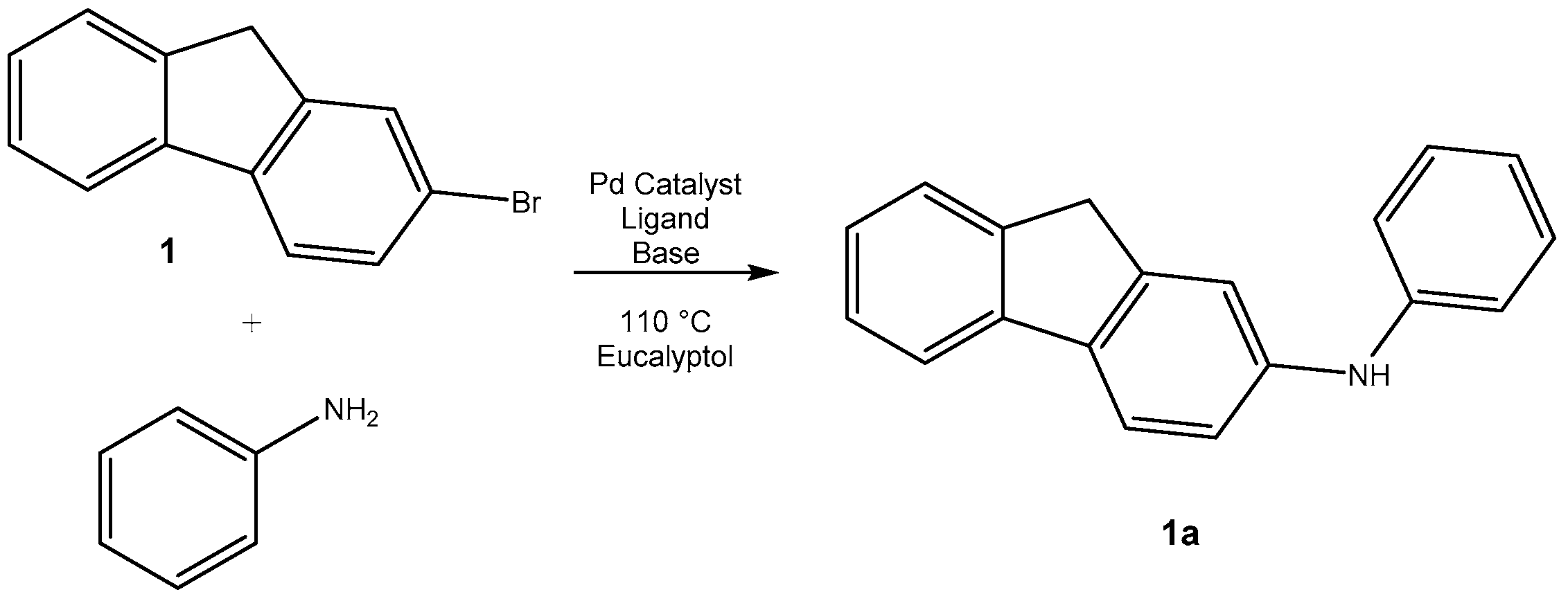
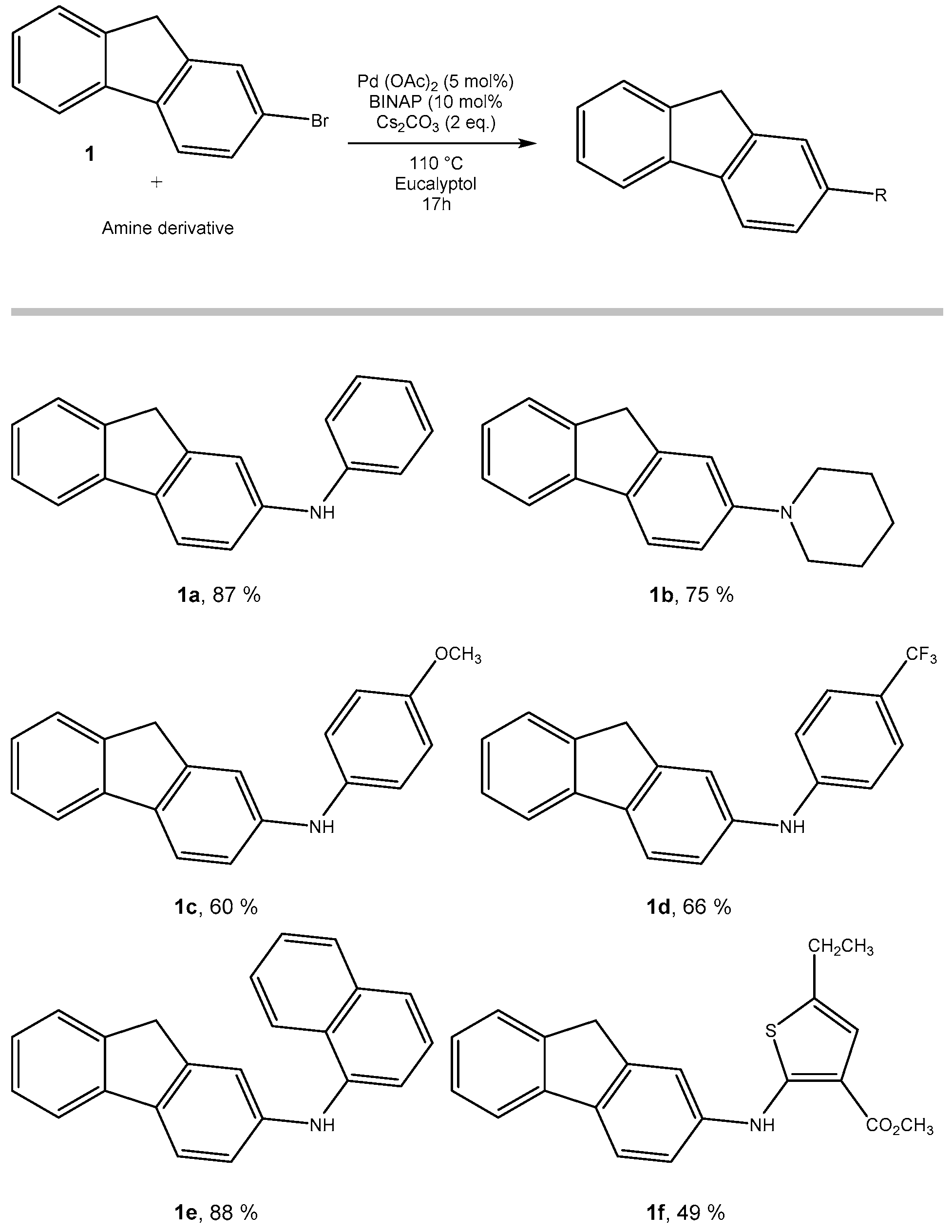
2.1. From 2-Bromofluorene
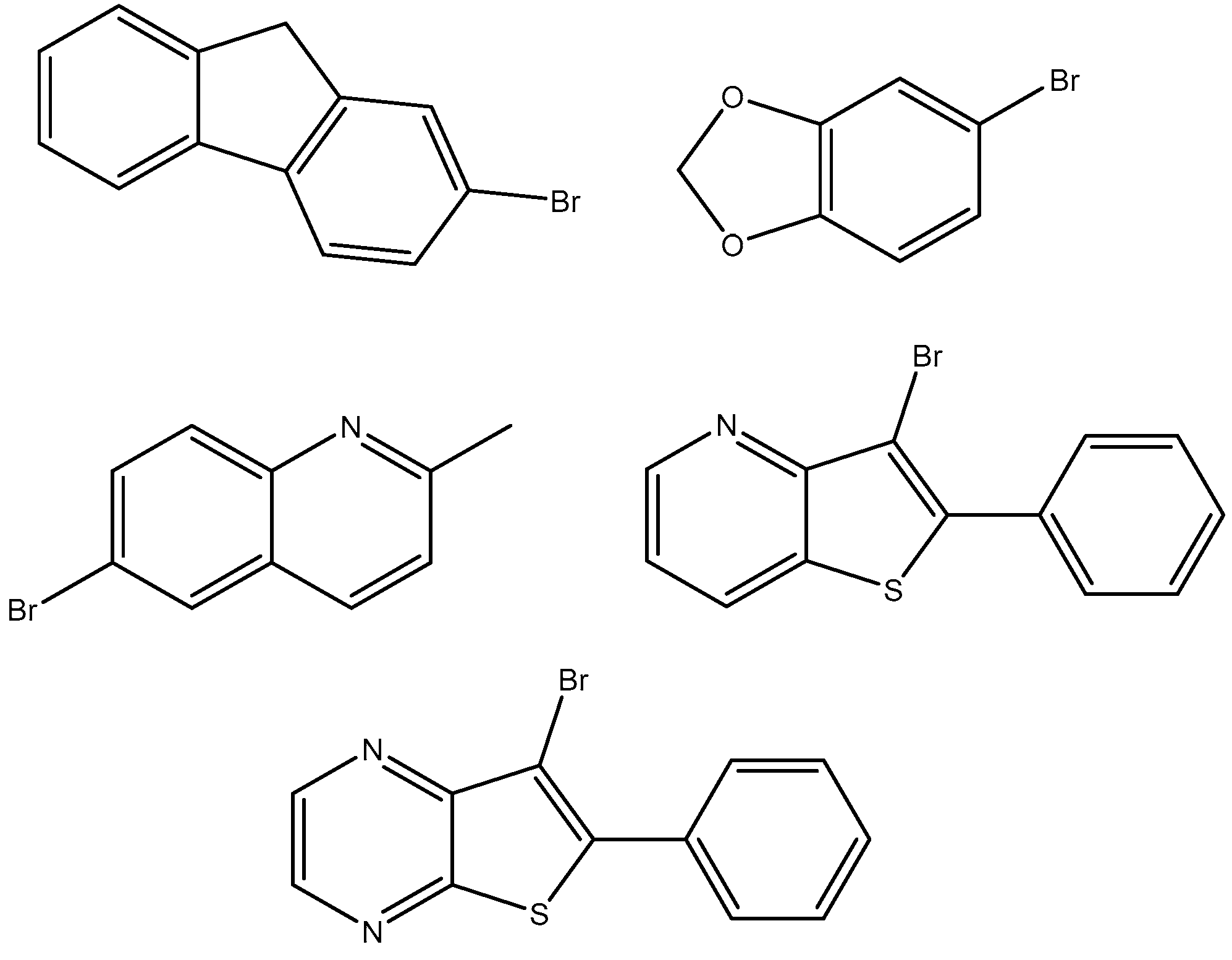
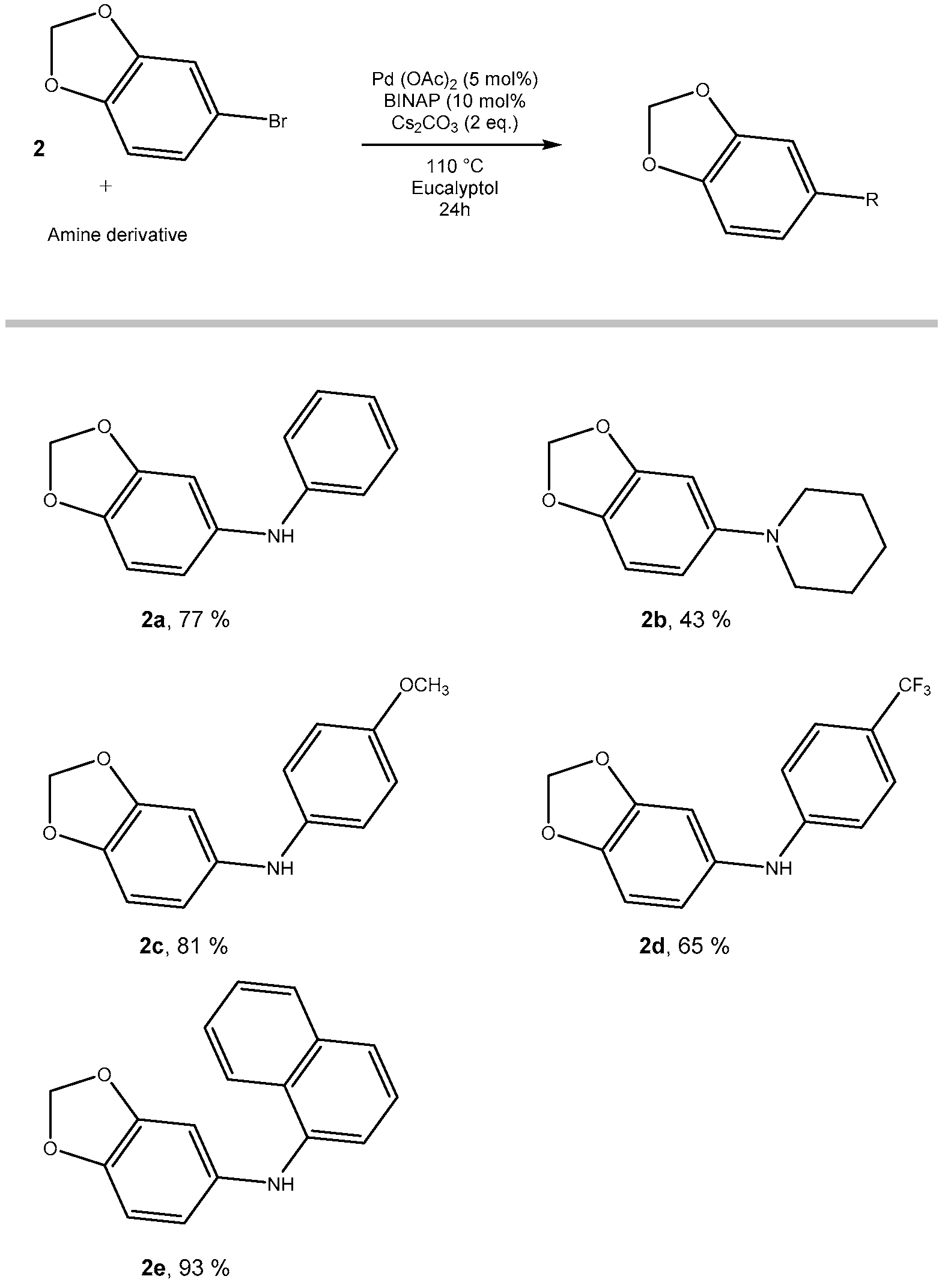
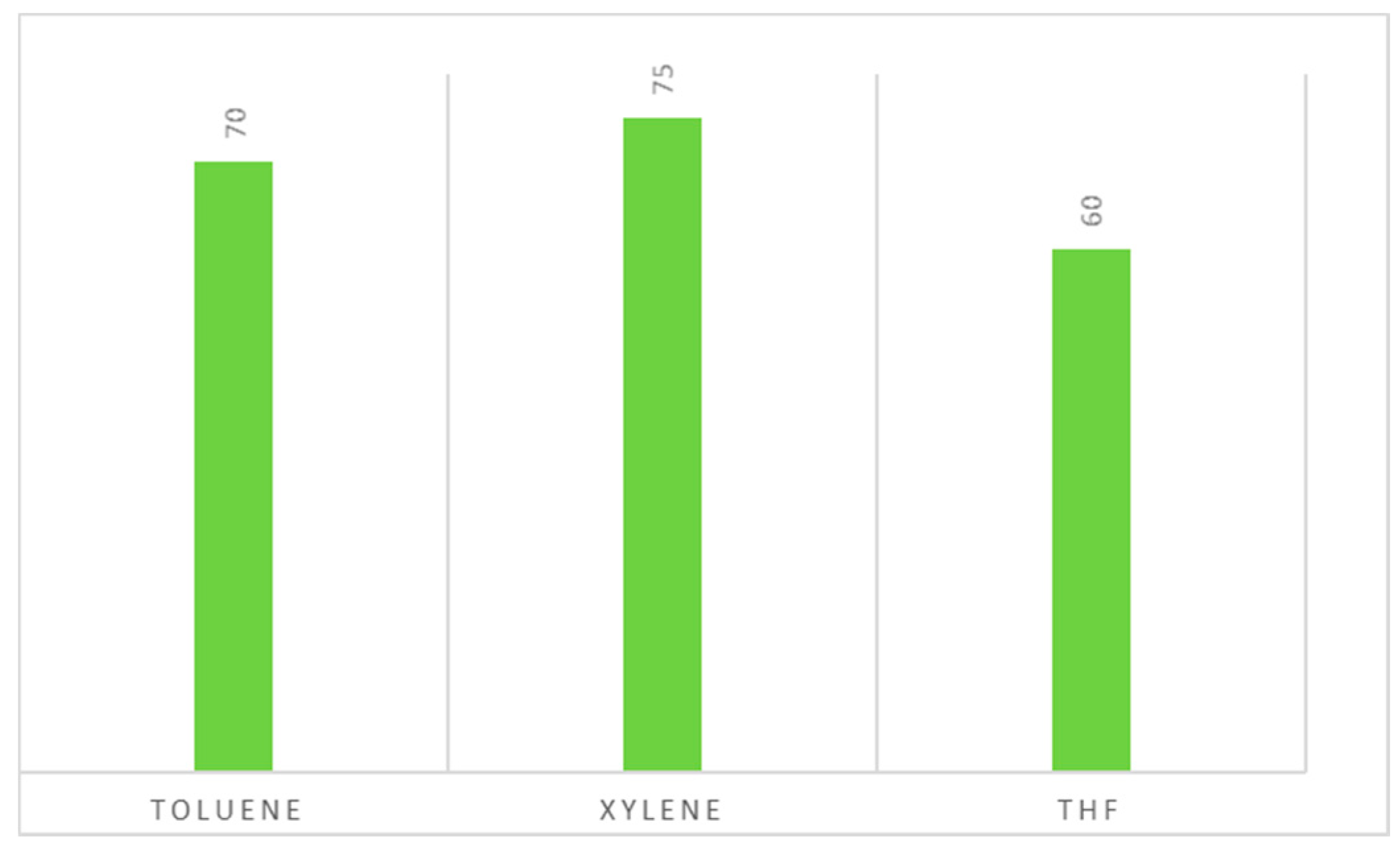
2.2. From 4-Bromo-1,2-methylenedioxybenzene
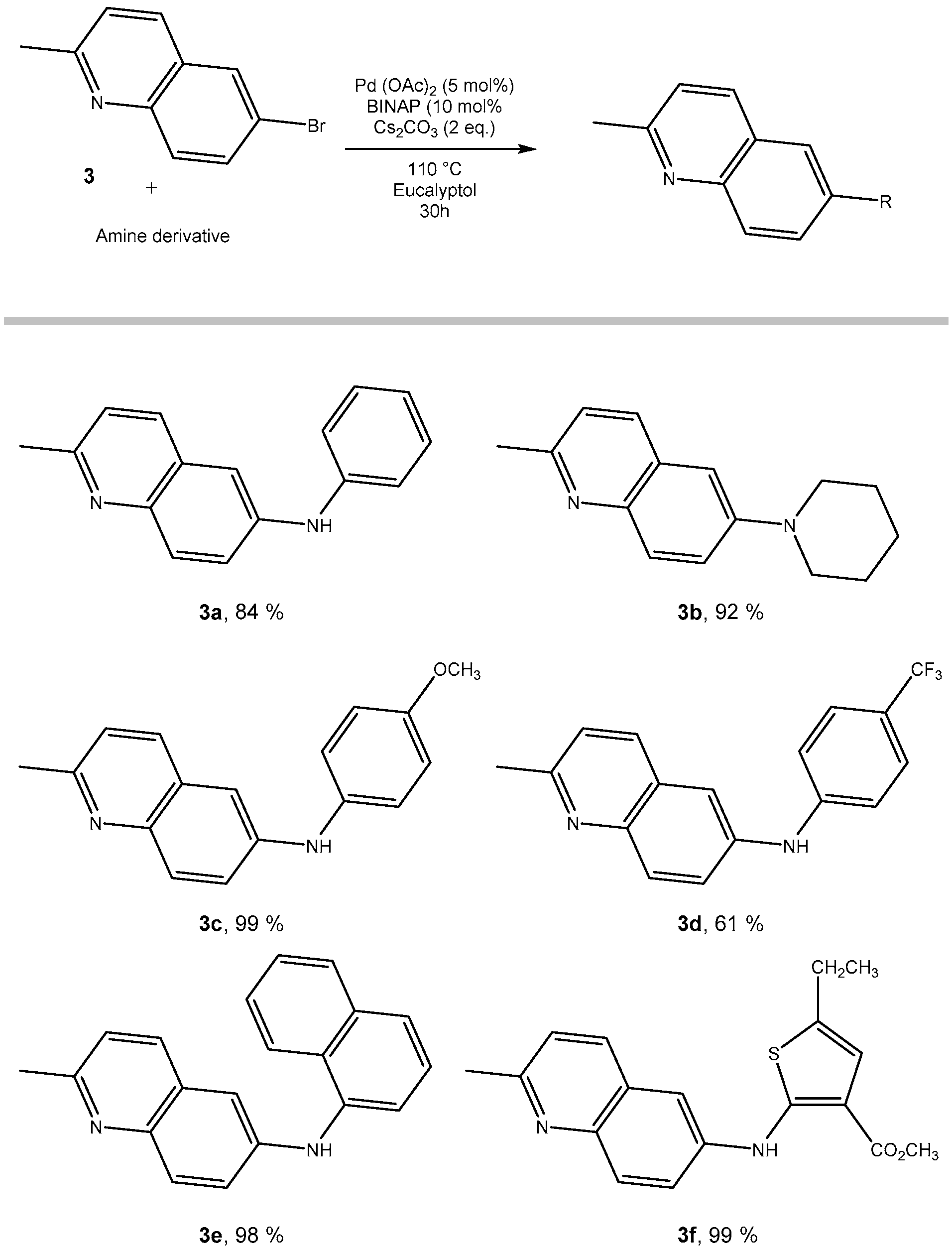
2.3. From 6-Bromo-2-methylquinoline
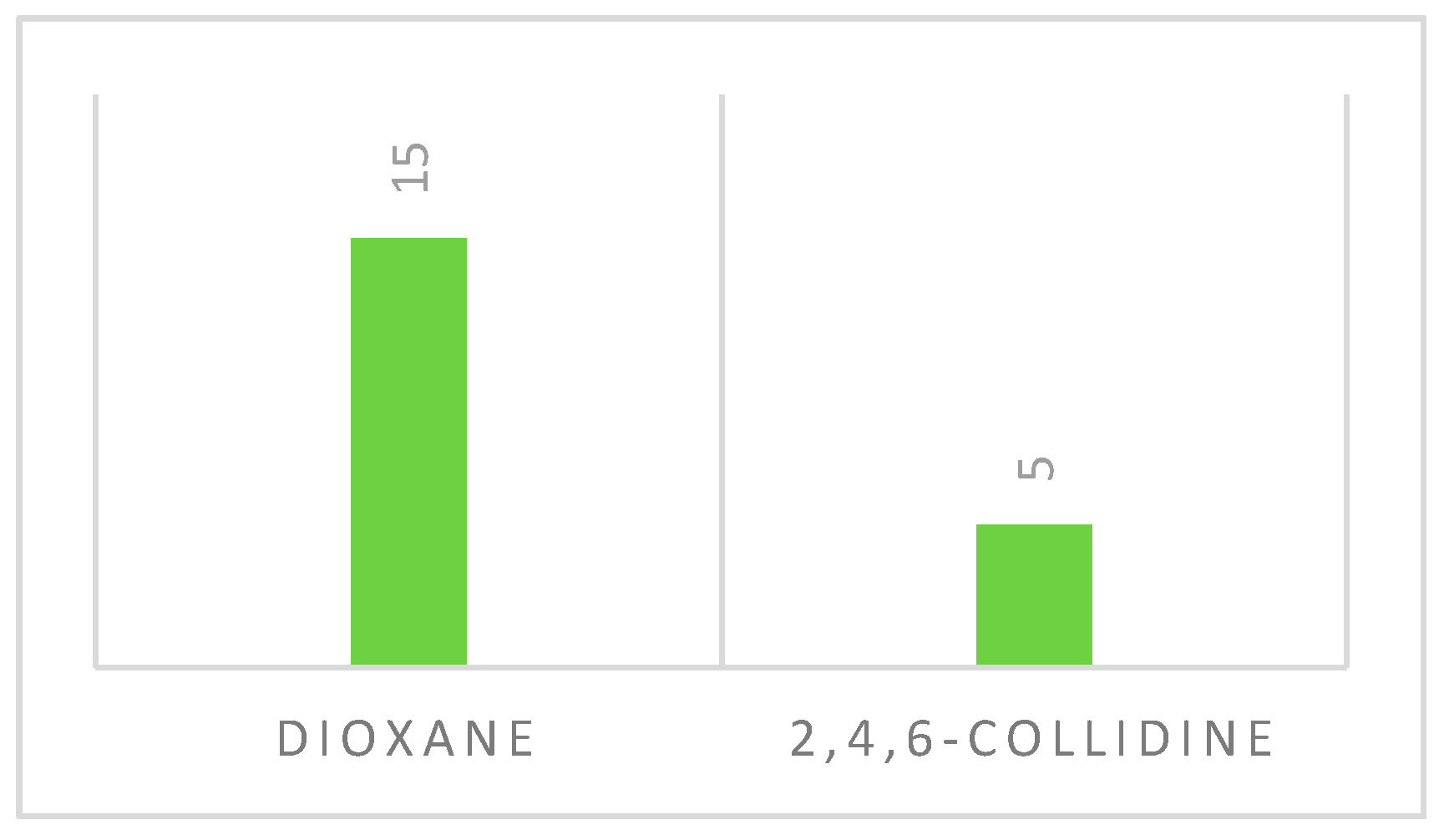
2.4. From 7-Bromo-6-phenylthieno[2,3-b]pyrazine and 3-Bromo-2-phenylthieno[3,2-b]pyridine
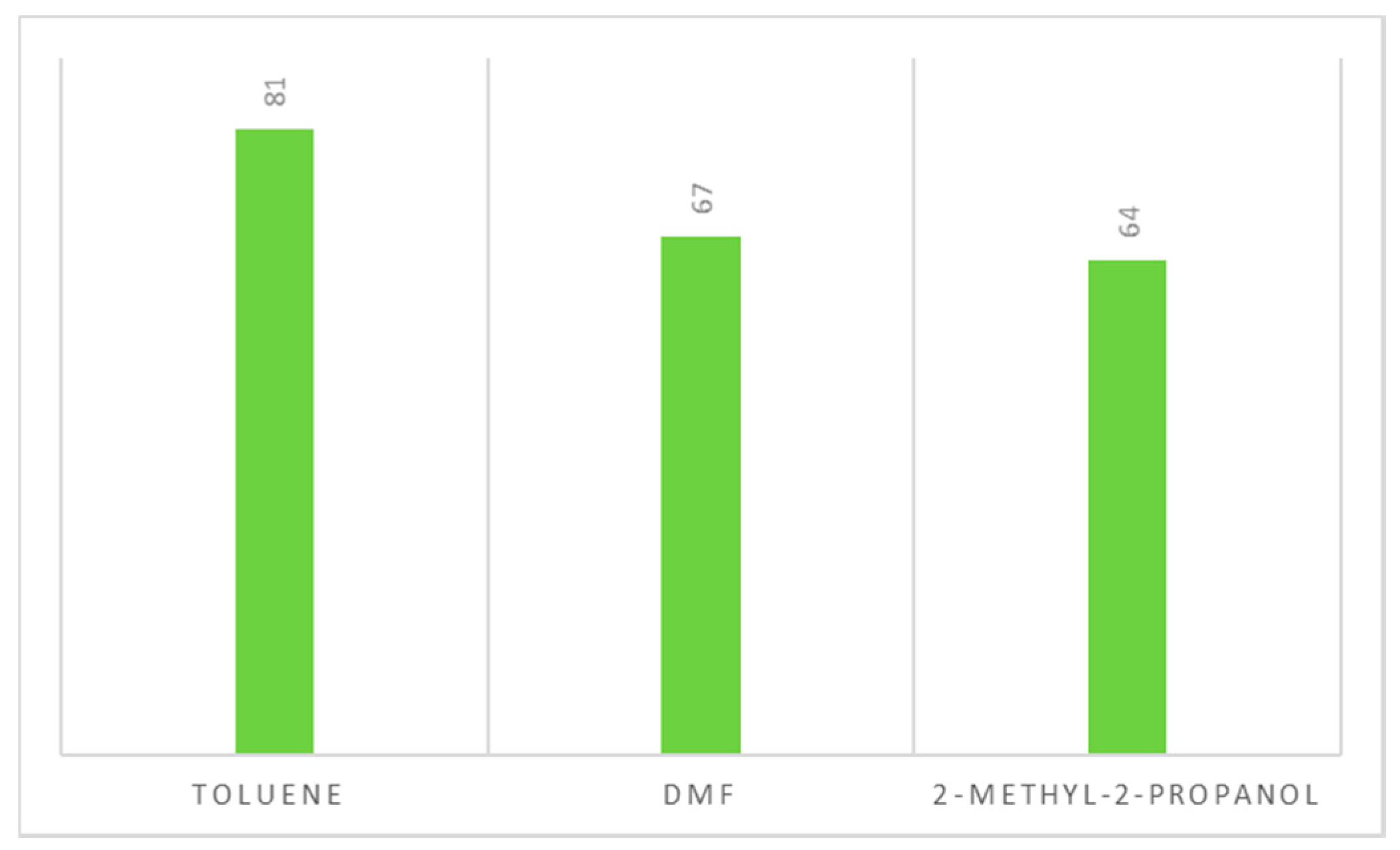
2.5. Recyclability of the Solvent
3. Materials and Methods
General Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Henderson, R.K.; Jiménez-González, C.; Constable, D.J.C.; Alston, S.R.; Inglis, G.G.A.; Fisher, G.; Sherwood, J.; Binks, S.P.; Curzons, A.D. Expanding GSK’s solvent selection guide—Embedding sustainability into solvent selection starting at medicinal chemistry. Green Chem. 2011, 13, 854–862. [Google Scholar] [CrossRef]
- Campos, J.F.; Scherrmann, M.-C.; Berteina-Raboin, S. Eucalyptol: A new solvent for the synthesis of heterocycles containing oxygen, sulfur and nitrogen. Green Chem. 2019, 21, 1531–1539. [Google Scholar] [CrossRef]
- Campos, J.F.; Loubidi, M.; Scherrmann, M.-C.; Berteina-Raboin, S. A Greener and Efficient Method for Nucleophilic Aromatic Substitution of Nitrogen-Containing Fused Heterocycles. Molecules 2018, 23, 684. [Google Scholar] [CrossRef] [PubMed]
- Dumonteil, G.; Hiebel, M.-A.; Scherrmann, M.-C.; Berteina-Raboin, S. Iodine-catalyzed formation of substituted 2-aminobenzothiazole derivatives in PEG400. RSC Adv. 2016, 6, 73517–73521. [Google Scholar] [CrossRef]
- Hiebel, M.-A.; Berteina-Raboin, S. Iodine-catalyzed regioselective sulfenylation of imidazoheterocycles in PEG400. Green Chem. 2015, 17, 937–944. [Google Scholar] [CrossRef]
- Hiebel, M.-A.; Fall, Y.; Scherrmann, M.-C.; Berteina-Raboin, S. Straightforward Synthesis of Various 2,3-Diarylimidazo[1,2-a]pyridines in PEG400 Medium through One-Pot Condensation and C–H Arylation. Eur. J. Org. Chem. 2014, 21, 4643–4650. [Google Scholar] [CrossRef]
- Fresneau, N.; Hiebel, M.-A.; Agrofoglio, L.A.; Berteina-Raboin, S. Efficient Synthesis of Unprotected C-5-Aryl/Heteroaryl-2’-deoxyuridine via a Suzuki-Miyaura Reaction in Aqueous Media. Molecules 2012, 17, 14409–14417. [Google Scholar] [CrossRef]
- Fresneau, N.; Hiebel, M.-A.; Agrofoglio, L.A.; Berteina-Raboin, S. One-pot Sonogashira-cyclization protocol to obtain substituted furopyrimidine nucleosides in aqueous conditions. Tetrahedron Lett. 2012, 53, 1760–1763. [Google Scholar] [CrossRef]
- Vybornyi, N.J.F.; Skabara, P.J. Scale-up Chemical Synthesis of Thermally-activated Delayed Fluorescence Emitters Based on the Dibenzothiophene-S,S-Dioxide Core. J. Vis. Exp. 2017, 128, 56501. [Google Scholar] [CrossRef]
- Han, X.; Gong, W.; Tong, Y.; Wei, D.; Wang, Y.; Ding, J.; Hou, H.; Song, Y. Synthesis and properties of benzothiadiazole-pyridine system: The modulation of optical feature. Dye. Pigm. 2017, 137, 135–142. [Google Scholar] [CrossRef]
- Pajtas, D.; Konya, K.; Kiss-Szikszai, A.; Dzubak, P.; Petho, Z.N.; Varga, Z.; Panyi, G.R.; Patonay, T. Optimization of the Synthesis of Flavone–Amino Acid and Flavone–Dipeptide Hybrids via Buchwald–Hartwig Reaction. J. Org. Chem. 2017, 82, 4578–4587. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Kim, S.H.; Kim, D.Y.; Kim, C.; Lee, H.W.; Lee, S.E.; Kim, Y.K.; Yoon, S.S. Blue organic light-emitting diodes based on diphenylamino dibenzo[g, p]chrysene derivatives. Thin Solid Films 2017, 636, 8–14. [Google Scholar] [CrossRef]
- Hie, L.; Fine Nathel, N.F.; Hong, X.; Yang, Y.F.; Houk, K.N.; Garg, N.K. Nickel-Catalyzed Activation of Acyl C−O Bonds of Methyl Esters. Angew.Chem. Int. Ed. 2016, 55, 2810–2814. [Google Scholar] [CrossRef] [PubMed]
- Saikia, P.; Sharma, G.; Gogoi, S.; Boruah, R.C. Cascade imination, Buchwald–Hartwig cross coupling and cycloaddition reaction: Synthesis of pyrido[2,3-d]pyrimidines. RSC Adv. 2015, 5, 23210–23212. [Google Scholar] [CrossRef]
- Copin, C.; Massip, S.; Leger, J.M.; Jarry, C.; Buron, F.; Routier, S. SNAr versus Buchwald–Hartwig Amination/Amidation in the Imidazo[2,1-b][1,3,4]thiadiazole Series. Eur. J. Org. Chem. 2015, 71, 6932–6942. [Google Scholar] [CrossRef]
- Schuster, C.; Borger, C.; Julich-Gruner, K.K.; Hesse, R.; Jager, A.; Kaufmann, G.; Schmidt, A.W.; Knolker, H.J. Synthesis of 2-Hydroxy-7-methylcarbazole, Glycozolicine, Mukoline, Mukolidine, Sansoakamine, Clausine-H, and Clausine-K and Structural Revision of Clausine-TY. Eur. J. Org. Chem. 2014, 22, 4741–4752. [Google Scholar] [CrossRef]
- Hesse, R.; Krahl, M.P.; Jager, A.; Kataeva, O.; Schmidt, A.W.; Knolker, H.J. Palladium(II)-Catalyzed Synthesis of the Formylcarbazole Alkaloids Murrayaline A–C, 7-Methoxymukonal, and 7-Methoxy-O-methylmukonal. Eur. J.Org. Chem. 2014, 19, 4014–4028. [Google Scholar] [CrossRef]
- Rao, R.K.; Karthikeyan, I.; Sekar, G. Domino aziridine ring opening and Buchwald–Hartwig type coupling-cyclization by palladium catalyst. Tetrahedron 2012, 68, 9090–9094. [Google Scholar] [CrossRef]
- Fei, X.D.; Zhou, Z.; Li, W.; Zhu, Y.M.; Shen, J.K. Buchwald–Hartwig Coupling/Michael Addition Reactions: One-Pot Synthesis of 1,2-Disubstituted 4-Quinolones from Chalcones and Primary Amines. Eur. J. Org. Chem. 2012, 3001–3008. [Google Scholar] [CrossRef]
- Krasavin, M. Novel diversely substituted 1-heteroaryl-2-imidazolines for fragment-based drug discovery. Tetrahedron Lett. 2012, 53, 2876–2880. [Google Scholar] [CrossRef] [Green Version]
- Bouhlel, A.; Curti, C.; Khoumeri, O.; Vanelle, P. Efficient one-pot double Buchwald–Hartwig coupling reaction on 5-phenyl-4-phenylsulfonyl-2,3-dihydrofuran derivatives. Tetrahedron Lett. 2011, 52, 1919–1923. [Google Scholar] [CrossRef]
- Lohou, E.; Collot, V.; Stiebing, S.; Rault, S. Direct Access to 3-Aminoindazoles by Buchwald-Hartwig C-N Coupling Reaction. Synthesis 2011, 16, 2651–2663. [Google Scholar] [CrossRef]
- Napolitano, C.; Borriello, M.; Cardullo, F.; Donati, D.; Paio, A.; Manfredini, S. First synthesis of 2,6-diazabicyclo[3.2.0]heptane derivatives. Tetrahedron Lett. 2009, 50, 7280–7282. [Google Scholar] [CrossRef]
- Audisio, D.; Messaoudi, S.; Peyrat, J.-F.; Brion, J.-D.; Alami, M. A convenient and expeditious synthesis of 3-(N-substituted) aminocoumarins via palladium-catalyzed Buchwald–Hartwig coupling reaction. Tetrahedron Lett. 2007, 48, 6928–6932. [Google Scholar] [CrossRef]
- Zhang, W.; Nagashima, T. Palladium-Catalyzed Buchwald-Hartwig Type Amination of Fluorous Arylsulfonates. J. Fluor. Chem. 2005, 127, 588–591. [Google Scholar] [CrossRef]
- Burgos, C.H.; Barder, T.E.; Huang, X.; Buchwald, S.L. Significantly Improved Method for the Pd-Catalyzed Coupling of Phenols with Aryl Halides: Understanding Ligand Effects. Angew. Chem. Int. Ed. 2006, 45, 4321–4326. [Google Scholar] [CrossRef]
- Willis, M.C.; Chauhana, J.; Whittingham, W.G. (2006) A new reactivity pattern for vinyl bromides: Cine-substitution via palladium catalysed C–N coupling/Michael addition reactions. Org. Biomol. Chem. 2005, 3, 3094–3095. [Google Scholar] [CrossRef]
- Wang, M.; Nalla, V.; Jeon, S.; Mamidala, V.; Ji, W.; Tan, L.-S.; Cooper, T.; Chiang, L.Y. Large Femtosecond Two-Photon Absorption Cross Sections of Fullerosome Vesicle Nanostructures Derived from a Highly Photoresponsive Amphiphilic C60-Light-Harvesting Fluorene Dyad. J. Phys. Chem. C 2011, 115, 18552–18559. [Google Scholar] [CrossRef] [Green Version]
- Nakano, T.; Yaegashi, T.; Tsuji, M. Chiral Macromolecules and Their Stationary Phases for Chromatography and Chromatography Packings for Optical Isomer Separation. JP 2008031223, 14 February 2008. [Google Scholar]
- Jeon, S.-H.; Anandakathir, R.; Chiang, J.; Chiang, L.Y. Alternative Synthesis of C60-Diphenylaminofluorene Derivatives for Nonlinear Photonic Applications: Method of Preparation and Characterization. J. Macromol. Sci. A 2007, 44, 1275–1282. [Google Scholar] [CrossRef]
- Prat, D.; Hayler, J.; Wells, A. A survey of solvent selection guides. Green Chem. 2014, 16, 4546–4551. [Google Scholar] [CrossRef]
- Kroth, H.; Hamel, C.; Benderitter, P.; Froestl, W.; Sreenivasachary, N.; Muhs, A. Preparation of 7-Azaindole, Indole, and Carbazole Compounds for the Treatment of Diseases Associated with Amyloid or Amyloid-Like Proteins. WO 2011128455, 20 October 2011. [Google Scholar]
- Xie, X.; Zhang, T.Y.; Zhang, Z. Synthesis of Bulky and Electron-Rich MOP-type Ligands and Their Applications in Palladium-Catalyzed C−N Bond Formation. J. Org. Chem. 2006, 71, 6522–6529. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Huang, M.; Yi, Z.; Du, D.; Zhu, X.; Wan, Y. Room-Temperature CuI-Catalyzed Amination of Aryl Iodides and Aryl Bromides. J. Org. Chem. 2017, 82, 5416–5423. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, S. Efficient Iron/Copper Cocatalyzed N-Arylation of Arylamines with Bromoarenes. Synlett 2011, 8, 1137–1142. [Google Scholar] [CrossRef]
- Suzuki, K.; Hori, Y.; Kobayashi, T. A New Hybrid Phosphine Ligand for Palladium-Catalyzed Amination of Aryl Halides. Adv. Synth. Catal. 2008, 350, 652–656. [Google Scholar] [CrossRef]
- Hirai, Y.; Yamamoto, Y.; Yoshioka, T.; Fukuzaki, E.; Yofu, K.; Tsukase, M.; Hamano, M.; Ichiki, T. Photoelectric Conversion Element, Method for Using Same, Imaging Element, Optical Sensor, and Chemical. , WO 2013133218, 12 September 2013. [Google Scholar]
- Cai, H.; Sun, K. From Faming Zhuanli Shenqing, Acridine Compounds and the Organic Luminescent Device Thereof. CN 107162974, 15 September 2017. [Google Scholar]
- Dalko, P.; Petit, M.; Ogden, D.; Acher, F. Multiphoton Activable Quinoline Derivatives, Their Preparation and Their Uses. WO 2011086469, 21 July 2011. [Google Scholar]
- Schmitt, M.; Klotz, E.; Macher, J.-P.; Bourguignon, J.-J. Preparation of Quinoline and Quinoxaline Derivatives as Inhibitors of Factor Xa with Therapeutic Uses. WO 2003010146, 6 February 2003. [Google Scholar]
- Chapdelaine, M.; Kemp, L.; McCauley, J. Preparation of Quinoline-4,6-diamines as N-Type Calcium Channel Antagonists for the Treatment of Pain. WO 2002036567, 10 May 2002. [Google Scholar]
- Tria, G.S.; Abrams, T.; Baird, J.; Burks, H.E.; Firestone, B.; Gaither, L.A.; Hamann, L.G.; He, G.; Kirby, C.A.; Kim, S.; et al. Discovery of LSZ102, a Potent, Orally Bioavailable Selective Estrogen Receptor Degrader (SERD) for the Treatment of Estrogen Receptor Positive Breast Cancer. J. Med. Chem. 2018, 61, 2837–2864. [Google Scholar] [CrossRef] [PubMed]
- Burks, H.E.; Dechantsreiter, M.A.; He, G.; Nunez, J.; Peukert, S.; Springer, C.; Sun, Y.; Thomsen, N.M.-F.; Tria, G.S.; Yu, B. Preparation of Substituted Benzothiophenes as Selective Estrogen Receptor Degraders. US 20140235660, 21 August 2014. [Google Scholar]
- Hauser, K.L.; Palkowitz, A.D.; Thrasher, K.J. Preparation of Benzothiophenes Useful for the Treatment of Postmenopausal Osteoporosis. US 5843963, 1 December 1998. [Google Scholar]
- Palkowitz, A.D.; Glasebrook, A.L.; Thrasher, K.J.; Hauser, K.L.; Short, L.L.; Phillips, D.L.; Muehl, B.S.; Sato, M.; Shetler, P.K.; Cullinan, G.J.; et al. Discovery and Synthesis of [6-Hydroxy-3-[4-[2-(1-piperidinyl)ethoxy]phenoxy]-2-(4-hydroxyphenyl)] benzo[b]thiophene: A Novel, Highly Potent, Selective Estrogen Receptor Modulator. J. Med. Chem. 1997, 40, 1407–1416. [Google Scholar] [CrossRef]
- Bhowal, M.; Gopal, M. Eucalyptol: Safety and Pharmacological Profile. RGUHS J. Pharm. Sci. 2015, 5, 125. [Google Scholar] [CrossRef]



| Entry | Pd (5 mol%) | Ligand (10 mol%) | Base (2 eq.) | t (h) | Yield(%) |
|---|---|---|---|---|---|
| 1 | Pd(OAc)2 | TTBP.HBF4 | K2CO3 | 30 | 72 |
| 2 | Pd(OAc)2 | TTBP.HBF4 | K3PO4 | 24 | 80 |
| 3 | Pd(OAc)2 | TTBP.HBF4 | Cs2CO3 | 24 | 81 |
| 4 | Pd(OAc)2 | BINAP | K2CO3 | 30 | 60 |
| 5 | Pd(OAc)2 | BINAP | K3PO4 | 17 | 36 |
| 6 | Pd(OAc)2 | BINAP | Cs2CO3 | 17 | 87 |
| 7 | Pd(OAc)2 | Xantphos | K2CO3 | 40 | 57 |
| 8 | Pd(OAc)2 | Xantphos | K3PO4 | 17 | 72 |
| 9 | Pd(OAc)2 | Xantphos | Cs2CO3 | 40 | 68 |
| 10 | Pd(OAc)2 | PPh3 | K2CO3 | 40 | Traces |
| 11 | Pd(OAc)2 | PPh3 | K3PO4 | 40 | 66 |
| 12 | Pd(OAc)2 | PPh3 | Cs2CO3 | 40 | 32 |
| 13 | Pd2dba3 | TTBP.HBF4 | K3PO4 | 48 | 40 |
| 14 | Pd2dba3 | TTBP.HBF4 | Cs2CO3 | 48 | 38 |
| 15 | Pd2dba3 | BINAP | Cs2CO3 | 20 | 67 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, J.F.; Berteina-Raboin, S. Eucalyptol as a Bio-Based Solvent for Buchwald-Hartwig Reaction on O,S,N-Heterocycles. Catalysts 2019, 9, 840. https://doi.org/10.3390/catal9100840
Campos JF, Berteina-Raboin S. Eucalyptol as a Bio-Based Solvent for Buchwald-Hartwig Reaction on O,S,N-Heterocycles. Catalysts. 2019; 9(10):840. https://doi.org/10.3390/catal9100840
Chicago/Turabian StyleCampos, Joana F., and Sabine Berteina-Raboin. 2019. "Eucalyptol as a Bio-Based Solvent for Buchwald-Hartwig Reaction on O,S,N-Heterocycles" Catalysts 9, no. 10: 840. https://doi.org/10.3390/catal9100840






