Highly Dispersed Co Nanoparticles Prepared by an Improved Method for Plasma-Driven NH3 Decomposition to Produce H2
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
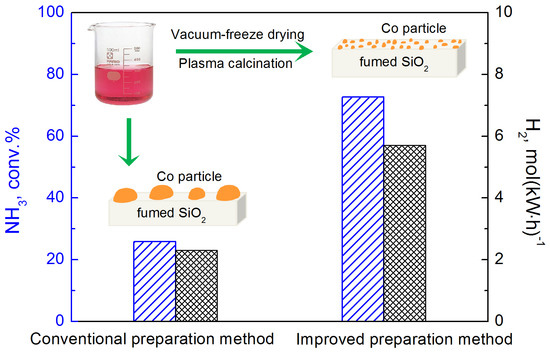
2.2. Performance of Prepared Catalyst in Plasma-Catalytic NH3 Decomposition
3. Materials and Methods
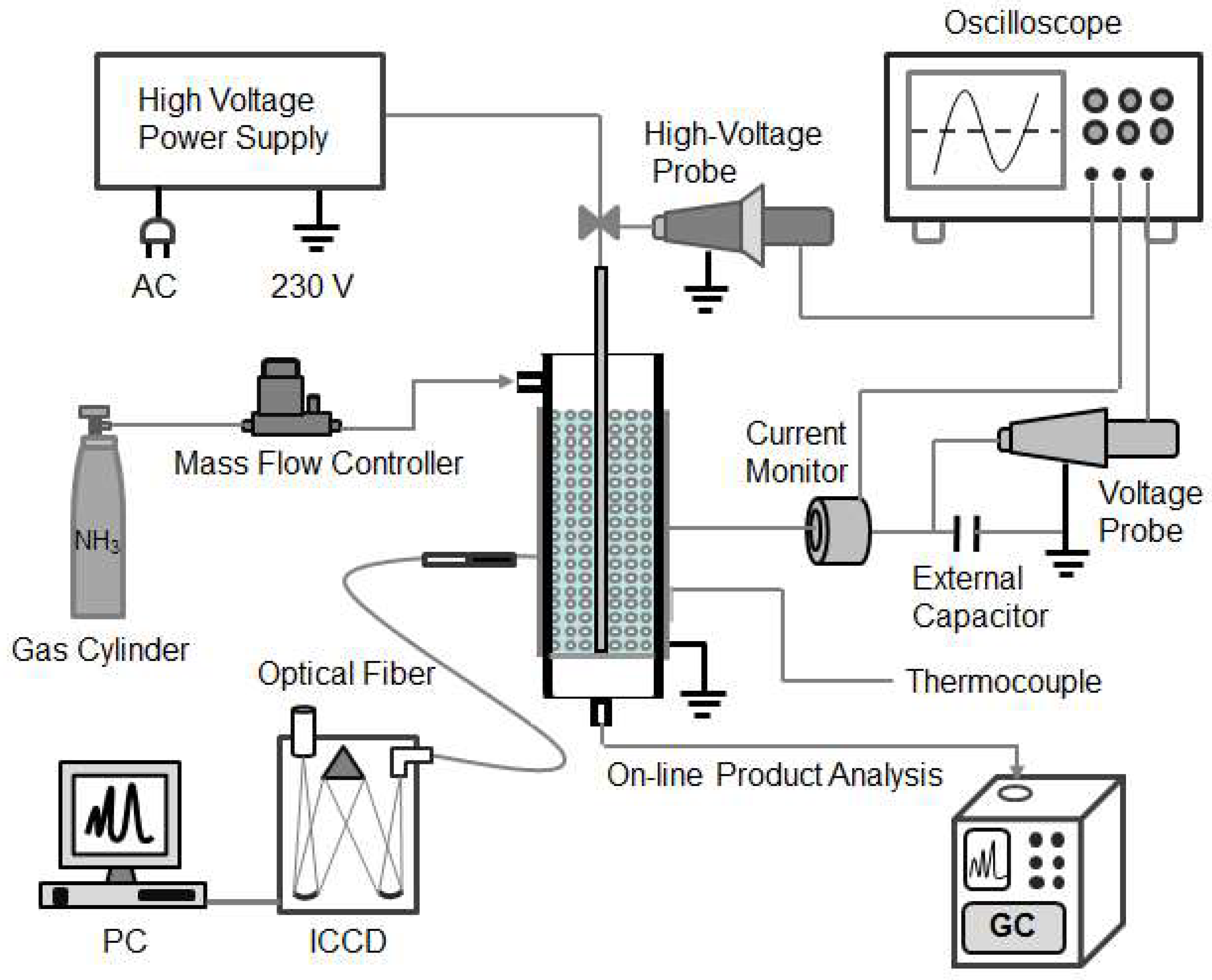
3.1. DBD Plasma-Catalytic Reactor
- : Energy efficiency of H2 formation, mol(kW∙h)−1
- : Conversion of NH3 , %
- : NH3 flow rate,
- P: Plasma power, kW
3.2. Catalyst Preparation
3.3. Catalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schüth, F.; Palkovits, R.; Schlögl, R.; Su, D.S. NH3 as a possible element in an energy infrastructure: Catalysts for NH3 decomposition. Energy Environ. Sci. 2012, 5, 6278–6289. [Google Scholar] [CrossRef]
- Lan, R.; Irvine, J.T.S.; Tao, S.W. NH3 and related chemicals as potential indirect H2 storage materials. Int. J. Hydrogen Energy 2012, 37, 1482–1494. [Google Scholar] [CrossRef]
- García-Bordejé, E.; Armenise, S.; Roldán, L. Toward practical application of H2 generation from NH3 decomposition guided by rational catalyst design. Catal. Rev. 2014, 56, 220–237. [Google Scholar] [CrossRef]
- Karim, A.M.; Prasad, V.; Mpourmpakis, G.; Lonergan, W.W.; Frenkel, A.I.; Chen, J.G.; Vlachos, D.G. Correlating particle size and shape of supported Ru/γ-Al2O3 Catalysts with NH3 Decomposition Activity. J. Am. Chem. Soc. 2009, 131, 12230–12239. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.F.; Xu, B.Q.; Wang, S.J.; Ng, C.F.; Au, C.T. Magnesia-carbon nanotubes (MgO-CNTs) nanocomposite: Novel support of Ru catalyst for the generation of COx-free hydrogen from ammonia. Catal. Lett. 2004, 96, 113–116. [Google Scholar] [CrossRef]
- Marco, Y.; Roldán, L.; Armenise, S.; García-Bordejé, E. Support-induced oxidation state of catalytic Ru nanoparticles on carbon nanofibers that were doped with heteroatoms (O, N) for the decomposition of NH3. ChemCatChem 2013, 5, 3829–3834. [Google Scholar] [CrossRef]
- Zheng, W.Q.; Zhang, J.; Ge, Q.J.; Xu, H.Y.; Li, W.Z. Effects of CeO2 addition on Ni/Al2O3 catalysts for the reaction of NH3 decomposition to H2. Appl. Catal. B Environ. 2008, 80, 98–105. [Google Scholar] [CrossRef]
- Donald, J.; Xu, C.B.; Hashimoto, H.; Byambajav, E.; Ohtsuka, Y. Novel carbon-based Ni/Fe catalysts derived from peat for hot gas NH3 decomposition in an inert helium atmosphere. Appl. Catal. A Gen. 2010, 375, 124–133. [Google Scholar] [CrossRef]
- Hansgen, D.A.; Vlachos, D.G.; Chen, J.G. Using first principles to predict bimetallic catalysts for the NH3 decomposition reaction. Nat. Chem. 2010, 2, 484–489. [Google Scholar] [CrossRef]
- Lu, A.H.; Nitz, J.J.; Comotti, M.; Weidenthaler, C.; Schlichte, K.; Lehmann, C.W.; Terasaki, O.; Schüth, F. Experimental and theoretical investigation of molybdenum carbide and nitride as catalysts for NH3 decomposition. J. Am. Chem. Soc. 2010, 132, 14152–14162. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Liu, C.Y.; Gong, W.M.; Guo, H.C. Plasma driven NH3 decomposition on a Fe-catalyst: Eliminating surface nitrogen poisoning. Chem. Commun. 2013, 49, 3787–3789. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yi, Y.H.; Zhao, Y.; Zhang, R.; Zhang, J.L.; Guo, H.C. NH3 decomposition for H2 generation: Effects of cheap metals and supports on plasma−catalyst synergy. ACS Catal. 2015, 5, 4167–4174. [Google Scholar] [CrossRef]
- Wang, L.; Yi, Y.H.; Guo, Y.J.; Zhao, Y.; Zhang, J.L.; Guo, H.C. Synergy of DBD plasma and Fe-based catalyst in NH3 decomposition: Plasma enhancing adsorption step. Plasma Process Polym. 2017, 14, e1600111. [Google Scholar] [CrossRef]
- Yi, Y.H.; Wang, L.; Guo, Y.J.; Sun, S.Q.; Guo, H.C. Plasma-assisted ammonia decomposition over Fe-Ni alloy catalysts for COx-free hydrogen. AIChE J. 2019, 65, 691–701. [Google Scholar]
- Munnik, P.; de Jongh, P.E.; de Jong, K.P. Recent developments in the synthesis of supported catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Flores, B.M.; Kharisov, B.I.; Jiménez-Pérez, V.M.; Martínez, P.E.; López, S.T. Recent advances in the synthesis and main applications of metallic nanoalloys. Ind. Eng. Chem. Res. 2011, 50, 7705–7721. [Google Scholar] [CrossRef]
- Zaera, F. Nanostructured materials for applications in heterogeneous catalysis. Chem. Soc. Rev. 2013, 42, 2746–2762. [Google Scholar] [CrossRef]
- Kogelschatz, U. Dielectric-barrier discharges: Their history, discharge physics, and industrial applications. Plasma Chem. Plasma Process 2003, 23, 1–45. [Google Scholar] [CrossRef]
- Wang, L.; Yi, Y.H.; Guo, H.C.; Tu, X. Atmospheric pressure and room temperature synthesis of methanol through plasma-catalytic hydrogenation of CO2. ACS Catal. 2018, 8, 90–100. [Google Scholar] [CrossRef]
- Kameshima, S.; Tamura, K.; Ishibashi, Y.; Nozaki, T. Pulsed dry methane reforming in plasma-enhanced catalytic reaction. Catal. Today 2015, 256, 67–75. [Google Scholar] [CrossRef]
- Wang, L.; Yi, Y.H.; Wu, C.F.; Guo, H.C.; Tu, X. One-step reforming of CO2 and CH4 into high-value liquid chemicals and fuels at room temperature by plasma-driven catalysis. Angew. Chem. Int. Ed. 2017, 56, 13679–13683. [Google Scholar] [CrossRef] [PubMed]
- Snoeckx, R.; Heijkers, S.; Wesenbeeck, K.V.; Lenaerts, S.; Bogaerts, A. CO2 conversion in a dielectric barrier discharge plasma: N2 in the mix as a helping hand or problematic impurity. Energy Environ. Sci. 2016, 9, 999–1011. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, S.; Sun, H.; Wang, R.; Tu, X.; Shao, T. Highly efficient conversion of methane using microsecond and nanosecond pulsed spark discharges. Appl. Energy 2018, 226, 534–545. [Google Scholar] [CrossRef]
- Guo, Z.F.; Yi, Y.H.; Wang, L.; Yan, J.H.; Guo, H.C. Pt/TS-1 catalyst promoted C-N coupling reaction in CH4-NH3 plasma for HCN synthesis at low temperature. ACS Catal. 2018, 8, 10219–10224. [Google Scholar] [CrossRef]
- Liu, C.J.; Vissokov, G.P.; Jang, B. Catalyst preparation using plasma technologies. Catal. Today 2002, 72, 173–184. [Google Scholar] [CrossRef]
- Zhang, H.; Chu, W.; Xu, H.Y.; Zhou, J. Plasma-assisted preparation of Fe–Cu bimetal catalyst for higher alcohols synthesis from carbon monoxide hydrogenation. Fuel 2010, 89, 3127–3131. [Google Scholar] [CrossRef]
- Cheng, D.G. Plasma decomposition and reduction in supported metal catalyst preparation. Catal. Surv. Asia 2008, 12, 145–151. [Google Scholar] [CrossRef]
- Hinokuma, S.; Misumi, S.; Yoshida, H.; Machida, M. Nanoparticle catalyst preparation using pulsed arc plasma deposition. Catal. Sci. Technol. 2015, 5, 4249–4257. [Google Scholar] [CrossRef]
- Di, L.B.; Zhang, J.S.; Zhang, X.L. A review on the recent progress, challenges, and perspectives of atmospheric-pressure cold plasma for preparation of supported metal catalysts. Plasma Process Polym. 2018, 15, e1700234. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Neyts, E.C.; Cao, X.X.; Zhang, X.S.; Jang, B.; Liu, C.J. Catalyst Preparation with Plasmas: How Does It Work? ACS Catal. 2018, 8, 2093–2110. [Google Scholar] [CrossRef]
- Wang, N.; Shen, K.; Yu, X.P.; Qian, W.Z.; Chu, W. Preparation and characterization of a plasma treated NiMgSBA-15 catalyst for methane reforming with CO2 to produce syngas. Catal. Sci. Technol. 2013, 3, 2278–2287. [Google Scholar] [CrossRef]
- Yan, X.L.; Zhao, B.R.; Liu, Y.; Li, Y.A. Dielectric barrier discharge plasma for preparation of Ni-based catalysts with enhanced coke resistance: Current status and perspective. Catal. Today 2015, 256, 29–40. [Google Scholar] [CrossRef]
- Guo, Z.L.; Huang, Q.S.; Luo, S.Z.; Chu, W. Atmospheric discharge plasma enhanced preparation of Pd/TiO2 catalysts for acetylene selective hydrogenation. Top. Catal. 2017, 60, 1009–1015. [Google Scholar] [CrossRef]
- Li, Y.A.; Jang, B. Selective hydrogenation of acetylene over Pd/Al2O3 catalysts: Effect of non-thermal RF plasma preparation methodologies. Top. Catal. 2017, 60, 997–1008. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Liu, C.J. Mechanism of template removal for the synthesis of molecular sieves using dielectric barrier discharge. Catal. Today 2015, 256, 137–141. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Laerb, K.V.; Neyts, E.C.; Bogaerts, A. Can plasma be formed in catalyst pores? A modeling investigation. Appl. Catal. B Environ. 2016, 185, 56–67. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.Z.; Zhang, L.; Jiang, D. Surface oxygen vacancies on Co3O4 mediated catalytic formaldehyde oxidation at room temperature. Catal. Sci. Technol. 2016, 6, 3845–3853. [Google Scholar] [CrossRef]
- Wang, Q.; Peng, Y.; Fu, J.; Kyzasd, G.Z.; Reduwan Billah, S.M.; An, S.Q. Synthesis, characterization, and catalytic evaluation of Co3O4/γ-Al2O3 as methane combustion catalysts: Significance of Co species and the redox cycle. Appl. Catal. B Environ. 2015, 168–169, 42–50. [Google Scholar] [CrossRef]
- Qazi, S.J.S.; Rennie, A.R.; Cockcroft, J.K.; Vickers, M. Use of wide-angle X-ray diffraction to measure shape and size of dispersed colloidal particles. J. Colloid Interf. Sci. 2009, 338, 105–110. [Google Scholar] [CrossRef]
- Luo, J.Y.; Meng, M.; Li, X.; Li, X.G.; Zha, Y.Q.; Hu, T.D.; Xie, Y.N.; Zhang, J. Mesoporous Co3O4–CeO2 and Pd/Co3O4–CeO2 catalysts: Synthesis, characterization and mechanistic study of their catalytic properties for low-temperature CO oxidation. J. Catal. 2008, 254, 310–324. [Google Scholar] [CrossRef]
- Teng, F.; Chen, M.D.; Li, G.Q.; Teng, Y.; Xu, T.G.; Hang, Y.C.; Yao, W.Q.; Santhanagopalan, S.; Meng, D.D.; Zhu, Y.F. High combustion activity of CH4 and catalluminescence properties of CO oxidation over porous Co3O4 nanorods. Appl. Catal. B Environ. 2011, 110, 133–140. [Google Scholar] [CrossRef]
- Vakros, J.; Kordulis, C.; Lycourghiotis, A. Cobalt oxide supported γ-Alumina catalyst with very high active surface area prepared by equilibrium deposition filtration. Langmuir 2002, 18, 417–422. [Google Scholar] [CrossRef]
- Arnoldy, P.; Moulijn, J.A. Temperature-programmed reduction of CoO/Al2O3 catalysts. J. Catal. 1985, 93, 38–54. [Google Scholar] [CrossRef]
- Hong, J.P.; Du, J.; Wang, B.; Zhang, Y.H.; Liu, C.C.; Xiong, H.F.; Sun, F.L.; Chen, S.F.; Li, J.L. Plasma-assisted preparation of highly dispersed Co enhanced Fischer–Tropsch synthesis performance. ACS Catal. 2018, 8, 6177–6185. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Yi, Y.; Guo, H.; Du, X.; Zhu, B.; Zhu, Y. Highly Dispersed Co Nanoparticles Prepared by an Improved Method for Plasma-Driven NH3 Decomposition to Produce H2. Catalysts 2019, 9, 107. https://doi.org/10.3390/catal9020107
Wang L, Yi Y, Guo H, Du X, Zhu B, Zhu Y. Highly Dispersed Co Nanoparticles Prepared by an Improved Method for Plasma-Driven NH3 Decomposition to Produce H2. Catalysts. 2019; 9(2):107. https://doi.org/10.3390/catal9020107
Chicago/Turabian StyleWang, Li, YanHui Yi, HongChen Guo, XiaoMin Du, Bin Zhu, and YiMin Zhu. 2019. "Highly Dispersed Co Nanoparticles Prepared by an Improved Method for Plasma-Driven NH3 Decomposition to Produce H2" Catalysts 9, no. 2: 107. https://doi.org/10.3390/catal9020107