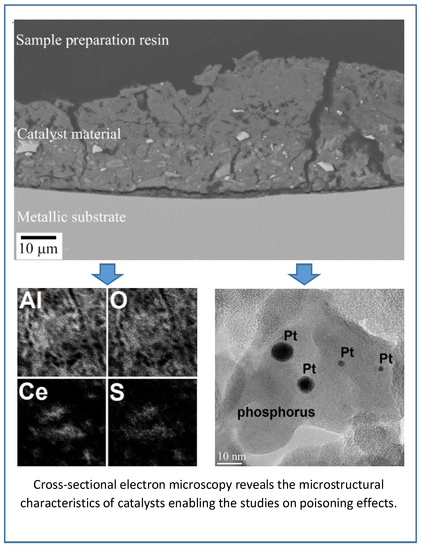
Microstructural Characteristics of Vehicle-Aged Heavy-Duty Diesel Oxidation Catalyst and Natural Gas Three-Way Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Diesel Oxidation Catalyst
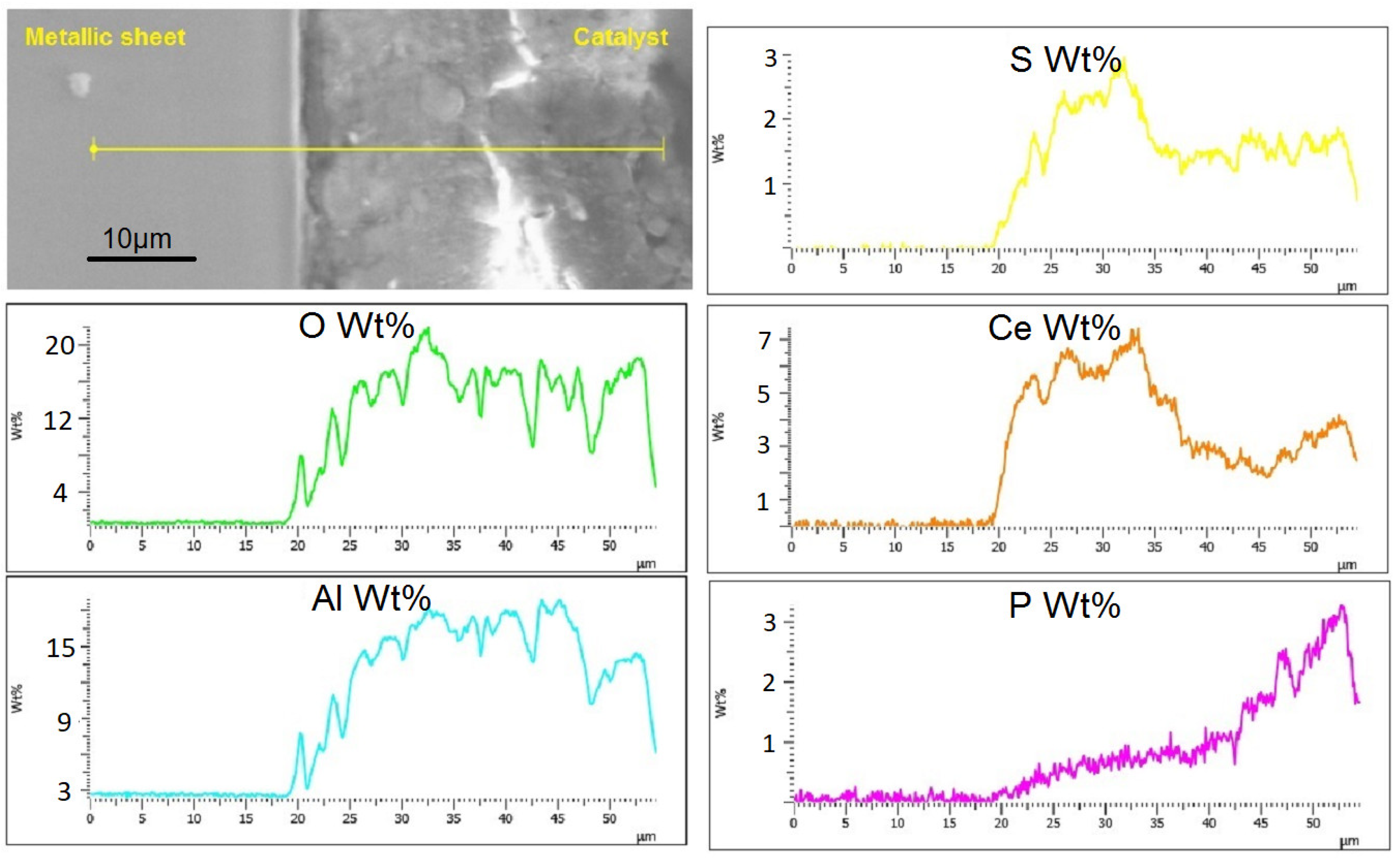
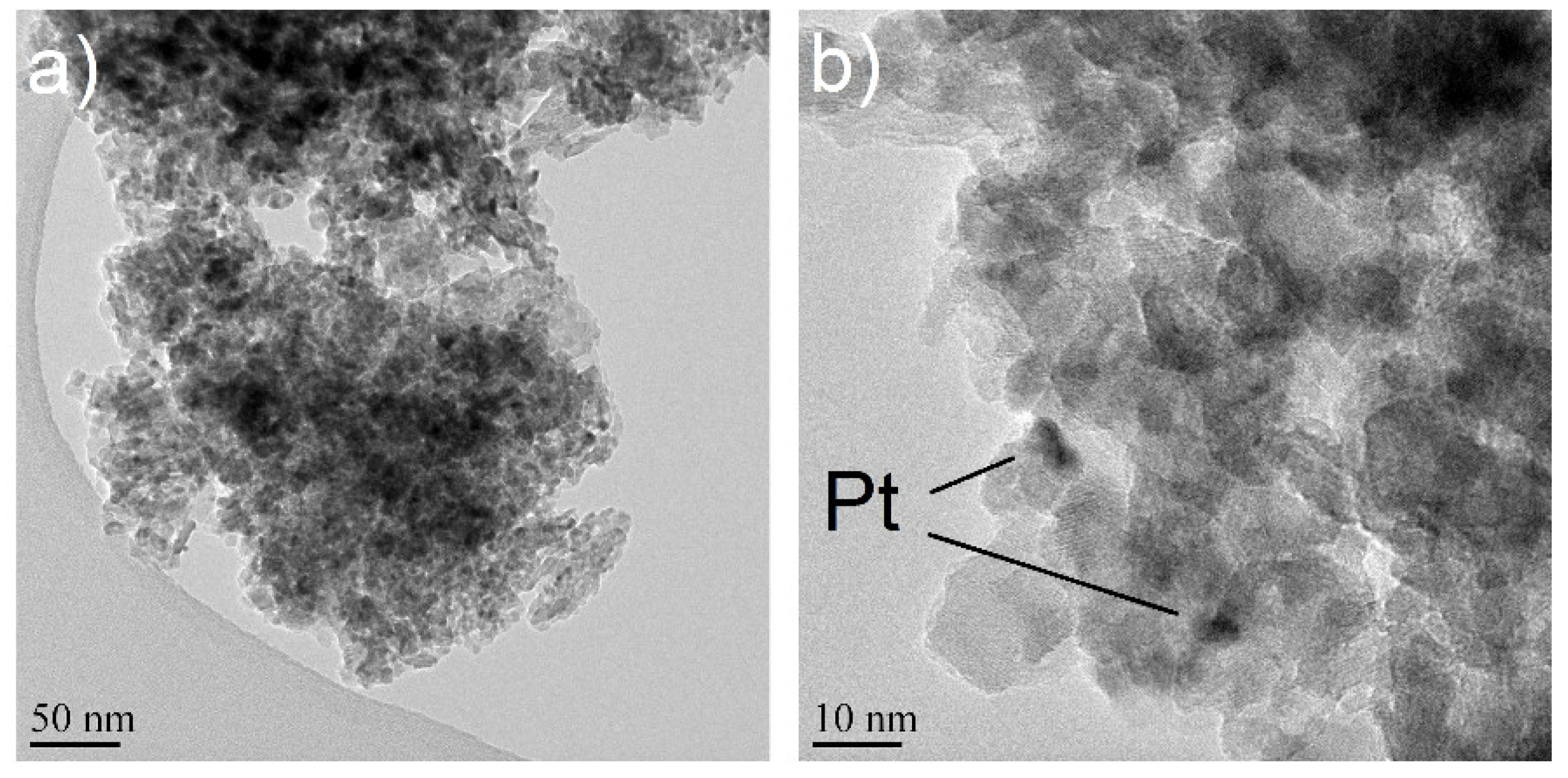
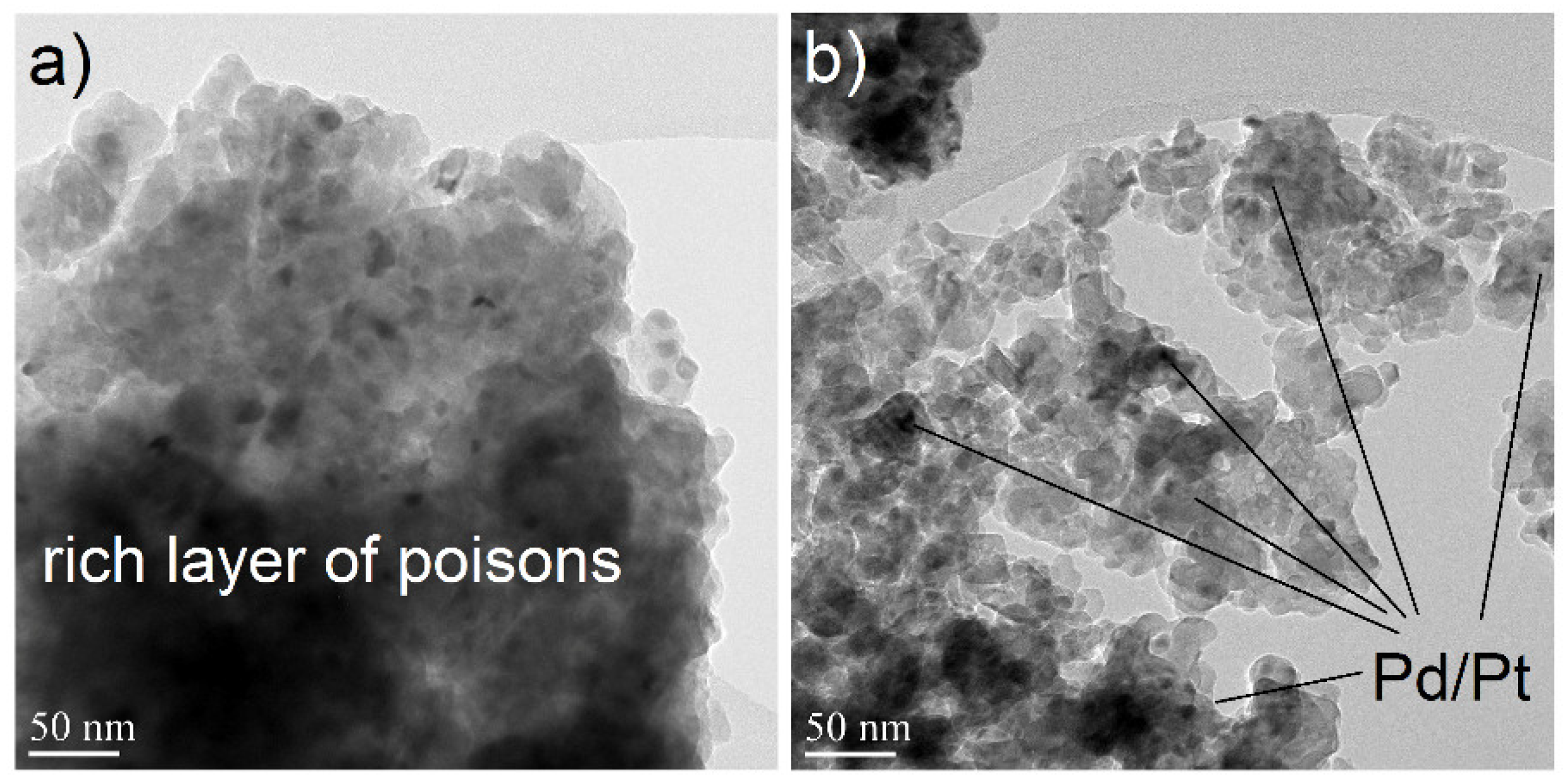
2.1.1. Electron Microscopy
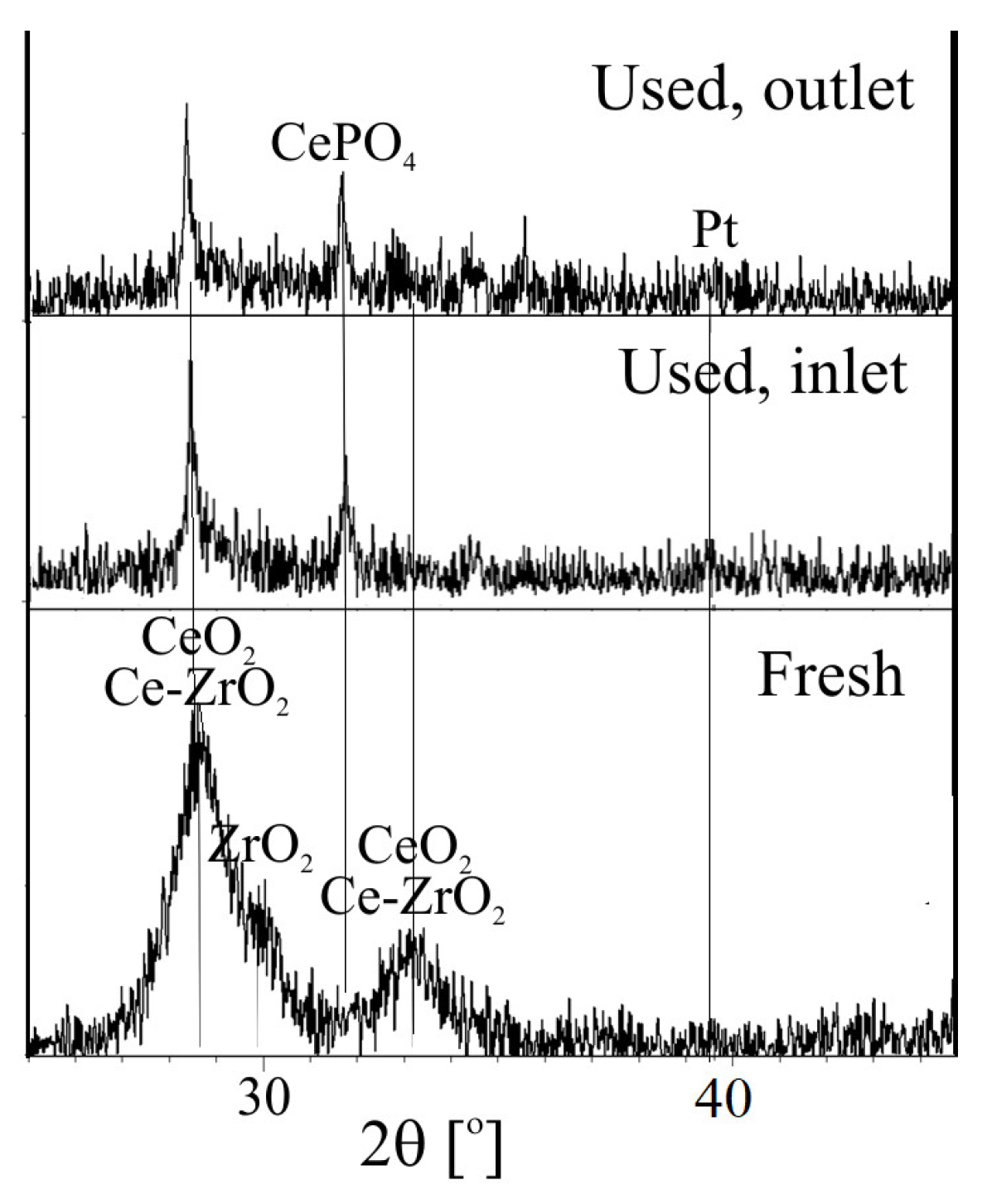
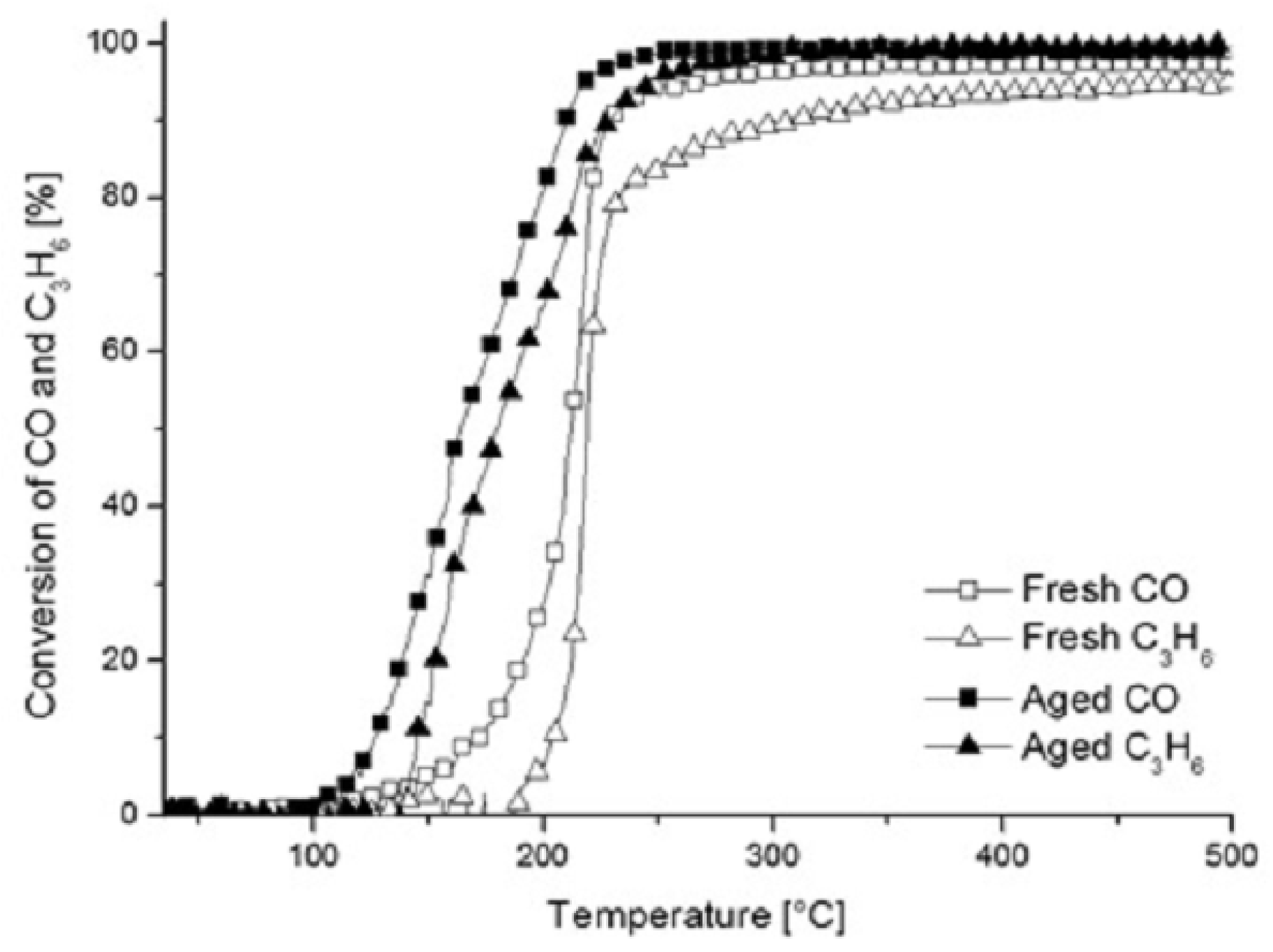
2.1.2. XRD and XPS Analysis, FTIR-ATR Measurements and Catalyst Performance Experiments
2.2. Natural Gas Catalyst
2.2.1. Electron Microscopy
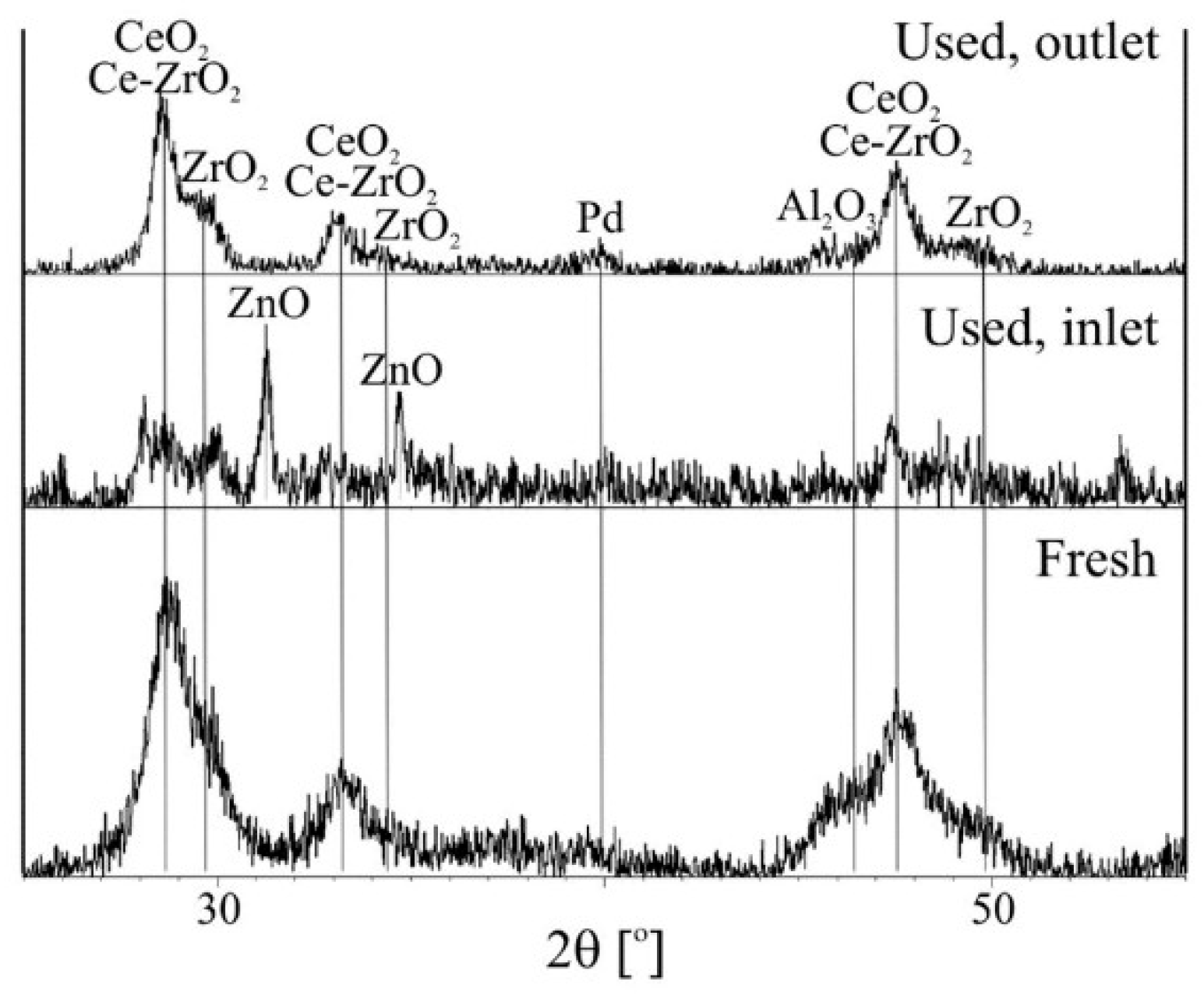
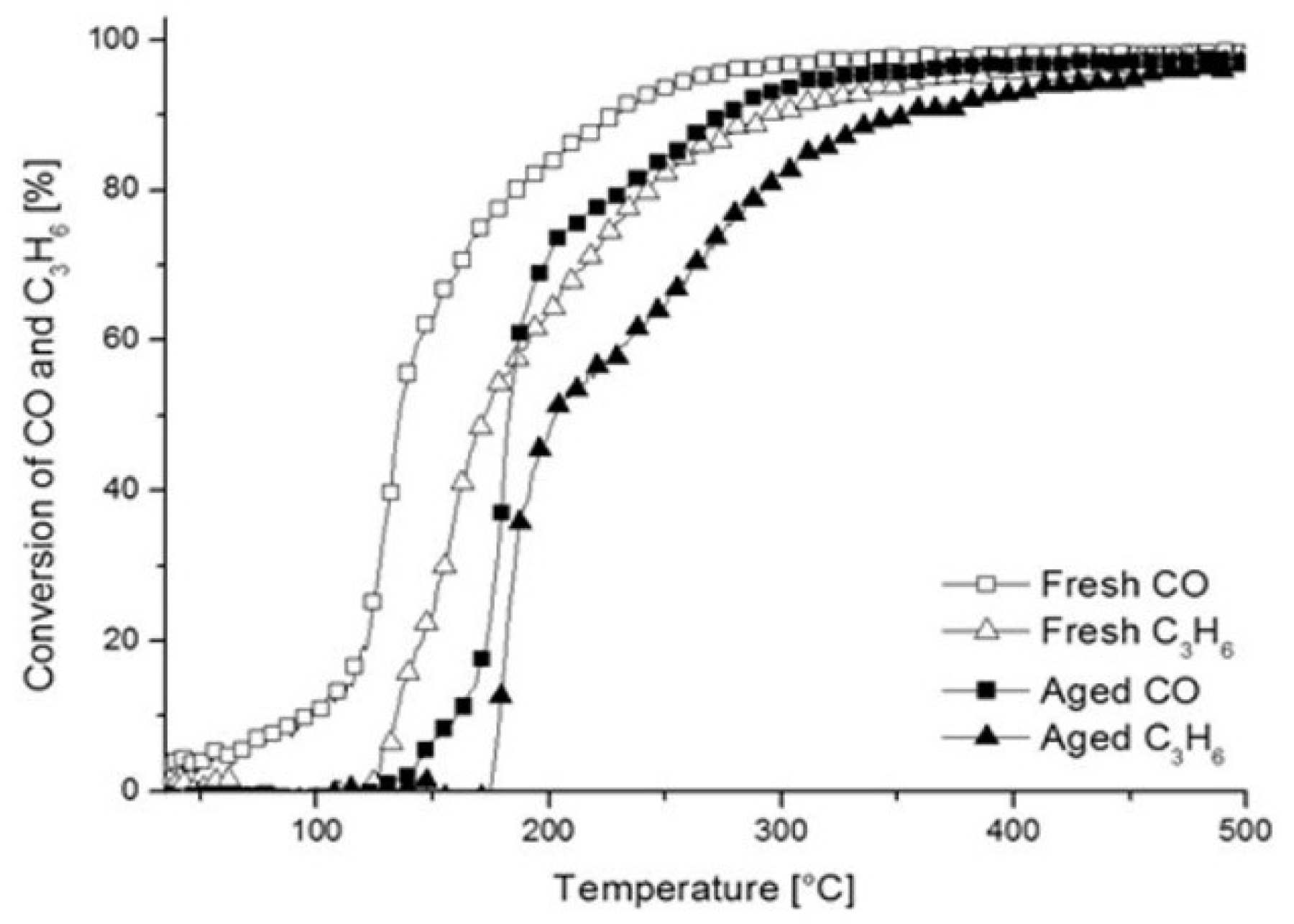
2.2.2. XRD and XPS Analysis, FTIR-ATR Measurements and Catalyst Performance Experiments
3. Materials and Methods
3.1. Electron Microscopy Characterization
3.2. Catalyst Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- DieselNet. Available online: https://www.dieselnet.com/ (accessed on 16 January 2019).
- Heck, R.M.; Farrauto, R.J. Automobile Exhaust catalysts. Appl. Catal. A 2001, 221, 443–457. [Google Scholar] [CrossRef]
- Gandhi, H.S.; Graham, G.W.; McCabe, R.W. Automotive exhaust catalysis. J. Catal. 2003, 216, 433–442. [Google Scholar] [CrossRef]
- Andersson, J.; Antonsson, M.; Eurenius, L.; Olsson, E.; Skoglundh, M. Deactivation of diesel oxidation catalysts: Vehicle- and synthetic ageing correlations. Appl. Catal. B 2007, 72, 71–81. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Air Topics. Available online: https://www.epa.gov/environmental-topics/air-topics (accessed on 20 February 2018).
- Wang, D.; Epling, B.; Nova, I.; Szanyi, J. Advances in Automobile Emissions Control Catalysis. Catal. Today 2016, 267, 1–2. [Google Scholar] [CrossRef]
- Wiebenga, M.H.; Kim, C.H.; Schmieg, S.J.; Oh, S.H.; Brown, D.B.; Kim, D.H.; Lee, J.-H.; Peden, C.H.F. Deactivation mechanisms of Pt/Pd-based diesel oxidation catalysts. Catal. Today 2012, 184, 197–204. [Google Scholar] [CrossRef]
- Matam, S.K.; Otal, E.H.; Aguirre, M.H.; Winkler, A.; Ulrich, A.; Rentsch, D.; Weidenkaff, A.; Ferri, D. Thermal and chemical aging of model three-way catalyst Pd/Al2O3 and its impact on the conversion of CNG vehicle exhaust. Catal. Today 2012, 184, 237–244. [Google Scholar] [CrossRef]
- Honkanen, M.; Kärkkäinen, M.; Viitanen, V.; Jiang, H.; Kallinen, K.; Huuhtanen, M.; Vippola, M.; Lahtinen, J.; Keiski, R.L.; Lepistö, T. Structural characteristics of natural-gas-vehicle-aged oxidation catalyst. Top. Catal. 2013, 56, 576–585. [Google Scholar] [CrossRef]
- Kärkkäinen, M.; Kolli, T.; Honkanen, M.; Heikkinen, O.; Huuhtanen, M.; Kallinen, K.; Lepistö, T.; Lahtinen, J.; Vippola, M.; Keiski, R.L. The effect of phosphorus exposure on diesel oxidation catalysts Part I: Activity measurements, elementary and surface analysis. Top. Catal. 2015, 58, 961–970. [Google Scholar] [CrossRef]
- Honkanen, M.; Kärkkäinen, M.; Heikkinen, O.; Kallinen, K.; Kolli, T.; Huuhtanen, M.; Lahtinen, J.; Keiski, R.L.; Lepistö, T.; Vippola, M. The effect of phosphorus exposure on diesel oxidation catalysts—Part II: Characterization of structural changes by transmission electron microscopy. Top. Catal. 2015, 58, 971–976. [Google Scholar] [CrossRef]
- Wilburn, M.S.; Ebling, W.S. Sulfur deactivation and regeneration of mono- and bimetallic Pd-Pt methane oxidation catalysts. Appl. Catal. B 2017, 206, 589–598. [Google Scholar] [CrossRef]
- Kanerva, T.; Rahkamaa-Tolonen, K.; Vippola, M.; Lepistö, T. Preparation of cross-sectional transmission electron microscopy samples from vehicle-aged and fresh diesel catalysts. In Proceedings of the EUROPACAT VIII, Turku, Finland, 26–31 August 2007. [Google Scholar]
- Millo, F.; Rafigh, M.; Andreata, M.; Vlachos, T.; Arya, P.; Miceli, P. Impact of high sulfur fuel and de-sulfaction process on a close-coupled diesel oxidation catalyst and diesel particulate filter. Fuel 2017, 198, 58–67. [Google Scholar] [CrossRef]
- Väliheikki, A.; Kärkkäinen, M.; Honkanen, M.; Heikkinen, O.; Kolli, T.; Kallinen, K.; Huuhtanen, M.; Vippola, M.; Lahtinen, J.; Keiski, R.L. Deactivation of Pt/SiO2-ZrO2 diesel oxidation catalysts by sulphur, phosphorus and their combinations. Appl. Catal. B 2017, 218, 409–419. [Google Scholar] [CrossRef]
- Gremminger, A.; Lott, P.; Menno, M.; Casapu, M.; Grunwaldt, J.-D.; Deutschmann, O. Sulfur poisoning and regeneration of bimetallic Pd-Pt methane oxidation catalysts. Appl. Catal. B 2017, 218, 833–843. [Google Scholar] [CrossRef]
- Sadokhina, N.; Smedler, G.; Nylén, U.; Olofsson, M.; Olsson, L. Deceleration of SO2 poisoning on PtPd/Al2O3 catalyst during complete methane oxidation. Appl. Catal. B 2018, 236, 384–395. [Google Scholar] [CrossRef]
- Luo, T.; Vohs, J.M.; Gorte, R.J. An Examination of Sulfur Poisoning on Pd/Ceria Catalysts. J. Catal. 2002, 210, 397–404. [Google Scholar] [CrossRef]
- Majumdar, S.S.; Alexander, A.-M.; Gawade, P.; Celik, G.; Ozkan, U.S. Effect of alumina incorporation on the sulfur tolerance of the dual-catalyst aftertreatment system for reduction of nitrogen oxides under lean conditions. Catal. Today 2019, 320, 204–213. [Google Scholar] [CrossRef]
- Beck, D.D.; Sommers, J.W.; Dimaggio, C.L. Axial characterization of oxygen storage capacity in close-coupled lightoff and underfloor catalytic converters and impact of sulfur. Appl. Catal. B 1997, 11, 273–290. [Google Scholar] [CrossRef]
- Beck, D.D.; Sommers, J.W. Impact of sulfur on the performance of vehicle-aged palladium monoliths. Appl. Catal. B 1994, 6, 185–200. [Google Scholar] [CrossRef]
- Beck, D.D. Impact of sulfur on three-way automotive catalyst performance and catalyst diagnostics. Catal. Deactiv. 1997, 111, 21–38. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy Database; version 4; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2012. Available online: http://srdata.nist.gov/xps/ (accessed on 1 July 2016).
- Gracia, F.J.; Guerrero, S.; Wolf, E.E.; Miller, J.T.; Kropf, A.J. Kinetics, operando FTIR, and controlled atmosphere EXAFS study of the effect of sulfur on Pt-supported catalysts during CO oxidation. J. Catal. 2005, 233, 372–387. [Google Scholar] [CrossRef]
- Coates, J. Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2000. [Google Scholar]














© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanerva, T.; Honkanen, M.; Kolli, T.; Heikkinen, O.; Kallinen, K.; Saarinen, T.; Lahtinen, J.; Olsson, E.; Keiski, R.L.; Vippola, M. Microstructural Characteristics of Vehicle-Aged Heavy-Duty Diesel Oxidation Catalyst and Natural Gas Three-Way Catalyst. Catalysts 2019, 9, 137. https://doi.org/10.3390/catal9020137
Kanerva T, Honkanen M, Kolli T, Heikkinen O, Kallinen K, Saarinen T, Lahtinen J, Olsson E, Keiski RL, Vippola M. Microstructural Characteristics of Vehicle-Aged Heavy-Duty Diesel Oxidation Catalyst and Natural Gas Three-Way Catalyst. Catalysts. 2019; 9(2):137. https://doi.org/10.3390/catal9020137
Chicago/Turabian StyleKanerva, Tomi, Mari Honkanen, Tanja Kolli, Olli Heikkinen, Kauko Kallinen, Tuomo Saarinen, Jouko Lahtinen, Eva Olsson, Riitta L. Keiski, and Minnamari Vippola. 2019. "Microstructural Characteristics of Vehicle-Aged Heavy-Duty Diesel Oxidation Catalyst and Natural Gas Three-Way Catalyst" Catalysts 9, no. 2: 137. https://doi.org/10.3390/catal9020137