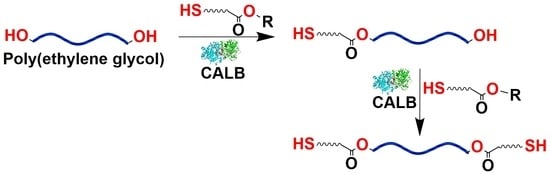
Synthesis of Mono- and Dithiols of Tetraethylene Glycol and Poly(ethylene glycol)s via Enzyme Catalysis
Abstract
:1. Introduction
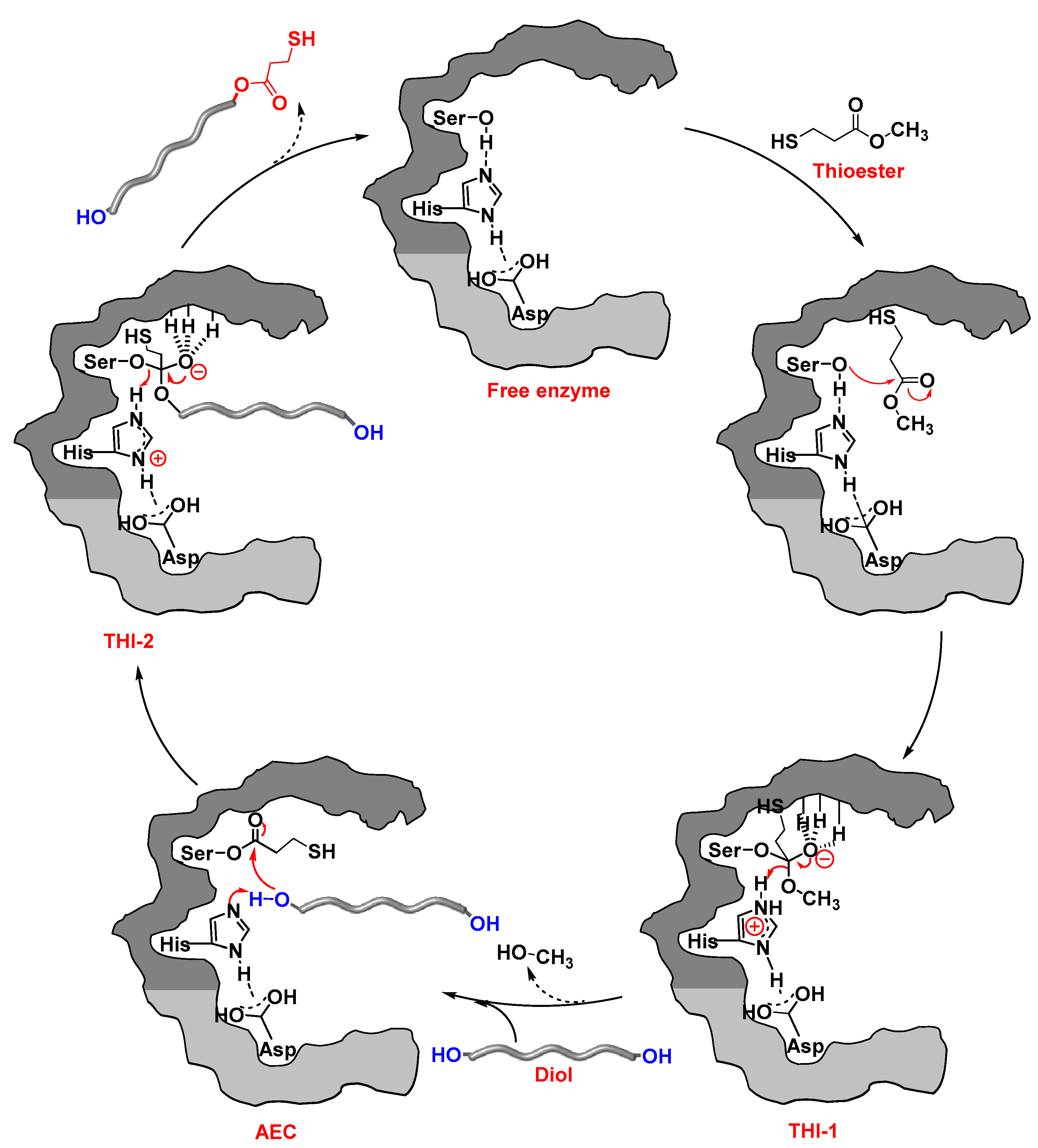
2. Results and Discussion
2.1. CALB-Catalyzed Transesterification of MP-SH with TEG
2.1.1. Kinetics of CALB-Catalyzed Transesterification of MP-SH with TEG
2.1.2. Synthesis of TEG-monothiol and TEG-dithiol
2.2. CALB-Catalyzed Transesterification of MP-SH with PEGs
2.2.1. Kinetics of CALB-catalyzed transesterification of MP-SH with PEG
2.2.2. Synthesis of PEG1000-monothiol
2.2.3. Synthesis of PEG1000-dithiol
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. CALB-catalyzed transesterification of MP-SH with TEG
- Kinetic study
- 2.
- Synthesis of TEG-monothiol
- 3.
- Synthesis of TEG-dithiol
3.2.2. CALB-catalyzed transesterification of MP-SH with PEG
- Kinetic study
- PEG1000
- PEG2050
- 2.
- Synthesis of PEG-monothiols
- PEG1000-monothiol
- PEG2050-monothiol
- 3.
- Synthesis of PEG-dithiols
- PEG1000-dithiol
- PEG2050-dithiol
3.3. Characterization
3.3.1. Nuclear Magnetic Resonance (NMR) Spectroscopy
3.3.2. Mass Spectrometry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zalipsky, S.; Harris, J.M. Introduction to Chemistry and Biological Applications of Poly (Ethylene Glycol). In Poly(Ethylene Glycol) Chemistry and Biological Applications; Harris, J.M., Zalipsky, S., Eds.; American Chemical Society: Washington, DC, USA, 1997; Volume 680, ISBN 978–0841235373. [Google Scholar]
- Dreborg, S.; Akerblom, E.B. Immunotherapy with Monomethoxypolyethylene Glycol Modified Allergens. Crit. Rev. Ther. Drug Carr. Syst. 1989, 6, 315–365. [Google Scholar]
- Yamaoka, T.; Tabata, Y.; Ikada, Y. Distribution and Tissue Uptake of Poly(Ethylene Glycol) with Different Molecular Weights after Intravenous Administration to Mice. J. Pharm. Sci. 1994, 83, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Puskas, J.E.; Castano, M.; Mulay, P.; Dudipala, V.; Wesdemiotis, C. Method for the Synthesis of γ-PEGylated Folic Acid and Its Fluorescein-Labeled Derivative. Macromolecules 2018, 51, 9069–9077. [Google Scholar] [CrossRef]
- Anseth, K.S.; Klok, H.A. Click Chemistry in Biomaterials, Nanomedicine, and Drug Delivery. Biomacromolecules 2016, 17, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Mather, B.D.; Viswanathan, K.; Miller, K.M.; Long, T.E. Michael Addition Reactions in Macromolecular Design for Emerging Technologies. Prog. Polym. Sci. 2006, 31, 487–531. [Google Scholar] [CrossRef]
- Nair, D.P.; Podgorski, M.; Chatani, S.; Gong, T.; Xi, W.; Fenoli, C.R.; Bowman, C.N. The Thiol-Michael Addition Click Reaction: A Powerful and Widely used Tool in Materials Chemistry. Chem. Mater. 2014, 26, 724–744. [Google Scholar] [CrossRef]
- Oliverio, M.; Perotto, S.; Messina, G.C.; Lovato, L.; De Angelis, F. Chemical Functionalization of Plasmonic Surface Biosensors: A Tutorial Review on Issues, Strategies, and Costs. ACS Appl. Mater. Interfaces 2017, 9, 29394–29411. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yu, C. Detection of Chemical Pollutants in Water using Gold Nanoparticles as Sensors: A Review. Rev. Anal. Chem. 2013, 32, 1–14. [Google Scholar] [CrossRef]
- Manson, J.; Kumar, D.; Meenan, B.J.; Dixon, D. Polyethylene glycol Functionalized Gold Nanoparticles: The Influence of Capping Density on Stability in Various Media. Gold Bull. 2011, 44, 99–105. [Google Scholar] [CrossRef]
- Mahou, R.; Wandrey, C. Versatile Route to Synthesize Heterobifunctional Poly(Ethylene Glycol) of Variable Functionality for Subsequent PEGylation. Polymers 2012, 4, 561–589. [Google Scholar] [CrossRef]
- Goessl, A.; Tirelli, N.; Hubbell, J.A. A Hydrogel System for Stimulus-Responsive, Oxygen-Sensitive In Situ Gelation. J. Biomater. Sci. Polym. Ed. 2004, 15, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, S.J.; Dijkstra, P.J.; Feijen, J. In Situ Forming Poly(Ethylene Glycol)-Poly(L-Lactide) Hydrogels via Michael Addition: Mechanical Properties, Degradation, and Protein Release. Macromol. Chem. Phys. 2012, 213, 766–775. [Google Scholar] [CrossRef]
- Hiemstra, C.; van der Aa, L.J.; Zhong, Z.; Dijkstra, P.J.; Feijen, J. Novel In Situ Forming, Degradable Dextran Hydrogels by Michael Addition Chemistry: Synthesis, Rheology, and Degradation. Macromolecules 2007, 40, 1165–1173. [Google Scholar] [CrossRef]
- Yoshimoto, K.; Hirase, T.; Nemoto, S.; Hatta, T.; Nagasaki, Y. Facile Construction of Sulfanyl-Terminated Poly(Ethylene Glycol)-Brushed Layer on a Gold Surface for Protein Immobilization by the Combined use of Sulfanyl-Ended Telechelic and Semitelechelic Poly(Ethylene Glycol)s. Langmuir 2008, 24, 9623–9629. [Google Scholar] [CrossRef] [PubMed]
- Hirase, T.; Nagasaki, Y. Construction of Mercapto-Ended Poly(Ethylene Glycol) Tethered Chain Surface for High Performance Bioconjugation. In Proceedings of the AIChE Annual Meeting, San Francisco, CA, USA, 12–17 November 2006. [Google Scholar]
- Nie, T.; Baldwin, A.; Yamaguchi, N.; Kiick, K.L. Production of Heparin-Functionalized Hydrogels for the Development of Responsive and Controlled Growth Factor Delivery Systems. J. Control. Release 2007, 122, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Belair, D.G.; Miller, M.J.; Wang, S.; Darjatmoko, S.R.; Binder, B.Y.; Sheibani, N.; Murphy, W.L. Differential Regulation of Angiogenesis using Degradable VEGF-Binding Microspheres. Biomaterials 2016, 93, 7–37. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Feng, Z.G.; Zhang, A.Y.; Sun, L.G.; Qian, L. Synthesis and Characterization of Three-Dimensional Crosslinked Networks Based on Self-Assemly of α-Cyclodextrins with Thiolated 4-arm PEG using a Three-Step Oxidation. Soft Matter 2006, 2, 343–349. [Google Scholar] [CrossRef]
- Du, Y.J.; Brash, J.L. Synthesis and Characterization of thiol-terminated Poly(Ethylene Oxide) for Chemisorption to Gold Surface. J. Appl. Polym. Sci. 2003, 90, 594–607. [Google Scholar] [CrossRef]
- Wan, J.K.S.; Depew, M.C. Some Mechanistic Insights in the Behaviour of Thiol Containing Antioxidant Polymers in Lignin Oxidation Processes. Res. Chem. Intermed. 1996, 22, 241–253. [Google Scholar] [CrossRef]
- Yang, T.; Long, H.; Malkoch, M.; Kristofer Gamstedt, E.; Berglund, L.; Hult, A. Characterization of Well-Defined Poly(Ethylene Glycol) Hydrogels Prepared by Thiol-Ene Chemistry. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 4044–4054. [Google Scholar] [CrossRef]
- Zhang, H.J.; Xin, Y.; Yan, Q.; Zhou, L.L.; Peng, L.; Yuan, J.Y. Facile and Efficient Fabrication of Photoresponsive Microgels via Thiol–Michael Addition. Macromol. Rapid Commun. 2012, 33, 1952–1957. [Google Scholar] [CrossRef] [PubMed]
- Zustiak, S.P.; Leach, J.B. Hydrolytically Degradable Poly(Ethylene Glycol) Hydrogel Scaffolds with Tunable Degradation and Mechanical Properties. Biomacromolecules 2010, 11, 1348–1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zustiak, S.P. Hydrolytically Degradable Polyethylene Glycol (PEG) Hydrogel: Synthesis, Gel Formation, and Characterization. In Extracellular Matrix; Leach, J., Powell, E., Eds.; Humana Press: New York, NY, USA, 2015; Volume 93, ISBN 978–1–4939–2082–2. [Google Scholar]
- Puskas, J.E.; Sen, M. Process of Preparing Functionalized Polymers via Enzymatic Catalysis. U.S. Patents 8,710,156, 29 April 2014. [Google Scholar]
- Puskas, J.E.; Sen, M.Y.; Seo, K.S. Green Polymer Chemistry using Nature’s Catalysts, Enzymes. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 2959–2976. [Google Scholar] [CrossRef]
- Castano, M.; Seo, K.S.; Guo, K.; Becker, M.L.; Wesdemiotis, C.; Puskas, J.E. Green Polymer Chemistry: Synthesis of Symmetric and Asymmetric Telechelic Ethylene Glycol Oligomers. Polym. Chem. 2015, 6, 1137–1142. [Google Scholar] [CrossRef]
- Puskas, J.E.; Sen, M.Y.; Kasper, J.R. Green Polymer Chemistry: Telechelic Poly(Ethylene Glycol)s via Enzymatic Catalysis. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3024–3028. [Google Scholar] [CrossRef]
- Puskas, J.E.; Seo, K.S.; Sen, M.Y. Green Polymer Chemistry: Precision Synthesis of Novel Multifunctional Poly(Ethylene Glycol)s using Enzymatic Catalysis. Eur. Polym. J. 2011, 47, 524–534. [Google Scholar] [CrossRef]
- Castano, M.; Seo, K.S.; Kim, E.H.; Becker, M.L.; Puskas, J.E. Green Polymer Chemistry VIII: Synthesis of Halo-Ester-Functionalized Poly(Ethylene Glycol)s via Enzymatic Catalysis. Macromol. Rapid Commun. 2013, 34, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Puskas, J.E.; Seo, K.S.; Castano, M.; Casiano, M.; Wesdemiotis, C. Green Polymer Chemistry: Enzymatic Functionalization of Poly(ethylene glycol)s under solventless conditions. In Green Polymer Chemistry: Biocatalysis and Materials II; Cheng, H.N., Gross, R.A., Smith, P.B., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2013; Volume 1144, ISBN 978–0–8412–2895–5. [Google Scholar]
- Sen, S.; Puskas, J.E. Green Polymer Chemistry: Enzyme Catalysis for Polymer Functionalization. Molecules 2015, 20, 9358–9379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zdarta, J.; Wysokowski, M.; Norman, M.; Kołodziejczak-Radzimska, A.; Moszyński, D.; Maciejewski, H.; Ehrlich, H.; Jesionowski, T. Candida antarctica Lipase B Immobilized onto Chitin Conjugated with POSS® Compounds: Useful Tool for Rapeseed Oil Conversion. Int. J. Mol. Sci. 2016, 17, 1581. [Google Scholar] [CrossRef] [PubMed]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme Immobilization by Adsorption: A Review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Zdarta, J.; Klapiszewski, L.; Jedrzak, A.; Nowicki, M.; Moszynski, D.; Jesionowski, T. Lipase B from Candida antarctica Immobilized on a Silica-Lignin Matrix as a Stable and Reusable Biocatalytic System. Catalyst 2017, 7, 14. [Google Scholar] [CrossRef]
- Wolfson, A.; Atyya, A.; Dlugy, C.; Tavor, D. Glycerol Triacetate as Solvent and Acyl Donor in the Production of Isoamyl Acetate with Candida antarctica Lipase B. Bioprocess Biosyst. Eng. 2010, 33, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Uppenberg, J.; Oehrner, N.; Norin, M.; Hult, K.; Kleywegt, G.J.; Patkar, S.; Waagen, V.; Anthonsen, T.; Jones, T.A. Crystallographic and Molecular-Modeling Studies of Lipase B from Candida antarctica reveal a Stereospecificity Pocket for Secondary Alcohols. Biochemistry 1995, 34, 16838–16851. [Google Scholar] [CrossRef] [PubMed]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulay, P.; Shrikhande, G.; Puskas, J.E. Synthesis of Mono- and Dithiols of Tetraethylene Glycol and Poly(ethylene glycol)s via Enzyme Catalysis. Catalysts 2019, 9, 228. https://doi.org/10.3390/catal9030228
Mulay P, Shrikhande G, Puskas JE. Synthesis of Mono- and Dithiols of Tetraethylene Glycol and Poly(ethylene glycol)s via Enzyme Catalysis. Catalysts. 2019; 9(3):228. https://doi.org/10.3390/catal9030228
Chicago/Turabian StyleMulay, Prajakatta, Gayatri Shrikhande, and Judit E. Puskas. 2019. "Synthesis of Mono- and Dithiols of Tetraethylene Glycol and Poly(ethylene glycol)s via Enzyme Catalysis" Catalysts 9, no. 3: 228. https://doi.org/10.3390/catal9030228