Photocatalytic Activity of Nanostructured Titania Films Obtained by Electrochemical, Chemical, and Thermal Oxidation of Ti6Al4V Alloy—Comparative Analysis
Abstract
:1. Introduction
2. Results
2.1. Morphological and Structural Characterization of Titania Nanomaterials
2.2. Wettability and Surface Energy of Titania Nanoarchitectures
2.3. Specific Surface Area According to BET Theory
2.4. Band Gap Characteristic
2.5. Photocatalytic Activity Results
3. Discussion
4. Materials and Methods
4.1. Electrochemical Oxidation of Ti6Al4V
4.2. Chemical Oxidation of Ti6Al4V
4.3. Thermal Oxidation of Ti6Al4V
4.4. Structure and Morphology Characterization
4.5. Wettability Studies and Surface Free Energy Estimation
4.6. Specific Surface Area Estimation According to BET analysis
4.7. Band Gap Characterization on the Basis of Diffuse Reflectance UV-Vis Spectroscopy
4.8. Photoactivity Test
4.9. Kinetic Calculations
5. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B Environ. 2015, 176–177, 396–428. [Google Scholar] [CrossRef]
- Erika Cedillo-González, E.I.; Riccò, R.; Montorsi, M.; Montorsi, M.; Falcaro, P.; Siligardi, C. Self-cleaning glass prepared from a commercial TiO2 nano-dispersion and its photocatalytic performance under common anthropogenic and atmospheric factors. Build. Environ. 2014, 71, 7–14. [Google Scholar] [CrossRef]
- Xu, F.; Wang, T.; Chen, H.Y.; Bohling, J.; Maurice, A.M.; Wu, L.; Zhou, S. Preparation of photocatalytic TiO2-based self-cleaning coatings for painted surface without interlayer. Prog. Org. Coat. 2017, 113, 15–24. [Google Scholar] [CrossRef]
- Takagi, K.; Makimoto, T.; Hiraiwa, H.; Negishi, T. Photocatalytic Antifogging Mirror. J. Vac. Sci. Technol. A 2001, 19, 2931–2935. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 photocatalysis—A historical overview and future prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Vitiello, G.; Pezzella, A.; Zanfardino, A.; Varcamonti, M.; Silvestri, B.; Costantini, A.; Luciani, G. Titania as a driving agent for DHICA polymerization: A novel strategy for the design of bioinspired antimicrobial nanomaterials. J. Mater. Chem. B 2015, 3, 2808–2815. [Google Scholar] [CrossRef]
- Fu, G.; Vary, P.S.; Lin, C.-T. Anatase TiO2 Nanocomposites for Antimicrobial Coatings. J. Phys. Chem. B 2005, 109, 8889–8898. [Google Scholar]
- Kubacka, A.; Diez, M.S.; Rojo, D.; Bargiel, R.; Ciordia, S.; Zapico, I.; Albar, J.P.; Barbas, C.; Martins dos Santos, V.; Fernandez-Garcia, M.; et al. Understanding the antimicrobial mechanism of TiO2-based nanocomposite films in a pathogenic bacterium. Sci. Rep. 2014, 4, 4134. [Google Scholar] [CrossRef]
- Vitiello, G.; Pezzella, A.; Zanfardino, A.; Silvestri, B.; Giudicianni, P.; Costantini, A.; Luciani, G. Antimicrobial activity of eumelanin-based hybrids: The role of TiO2 in modulating the structure and biological performance. Mater. Sci. Eng. C 2017, 75, 454–462. [Google Scholar] [CrossRef]
- Ghicov, A.; Tsuchiya, H.; Hahn, R.; Macak, J.M.; Munoz, A.G.; Schmuki, P. TiO2 nanotubes: H+-insertion and strong electrochromic effects. Electrochem. Commun. 2006, 8, 528–532. [Google Scholar] [CrossRef]
- Garcia Canadas, J.; Fabregat-Santiago, F.; Kapla, J.; Bisquert, J.; Garcia-Belmonte, G.; Mora-Sero, I.; Edwards, M.O.M. Dynamic behaviour of viologen-activated nanostructured TiO2: Correlation between kinetics of charging and coloration. Electrochim. Acta 2004, 49, 745–752. [Google Scholar] [CrossRef]
- Turhan, I.; Tepehan, F.Z.; Tepehan, G.G. Effect of V2O5 content on the optical, structural and electrochromic properties of TiO2 and ZrO2 thin films. J. Mater. Sci. 2005, 40, 1359–1362. [Google Scholar] [CrossRef]
- Yan, X.; Wang, Z.; He, M.; Hou, Z.; Xia, T.; Liu, G.; Chen, X. TiO2 Nanomaterials as Anode Materials for Lithium-Ion Rechargeable Batteries. Energy Technol. 2015, 3, 801–814. [Google Scholar] [CrossRef]
- Lahan, H.; Boruah, R.; Hazarika, A.; Das, S.K. Anatase TiO2 as an Anode Material for Rechargeable Aqueous Aluminum-Ion Batteries: Remarkable Graphene Induced Aluminum Ion Storage Phenomenon. J. Phys. Chem. C 2017, 121, 26241–26249. [Google Scholar] [CrossRef]
- Bai, J.; Zhou, B. Titanium Dioxide Nanomaterials for Sensor Applications. Chem. Rev. 2014, 114, 10131–10176. [Google Scholar] [CrossRef]
- Maziarz, W.; Kusior, A.; Trenczek-Zajac, A. Nanostructured TiO2-based gas sensors with enhanced sensitivity to reducing gases. Beilstein J. Nanotechnol. 2016, 7, 1718–1726. [Google Scholar] [CrossRef]
- Rehman, F.U.; Zhao, C.; Jiang, H.; Wang, X. Biomedical applications of nano-titania in theranostics and photodynamic therapy. Biomater Sci. 2016, 4, 40–54. [Google Scholar] [CrossRef]
- Jukapli, N.M.; Bagheri, S. Recent developments on Titania nanoparticle as photocatalytic cancer cells treatment. J. Photochem. Photobiol. B 2016, 163, 421–430. [Google Scholar] [CrossRef]
- Yin, F.Z.; Wu, L.; Yang, H.G.; Su, Y.S. Recent progress in biomedical applications of titanium dioxide. Phys. Chem. Chem. Phys. 2013, 15, 4844–4858. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Gongadze, E.; Perutkova, Š.; Kralj-Iglič, V.; Milošev, I.; Schmuki, P.; Iglič, A.; Mozetič, M. Titanium nanostructures for biomedical applications. Nanotechnology 2015, 26, 062002. [Google Scholar] [CrossRef] [Green Version]
- Cao, P.J.; Wu, H.D.; Dong, J.L. Research on Application of Nano-TiO2 in Automotive Coating. Appl. Mech. Mater. 2012, 160, 216–222. [Google Scholar] [CrossRef]
- Francioso, L.; Presicce, D.S.; Taurino, A.M.; Rella, R.; Siciliano, P.; Ficarella, A. Automotive application of sol–gel TiO2 thin film-based sensor for lambda measurement. Sens. Actuators B Chem. 2003, 95, 66–72. [Google Scholar] [CrossRef]
- Lazar, M.; Varghese, S.; Nair, S. Photocatalytic Water Treatment by Titanium Dioxide: Recent Updates. Catalysts 2012, 2, 572–601. [Google Scholar] [CrossRef] [Green Version]
- Gamage, J.; Zhang, Z. Applications of Photocatalytic Disinfection. Int. J. Photoenergy 2010, 2010, 1–11. [Google Scholar] [CrossRef]
- Binas, V.; Venieri, D.; Kotzias, D.; Kiriakidis, G. Modified TiO2 based photocatalysts for improved air and health quality. J. Mater. 2017, 3, 3–16. [Google Scholar]
- Stefanov, B.I.; Lebrun, D.; Mattsson, A.; Granqvist, C.G.; Österlund, L. Demonstrating Online Monitoring of Air Pollutant Photodegradation in a 3D Printed Gas-Phase Photocatalysis Reactor. J. Chem. Educ. 2015, 92, 678–682. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef]
- Clarizia, L.; Vitiello, G.; Pallotti, D.K.; Silvestri, B.; Nadagouda, M.; Lettieri, S.; Marotta, R. Effect of surface properties of copper-modified commercial titanium dioxide photocatalysts on hydrogen production through photoreforming of alcohols. Int. J. Hydrogen Energy 2017, 42, 28349–28362. [Google Scholar] [CrossRef]
- Araiedh, F.; Ducos, F.; Houas, A.; Chaoui, N. Kinetic study of the photocatalytic degradation of the C-polymorph of a stearic acid microcrystal grown on an amorphous titania surface scattered with anatase microdomains. J. Photochem. Photobiol. A Chem. 2018, 353, 458–463. [Google Scholar] [CrossRef]
- Thao, L.; Dang, T.; Khanitchaidecha, W.; Channei, D.; Nakaruk, A. Photocatalytic Degradation of Organic Dye under UV-A Irradiation Using TiO2-Vetiver Multifunctional Nano Particles. Materials 2017, 10, 122. [Google Scholar] [CrossRef]
- Zywitzki, D.; Jing, H.; Tüysüz, H.; Chan, C.K. High surface area, amorphous titania with reactive Ti3+ through a photo-assisted synthesis method for photocatalytic H2 generation. J. Mater. Chem. A 2017, 5, 10957–10967. [Google Scholar] [CrossRef]
- Matsuzawa, S.; Tanaka, J.; Sato, S.; Ibusuki, T. Photocatalytic oxidation of dibenzothiophenes in acetonitrile using TiO2: Effect of hydrogen peroxide and ultrasound irradiation. J. Photochem. Photobiol. A Chem. 2002, 149, 183–189. [Google Scholar] [CrossRef]
- Krylova, G.; Na, C. Photoinduced crystallization and activation of amorphous titanium dioxide. J. Phys. Chem. C 2015, 119, 12400–12407. [Google Scholar] [CrossRef]
- Xiong, L.B.; Li, J.L.; Yang, B.; Yu, Y. Ti3+ in the surface of titanium dioxide: Generation, properties and photocatalytic application. J. Nanomater. 2012, 2012, 831524. [Google Scholar] [CrossRef]
- Ghuman, K.K.; Singh, C.V. Self-Trapped Charge Carriers in Defected Amorphous TiO2. J. Phys. Chem. C 2016, 120, 27910–27916. [Google Scholar] [CrossRef]
- Ohtani, B.; Ogawa, Y.; Nishimoto, S. Photocatalytic activity of amorphous-anatase mixture of titanium(IV) oxide particles suspended in aqueous solutions. J. Phys. Chem. B 1997, 101, 3746–3752. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A 2010, 216, 179–182. [Google Scholar] [CrossRef] [Green Version]
- Scolan, E.; Sanchez, C. Synthesis and Characterization of Surface-Protected Nanocrystalline Titania Particles. Chem. Mater. 1998, 10, 3217–3223. [Google Scholar] [CrossRef]
- Gupta, S.M.; Tripathi, M. A review of TiO2 nanoparticles. Chin. Sci. Bull. 2011, 56, 1639–1657. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, N.; Schmuki, P. Photocatalysis with TiO2 Nanotubes: “Colorful” Reactivity and Designing Site-Specific Photocatalytic Centers into TiO2 Nanotubes. ACS Catal. 2017, 7, 3210–3235. [Google Scholar] [CrossRef]
- Meriam Suhaimy, S.; Lai, C.; Tajuddin, H.; Samsudin, E.; Johan, M. Impact of TiO2 Nanotubes’ Morphology on the Photocatalytic Degradation of Simazine Pollutant. Materials 2018, 11, 2066. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Fabrication of Titania Nanofibers by Electrospinning. Nano Lett. 2003, 3, 555–560. [Google Scholar]
- Wang, X.; Li, Z.; Shi, J.; Yu, Y. One-Dimensional Titanium Dioxide Nanomaterials: Nanowires, Nanorods, and Nanobelts. Chem. Rev. 2014, 114, 9346–9384. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-M.; Zhang, T.-W.; Zeng, Y.-W.; Hayakawa, S.; Tsuru, K.; Osaka, A. Large-Scale Preparation of Ordered Titania Nanorods with Enhanced Photocatalytic Activity. Langmuir 2005, 21, 6995–7002. [Google Scholar] [CrossRef]
- Wu, N.; Wang, J.; Tafen, D.; Wang, H.; Zheng, J.G.; Lewis, J.P.; Liu, X.; Leonard, S.S.; Manivannan, A. Shape-enhanced photocatalytic activity of single-crystalline anatase TiO2 (101) nanobelts. J. Am. Chem. Soc. 2010, 132, 6679–6685. [Google Scholar] [CrossRef] [PubMed]
- Rutar, M.; Rozman, N.; Pregelj, M.; Bittencourt, C.; Cerc Korošec, R.; Sever Škapin, A.; Mrzel, A.; Škapin, S.D.; Umek, P. Transformation of hydrogen titanate nanoribbons to TiO2 nanoribbons and the influence of the transformation strategies on the photocatalytic performance. Beilstein J. Nanotechnol. 2015, 6, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Stengl, V.; Bakardjieva, S.; Murafa, N.; Houskova, V. Hydrothermal synthesis of Titania powders and their photocatalytic properties. Ceram. Silik. 2008, 52, 278–290. [Google Scholar]
- Nam, C.T.; Yang, W.-D.; Duc, L.M. Solvothermal Synthesis of TiO2 Photocatalysts in Ketone Solvents with Low Boiling Points. J. Nanomater. 2013, 2013, 627385. [Google Scholar] [CrossRef]
- Su, C.; Hong, B.-Y.; Tseng, C.-M. Sol-gel preparation and photocatalysis of titanium dioxide. Catal. Today 2004, 96, 119–126. [Google Scholar] [CrossRef]
- Michalcik, Z.; Horakova, M.; Spatenka, P.; Klementova, S.; Zlamal, M.; Martin, N. Photocatalytic Activity of Nanostructured Titanium Dioxide Thin Films. Int. J. Photoenergy 2012, 2012, 689154. [Google Scholar] [CrossRef]
- Lee, K.; Mazare, A.; Schmuki, P. One-Dimensional Titanium Dioxide Nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef] [Green Version]
- Yi, J.-H.; Bernard, C.; Variola, F.; Zalzal, S.F.; Wuest, J.D.; Rosei, F.; Nanci, A. Characterization of a bioactive nanotextured surface created by controlled chemical oxidation of titanium. Surf. Sci. 2006, 600, 4613–4621. [Google Scholar] [CrossRef]
- Jamesh, M.; Sankara Narayanan, T.S.N.; Chu, P.K. Thermal oxidation of titanium: Evaluation of corrosion resistance as a function of cooling rate. Mater. Chem. Phys. 2013, 138, 565–572. [Google Scholar] [CrossRef]
- Mills, A.; Lepre, A.; Elliott, N.; Bhopal, S.; Parkin, I.P.; O’Neill, S.A. Characterisation of the photocatalyst Pilkington ActivTM: A reference film photocatalyst? J. Photochem. Photobiol. A Chem. 2003, 160, 213–224. [Google Scholar] [CrossRef]
- Sheel, D.W.; McCurdy, R.J.; Hurst, S.J. Method of Depositing Tin Oxide and Titanium Oxide Coatings on Flat Glass and the Resulting Coated Glass. Patent Application WO 1998/06675, 1998. [Google Scholar]
- Zhang, W.F.; He, Y.L.; Zhang, M.S.; Yin, Z.; Chen, Q. Raman scattering study on anatase TiO2 nanocrystals. J. Phys. D Appl. Phys. 2000, 33, 912–916. [Google Scholar] [CrossRef]
- Mazza, T.; Barborini, E.; Piseri, P.; Milani, P.; Cattaneo, D.; Li Bassi, A.; Bottani, C.E.; Ducati, C. Raman spectroscopy characterization of TiO2 rutile nanocrystals. Phys. Rev. B Condens. Matter Mater. Phys. 2007, 75, 045416. [Google Scholar] [CrossRef]
- Saalinraj, S.; Ajithprasad, K.C. Effect of Calcination Temperature on Non-linear Absorption Co-efficient of Nano Sized Titanium Dioxide (TiO2) Synthesised by Sol-Gel Method. Mater. Today Proc. 2017, 4, 4372–4379. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Regonini, D.; Jaroenworaluck, A.; Stevens, R.; Bowen, C.R. Effect of heat treatment on the properties and structure of TiO2 nanotubes: Phase composition and chemical composition. Surf. Interface Anal. 2010, 42, 139–144. [Google Scholar] [CrossRef]
- Ghosh, M.; Lohrasbi, M.; Chuang, S.S.C.; Jana, S.C. Mesoporous Titanium Dioxide Nanofibers with a Significantly Enhanced Photocatalytic Activity. ChemCatChem 2016, 8, 2525–2535. [Google Scholar] [CrossRef]
- Lee, C.; Shul, Y.-G.; Einaga, H. Structural Analysis of Mesoporous ZrO2 and TiO2 Nanofiber Mats Prepared by Electrospinning Methods; Engineering Sciences Reports; Kyushu University: Fukuoka, Japan, 2016; Volume 37, pp. 1–5. [Google Scholar]
- Radtke, A.; Piszczek, P.; Topolski, A.; Lewandowska, Ż.; Talik, E.; Andersen, I.H.; Nielsen, L.P.; Heikkilä, M.; Leskelä, M. The structure and the photocatalytic activity of Titania nanotube and nanofiber coatings. Appl. Surf. Sci. 2016, 368, 165–172. [Google Scholar] [CrossRef]
- Padiyan, D.P.; Raja, D.H. Synthesis of Various Generations Titania Nanotube Arrays by Electrochemical Anodization for H2 Production. Energy Procedia 2012, 22, 88–100. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Q.; Qin, L.-C. Reduction in the electronic band gap of titanium oxide nanotubes. Solid State Commun. 2007, 141, 168–171. [Google Scholar] [CrossRef]
- Dette, C.; Pérez-Osorio, M.A.; Kley, C.S.; Punke, P.; Patrick, C.E.; Jacobson, P.; Kern, K. TiO2 Anatase with a Bandgap in the Visible Region. Nano Lett. 2014, 14, 6533–6538. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Sokol, A.A. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2010, 46, 855–874. [Google Scholar] [CrossRef] [Green Version]
- Dariani, R.S.; Esmaeili, A.; Mortezaali, A.; Dehghanpour, S. Photocatalytic reaction and degradation of methylene blue on TiO2 nano-sized particles. Opt. Int. J. Light Electron. Opt. 2016, 127, 7143–7154. [Google Scholar] [CrossRef]
- Hou, C.; Hu, B.; Zhu, J. Photocatalytic Degradation of Methylene Blue over TiO2 Pretreated with Varying Concentrations of NaOH. Catalysts 2018, 8, 575. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Houas, A. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Herrmann, J.M. Water treatment by heterogeneous photocatalysis. In Environmental Catalysis; Catalytic Science Series; Jansen, F., van Santen, R.A., Eds.; Imperial College Press: London, UK, 1999; Volume 1, Chapter 9; pp. 171–194. [Google Scholar]
- Radtke, A.; Ehlert, M.; Jędrzejewski, T.; Sadowska, B.; Więckowska-Szakiel, M.; Holopainen, J.; Ritala, M.; Leskelä, M.; Bartmański, M.; Szkodo, M.; et al. Titania Nanotubes/Hydroxyapatite Nanocomposites Produced with the Use of the Atomic Layer Deposition Technique: Estimation of Bioactivity and Nanomechanical Properties. Nanomaterials 2019, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Grodzicka, M.; Ehlert, M.; Muzioł, T.; Szkodo, M.; Bartmański, M.; Piszczek, P. Studies on Silver Ions Releasing Processes and Mechanical Properties of Surface-Modified Titanium Alloy Implants. Int. J. Mol. Sci. 2018, 19, 3962. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.V.; Porkodi, K.; Rocha, F. Langmuir–Hinshelwood kinetics—A theoretical study. Catal. Commun. 2008, 9, 82–84. [Google Scholar] [CrossRef]
- Radtke, A.; Bal, M.; Jędrzejewski, T. Novel Titania Nanocoatings Produced by Anodic Oxidation with the Use of Cyclically Changing Potential: Their Photocatalytic Activity and Biocompatibility. Nanomaterials 2018, 8, 712. [Google Scholar] [CrossRef] [PubMed]
- Piszczek, P.; Lewandowska, Ż.; Radtke, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B.; Szubka, M.; Talik, E.; Fiori, F. Biocompatibility of Titania Nanotube Coatings Enriched with Silver Nanograins by Chemical Vapor Deposition. Nanomaterials 2017, 7, 274. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Topolski, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B.; Więckowska-Szakiel, M.; Szubka, M.; Talik, E.; Pleth Nielsen, L.; Piszczek, P. The Bioactivity and Photocatalytic Properties of Titania Nanotube Coatings Produced with the Use of the Low-Potential Anodization of Ti6Al4V Alloy Surface. Nanomaterials 2017, 7, 197. [Google Scholar] [CrossRef]



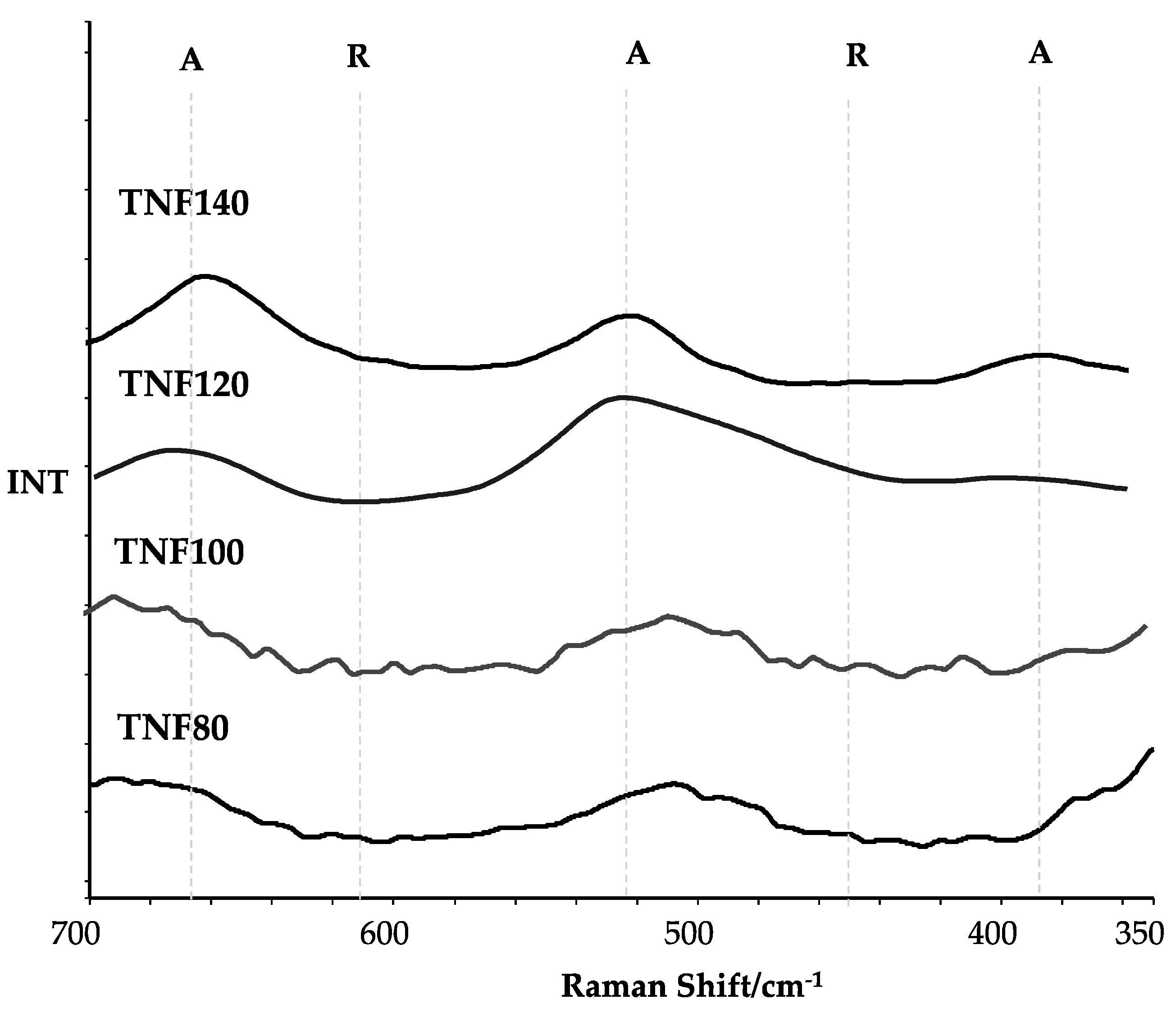



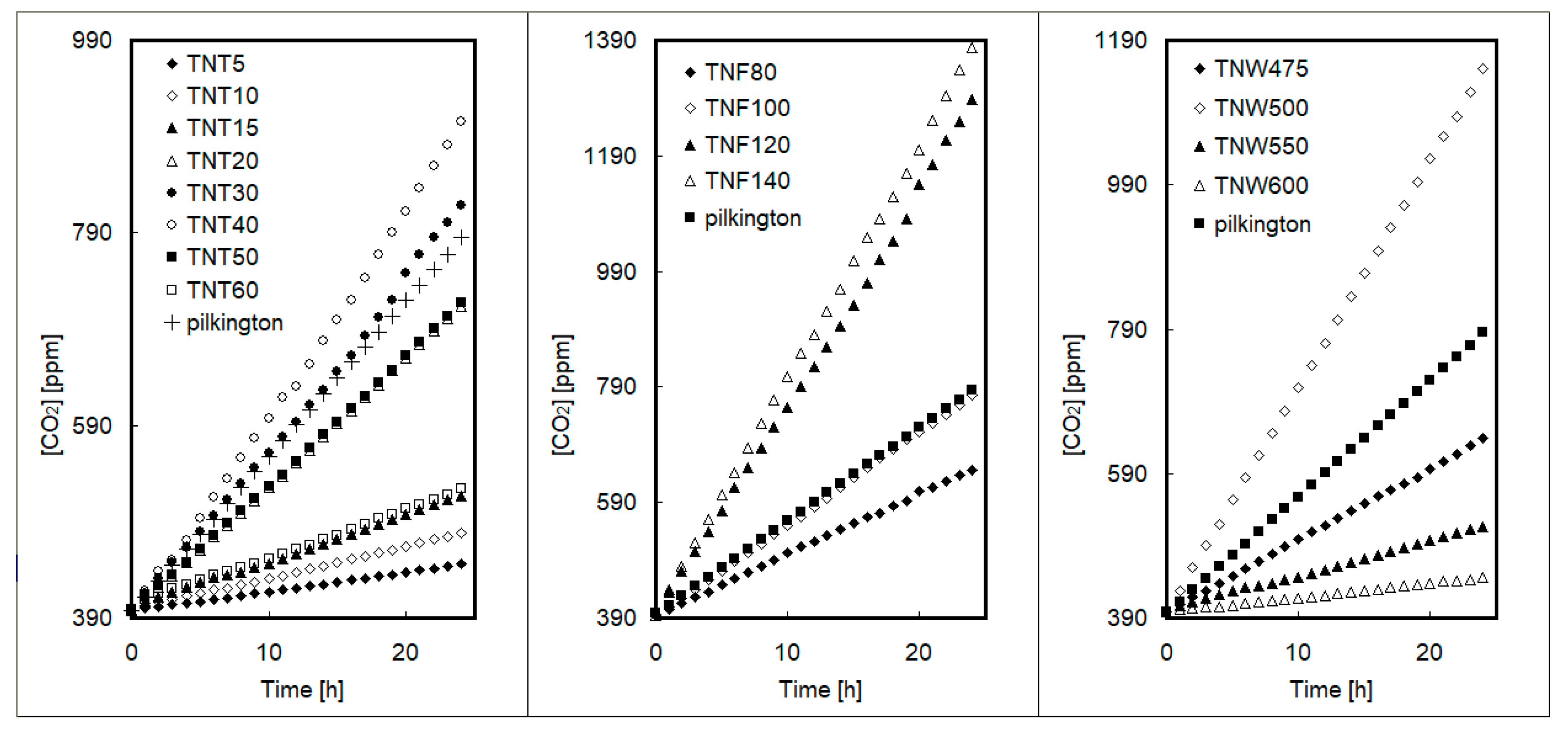

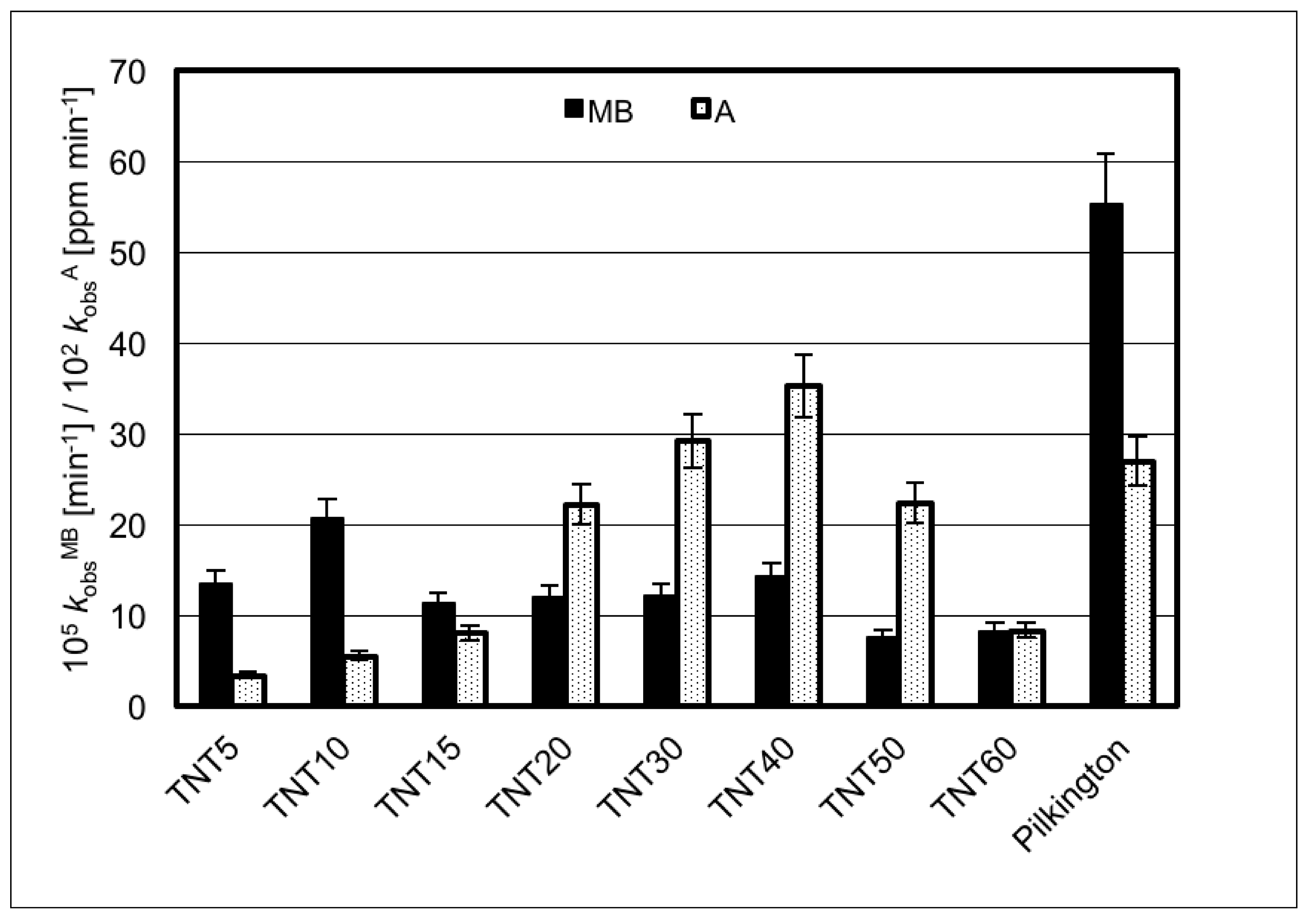



| Sample | Average Contact Angle [°] ± Standard Deviation | SFE [mJ/m2] | |
|---|---|---|---|
| Measuring Liquid | |||
| Water | Diiodomethane | ||
| Ti6Al4V | 108.3 ± 0.1 | 37 ± 0.2 | 45.4 ± 0.1 |
| Ti6Al4V/TNT5 | 64.5 ± 0.8 | 48.5 ± 2.3 | 42.3 ± 2.4 |
| Ti6Al4V/TNT10 | 52.2 ± 1.2 | 40.8 ± 1.3 | 51.09 ± 1.3 |
| Ti6Al4V/TNT15 | 43.3 ± 1.8 | 31.8 ± 0.9 | 57.98 ± 1.3 |
| Ti6Al4V/TNT20 | 31.2 ± 0.8 | 27.8 ± 1.3 | 65.01 ± 1.3 |
| Ti6Al4V/TNT30 | 21.9 ± 1.3 | <10 | >70.63 ± 1.3 |
| Ti6Al4V/TNT40 | 19.3 ± 1.0 | <10 | >71.52 ± 0.1 |
| Ti6Al4V/TNT50 | 55.6 ± 1.2 | 40.9 ± 0.6 | 49.14 ± 1.2 |
| Ti6Al4V/TNT60 | 75.10 ± 1.1 | 51.8 ± 1.5 | 36.45 ± 1.5 |
| Ti6Al4V/TNF80 | 80.9 ± 0.8 | 46.9 ± 1.2 | 36.87 ± 1.2 |
| Ti6Al4V/TNF100 | 75.7 ± 1.1 | 49.8 ± 1.3 | 37.02 ± 1.3 |
| Ti6Al4V/TNF120 | 60.9 ± 1.3 | 40.2 ± 1.0 | 46.55 ± 1.1 |
| Ti6Al4V/TNF140 | 52.8 ± 1.0 | 57.9 ± 0.7 | 47.55 ± 1.0 |
| Ti6Al4V/TNW475 | 118.7 ± 0.8 | 49.6 ± 0.2 | 42.36 ± 0.8 |
| Ti6Al4V/TNW500 | 123.3 ± 0.3 | 51.9 ± 0.4 | 42.88 ± 0.4 |
| Ti6Al4V/TNW550 | 126.8 ± 0.5 | 60.3 ± 0.1 | 37.25 ± 0.5 |
| Ti6Al4V/TNW600 | 129.8 ± 0.2 | 68.3 ± 0.1 | 31.50 ± 0.2 |
| TNT | SBET [m2/g] | TNF | SBET [m2/g] | TNW | SBET [m2/g] |
|---|---|---|---|---|---|
| TNT5 | 18.0 ± 0.2 | TNF80 | 22.5 ± 0.3 | TNW475 | 45.2 ± 0.2 |
| TNT10 | 16.9 ± 0.2 | TNF100 | 21.3 ± 0.2 | TNW500 | 22.1 ± 0.3 |
| TNT15 | 13.4 ± 0.1 | TNF120 | 23.7 ± 0.1 | TNW550 | 15.0 ± 0.1 |
| TNT20 | 10.4 ± 0.2 | TNF140 | 31.2 ± 0.2 | TNW600 | 9.7 ± 0.1 |
| TNT30 | 9.3 ± 0.1 | - | - | - | - |
| TNT40 | 8.5 ± 0.2 | - | - | - | - |
| TNT50 | 11.4 ± 0.3 | - | - | - | - |
| TNT60 | 12.8 ± 0.2 | - | - | - | - |
| TNT Samples | Eg [eV] | TNF Samples | Eg [eV] | TNW Samples | Eg [eV] |
|---|---|---|---|---|---|
| TNT5 | 3.64 | TNF80 | 3.55 | TNW475 | 3.24 |
| TNT10 | 3.59 | TNF100 | 3.43 | TNW500 | 3.11 |
| TNT15 | 3.49 | TNF120 | 3.32 | TNW550 | 3.01 |
| TNT20 | 3.42 | TNF140 | 3.22 | TNW600 | 2.96 |
| TNT30 | 3.36 | - | - | - | - |
| TNT40 | 3.40 | - | - | - | - |
| TNT50 | 3.44 | - | - | - | - |
| TNT60 | 3.56 | - | - | - | - |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radtke, A. Photocatalytic Activity of Nanostructured Titania Films Obtained by Electrochemical, Chemical, and Thermal Oxidation of Ti6Al4V Alloy—Comparative Analysis. Catalysts 2019, 9, 279. https://doi.org/10.3390/catal9030279
Radtke A. Photocatalytic Activity of Nanostructured Titania Films Obtained by Electrochemical, Chemical, and Thermal Oxidation of Ti6Al4V Alloy—Comparative Analysis. Catalysts. 2019; 9(3):279. https://doi.org/10.3390/catal9030279
Chicago/Turabian StyleRadtke, Aleksandra. 2019. "Photocatalytic Activity of Nanostructured Titania Films Obtained by Electrochemical, Chemical, and Thermal Oxidation of Ti6Al4V Alloy—Comparative Analysis" Catalysts 9, no. 3: 279. https://doi.org/10.3390/catal9030279






