Hydroxymethylation of Furfural to HMF with Aqueous Formaldehyde over Zeolite Beta Catalyst
Abstract
:1. Introduction
2. Results and Discussions
2.1. Catalyst Structure and Physicochemical Properties
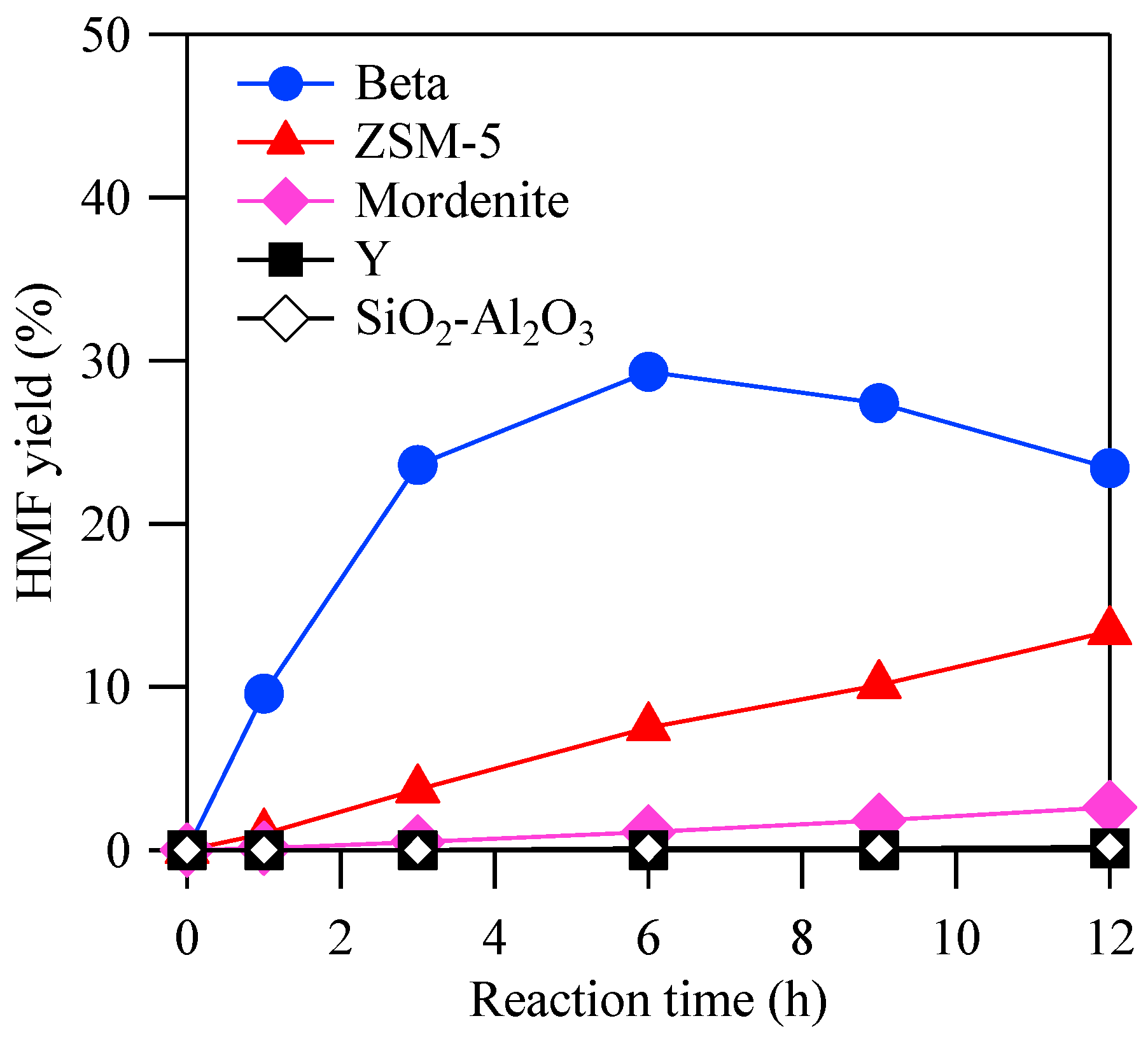
2.2. Reactivity among Different Zeolite Structures
2.3. Reusability of Zeolite Beta
2.4. Effect of SiO2/Al2O3 Ratio on Zeolite Beta
3. Materials and Methods
3.1. Batch Reactor System
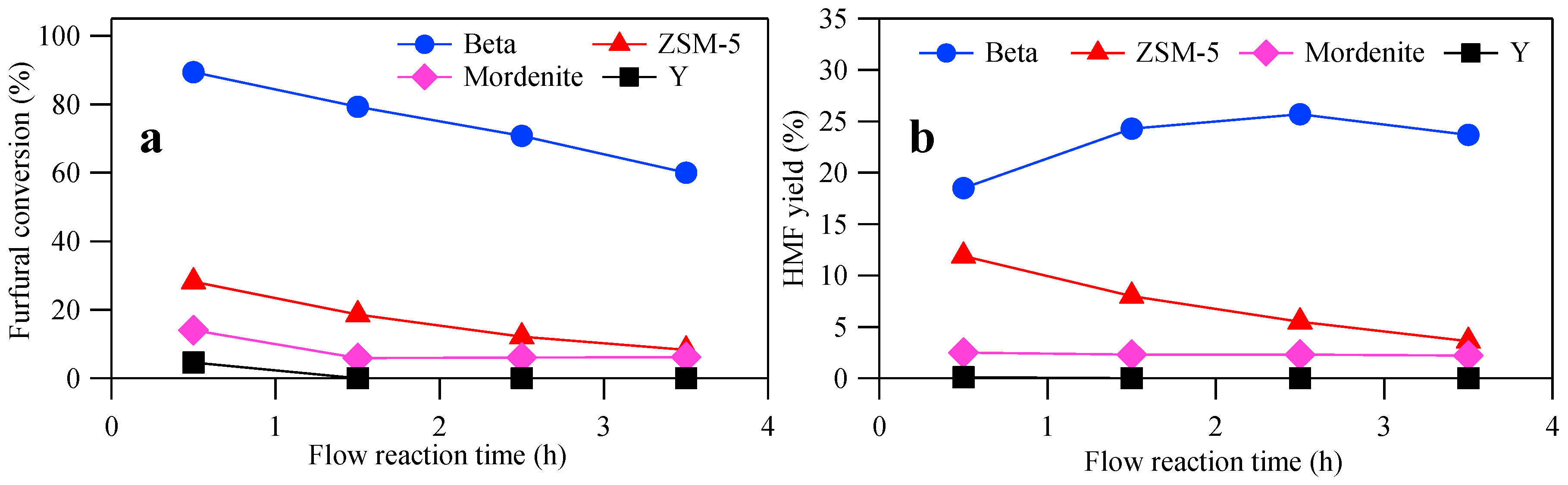
3.2. Flow Reactor System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Putten, R.J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Delidovich, I.; Hausoul, P.J.C.; Deng, L.; Pfutzenreuter, R.; Rose, M.; Palkovits, R. Alternative Monomers Based on Lignocellulose and Their Use for Polymer production. Chem. Rev. 2016, 116, 1540–1599. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Fukuoka, A. Synthesis and utilization of sugar compounds derived from lignocellulosic biomass. Green Chem. 2013, 15, 1740–1763. [Google Scholar] [CrossRef]
- Nishimura, S.; Ebitani, K. Selective Oxidation of Biomass-derived Alcohols with Supported Metal Catalysts. J. Jpn. Petroleum Inst. 2017, 60, 72–84. [Google Scholar] [CrossRef] [Green Version]
- Lange, J.P.; van der Heide, E.; van Buijtenen, J.; Price, R. Furfural—A Promising Platform for Lignocellulosic biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhou, P.; Zhang, Z.; Yang, C.; Zhang, B.; Deng, K.; Bottle, S.; Zhu, H. Selective Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid using O2 and a Photocatalyst of Cothioporphyrazine Bonded to g-C3N4. J. Am. Chem. Soc. 2017, 139, 14775–14782. [Google Scholar] [CrossRef]
- Gupta, N.K.; Nishimura, S.; Takagaki, A.; Ebitani, K. Hydrotalcite supported gold-nanoparticle-catalyzed highly efficient base-free aqueous oxidation of 5-hydroxymethylfurfural into 2,5-fudandicarboxylic acid under atmospheric oxygen pressure. Green Chem. 2011, 13, 824–827. [Google Scholar] [CrossRef]
- Yuan, Z.; Liu, B.; Zhou, P.; Zhang, Z.; Chi, Q. Aerobic oxidation of biomass-derived 5-hydrxymtehylfurfural to 2,5-diformylfuran with cesium-doped manganese dioxide. Catal. Sci. Techonol. 2018, 8, 4430–4439. [Google Scholar] [CrossRef]
- Takagaki, A.; Takahashi, M.; Nishimura, S.; Ebitani, K. One-pot synthesis of 2,5-diformylfuran from carbohydrate derivatives by sulfonated resin and hydrotalcite-supported ruthenium catalysts. ACS Catal. 2011, 1, 1562–1565. [Google Scholar] [CrossRef]
- Perret, N.; Grigoropoulos, A.; Zanella, M.; Manning, T.D.; Claridge, J.B.; Rosseinsky, M.J. Catalytic Response and Stability of Nickel/Alumina for the Hydrogenation of 5-Hydroxymethylfurfural in water. ChemSusChem 2016, 8, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, J.; Hayashi, Y.; Ueda, K.; Yamamoto, Y.; Arai, S.; Satsuma, A. Effect of FeOx modification of Al2O3 on its supported Au catalyst for hydrogenation of 5-hydroxymethylfurfural. J. Phys. Chem. C 2016, 120, 15129–15136. [Google Scholar] [CrossRef]
- Roman-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J.A. Production of dimethylfuran for liquid fuels from biomass-derived carbonhydrates. Nature 2007, 447, 982–985. [Google Scholar] [CrossRef]
- Thananatthanachon, T.; Rauchfuss, T.B. Efficient Production of the liquid fuel 2,5-Dimethylfuran from fructose using formic Acid as a reagent. Angew. Chem. Int. Ed. 2010, 49, 6616–6618. [Google Scholar] [CrossRef]
- Nishimura, S.; Ikeda, N.; Ebitani, K. Selective hydrogenation of biomass-derived 5-hydroxymethylfurfural (HMF) to 2,5-dimethylfuran (DMF) under atomospheric hydrogen pressure over carbon supported PdAu bimetallic catalyst. Catal. Today 2014, 232, 89–98. [Google Scholar] [CrossRef]
- Chandra, D.; Inoue, Y.; Sasase, M.; Kitano, M.; Bhaumik, A.; Kamata, K.; Hosono, H.; Hara, M. A high performance catalyst of shape-specific ruthenium nanoparticles for production pf primary amines by reductive amination of carbonyl compounds. Chem. Sci. 2018, 9, 5949–5956. [Google Scholar] [CrossRef]
- Tuteja, J.; Choudhary, H.; Nishimura, S.; Ebitani, K. Direct synthesis of 1,6-hexanediol from HMF over a heterogeneous Pd/ZrP catalyst using formic acid as hydrogen source. ChemSusChem 2014, 7, 96–100. [Google Scholar] [CrossRef]
- Weingarten, R.; Conner, W.C., Jr.; Huber, G.W. Production of levulinic acid from cellulose by hydrothermal decomposition combined with aqueous phase dehydration with a solid acid catalyst. Energy Environ. Sci. 2012, 5, 7559–7574. [Google Scholar] [CrossRef]
- Son, P.A.; Nishimura, S.; Ebitani, K. Synthesis of levulinic acid from fructose using Amberlyst-15 as a solid acid catalyst. React. Kinet. Mech. Catal. 2012, 106, 185–192. [Google Scholar] [CrossRef]
- Pupovac, K.; Palkovits, R. Cu/MgAl2O4 as Bifunctional Catalyst for Aldol Condensation of 5-Hydroxymethylfurfural and selective Transfer Hydrogenation. ChemSusChem 2013, 6, 2103–2110. [Google Scholar] [CrossRef] [PubMed]
- Buntara, T.; Noel, S.; Phua, P.H.; Melian-Cabrera, I.; de Vries, J.G. Caprolactam from Renewable Resources: Catalytic Conversion of 5-Hydroxymethylfurfural into Caprolactone. Angew. Chem. Int. Ed. 2011, 50, 7083–7087. [Google Scholar] [CrossRef]
- Wozniak, B.; Spannenberg, A.; Li, Y.; Hinze, S.; de Vries, J.G. Cyclopentanone Derivatives from 5-Hydroxymethylfurfural via 1-Hydroxyhexane-2,5-dione as Intermediate. ChemSusChem 2018, 11, 356–359. [Google Scholar] [CrossRef]
- Gomes, R.F.A.; Coelho, J.A.S.; Afonso, C.A.M. Direct Conversion of Activated 5-Hydroxymethylfurfural into d-Lactone-fused Cyclopentenones. ChemSusChem 2019, 12, 420–425. [Google Scholar] [CrossRef]
- Gupta, N.K.; Fukuoka, A.; Nakajima, K. Metal-Free and Selective Oxidation of furfural to Furoic Acid with an N-heterocyclic Carbone Catalyst. ACS Sustain. Chem. Eng. 2018, 6, 3434–3442. [Google Scholar] [CrossRef]
- Douthwaite, M.; Huang, X.; Iqbal, S.; Miedziak, P.J.; Brett, G.L.; Kondrat, S.A.; Edwards, J.K.; Sankar, M.; Knight, D.W.; Bethell, D.; et al. The controlled catalytic oxidation of furfural to furoic acid using AuPd/Mg(OH)2. Catal. Sci. Technol. 2017, 7, 5284–5293. [Google Scholar] [CrossRef]
- Lopez-Asensio, R.; Cecilia, J.A.; Jimenez-Gomez, C.P.; Garcia-Sancho, C.; Moreno-Rost, R.; Maireles-Torres, P. Selective production of furfuryl alcohol from furfural by catalytic transfer hydrogenation over commercial aluminas. Appl. Catal. A Gen. 2018, 556, 1–9. [Google Scholar] [CrossRef]
- Nishimura, S.; Shimura, T.; Ebitani, K. Transfer hydrogenation of furaldehydes with sodium phosphinate as a hydrogen source using Pd-supported alumina catalyst. J. Taiwan Inst. Chem. Eng. 2017, 79, 97–102. [Google Scholar] [CrossRef]
- Gandarias, I.; Garcia-Fernandez, S.; Obregon, I.; Agirrezabal-Telleria, I.; Arias, P.L. Production of 2-methylfuran from biomass through an integrated biorefinery approach. Fuel Proc. Technol. 2018, 178, 336–343. [Google Scholar] [CrossRef]
- Sitthisa, S.; An, W.; Resasco, D.E. Selective conversion of furfural to methylfuran over silica-supported Ni-Fe bimetallic catalysts. J. Catal. 2011, 284, 90–101. [Google Scholar] [CrossRef]
- Komanoya, T.; Kinemura, T.; Kita, K.; Kamata, K.; Hara, M. Electronic effect of ruthenium nanoparticles on efficient reductive amination of carbonyl compounds. J. Am. Chem. Soc. 2017, 139, 11493–11499. [Google Scholar] [CrossRef]
- Nishimura, S.; Mizuhori, K.; Ebitani, K. Reductive amination of furfural toward furfurylamine with aqueous ammonia under hydrogen over Ru-supported catalyst. Res. Chem. Intermed. 2016, 42, 19–30. [Google Scholar] [CrossRef]
- Liu, S.; Amada, Y.; Tamura, M.; Nakagawa, Y.; Tomishige, K. Performance and characterization of rhenium-modified Rh-Ir alloy catalyst for one-pot conversion of furfural into 1,5-oentandiol. Catal. Sci. Technol. 2014, 4, 2535–3549. [Google Scholar] [CrossRef]
- Lee, J.; Burt, S.P.; Carrero, C.A.; Alba-Rubio, A.C.; Ro, I.; O’Neil, B.J.; Kim, H.J.; Jackson, D.H.K.; Kuech, T.F.; Hermans, I.; et al. Stabilizating cobalt catalyst for aqueous-phase reactions by strong metal-support interaction. J. Catal. 2015, 330, 19–27. [Google Scholar] [CrossRef]
- Choudhary, H.; Nishimura, S.; Ebitani, K. Highly efficient aqueous oxidation of furfural to succinic acid using reusable heterogeneous acid catalyst with hydrogen peroxide. Chem. Lett. 2012, 41, 409–411. [Google Scholar] [CrossRef]
- Yang, J.; Li, N.; Li, G.; Wang, W.; Wang, A.; Wang, X.; Cong, Y.; Zhang, T. Solvent-Free Synthesis of C10 and C11 Branched Alkanes from Furfural and Methyl Isobutyl Ketone. ChemSusChem 2013, 6, 1149–1152. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xia, Q.; Zhang, Y.; Guo, Y.; Wang, Y.; Lu, G. Effective Production of Octane from Biomass Derivatives under Mild Conditions. ChemSusChem 2011, 4, 1758–1761. [Google Scholar] [CrossRef]
- Shirotori, M.; Nishimura, S.; Ebitani, K. One-Pot Synthesis of Furfural Derivatives from Pentoses using Solid Acid and Base Catalysts. Catal. Sci. Technol. 2014, 4, 971–978. [Google Scholar] [CrossRef]
- Nunes, J.P.M.; Afonso, C.A.M.; Caddick, S. Synthesis of 2,4-bifunctionalized cyclopentenones from 2-furaldehyde. RSC Adv. 2013, 3, 14975–14978. [Google Scholar] [CrossRef]
- Nardi, M.; Costanzo, P.; Nino, A.D.; Gioia, M.L.D.; Olivito, F.; Sindona, G.; Procopio, A. Water excellent solvent for the synthesis of bifunctionalized cyclopentenones from furfuram. Green Chem. 2017, 19, 5403–5411. [Google Scholar] [CrossRef]
- Gioia, M.L.D.; Nardi, M.; Costanzo, P.; Nino, A.D.; Maiuolo, L.; Oliverio, M.; Procopio, A. Biorenewable Deep Eutectic Solvent for Selective and Scalable Conversion of Furfural into Cyclopenenone Derivatives. Molecules 2018, 23, 1891. [Google Scholar] [CrossRef]
- Koh, P.F.; Wang, P.; Huang, J.M.; Loh, T.P. Biomass derived furfural-based facile synthesis of protected (2S)-phenyl-3-piperidone, a common intermediate for many drugs. Chem. Commun. 2014, 50, 8324–8327. [Google Scholar] [CrossRef]
- Nishimura, S.; Shibata, A.; Ebitani, K. Direct Hydroxymethylation of Furaldehydes with Aqueous Formaldehyde over a Reusable Sulfuric Functionalized Resin Catalyst. ACS Omega 2018, 3, 5988–5993. [Google Scholar] [CrossRef]
- Lecomte, J.; Finiels, A.; Geneste, P.; Moreau, C. Kinetics of furfuryl alcohol hydroxymethylation with aqueous formaldehyde over a highly dealuminated H-mordenite. J. Mol. Catal. A Chem. 1998, 133, 283–288. [Google Scholar] [CrossRef]
- Lecomte, J.; Finiels, A.; Geneste, P.; Moreau, C. Selective hydroxymethylation of furfuryl alcohol with aqueous formaldehyde in the presence of dealuminated mordenites. Appl. Catal. A Gen. 1998, 168, 235–241. [Google Scholar] [CrossRef]
- Lecomte, J.; Finiels, A.; Moreau, C. A new selective route to 5-hydroxymethylfurfural from furfural and furfural derivatives over microporous solid acidic catalysts. Ind. Crops Prod. 1999, 19, 235–241. [Google Scholar] [CrossRef]
- Lecomte, J.; Finiels, A.; Geneste, P.; Moreau, C. Attempt to quantify the hydrophobic character of highly dealuminated H-mordenites in hydroxymethylation of furfuryl alcohol with aqueous formaldehyde. J. Mol. Catal. A Chem. 1999, 140, 157–163. [Google Scholar] [CrossRef]
- Everett, D.H.; Powl, J.C. Adsorption in slit-like and cylindrical micropores in the henry’s law region. A model for the microporosity of carbons. J. Chem. Soc. Faraday Trans. 1976, 72, 619–636. [Google Scholar] [CrossRef]
- Ono, Y.; Hattori, H. Solid Base Catalysis. In Springer Series in Chemical Physics 101; Springer: Berlin/Heidelberg, Germany; Tokyo Institute of Technolgy Press: Tokyo, Japan, 2011. [Google Scholar] [Green Version]
- Imelik, B.; Naccache, C.; Taarit, Y.B.; Vedrine, J.C.; Coudurier, G.; Praliaud, H. Catalysis by Zeolites. In Studies in Surface Science and Catalysus 5; Elsevier: Amsterdam, The Netherlands, 1980. [Google Scholar]
- He, Y.; Hoff, T.C.; Emaadi, L.; Wu, Y.; Bouraima, J.; Liu, D. Catalytic consequences of micropore topology, mesoporosity, and acidity on the hydrolysis of sucrose over zeolite catalysts. Catal. Sci. Technol. 2014, 4, 3064–3073. [Google Scholar] [CrossRef]
- Li, H.; Yang, S.; Riisager, A.; Pandey, A.; Sangwan, R.S.; Saravanamurugan, S.; Luque, R. Zeolite and zeotype-catalysed transformations of biofuranic compounds. Green Chem. 2016, 18, 5701–5735. [Google Scholar] [CrossRef]
- Otomo, R.; Yokoi, T.; Kondo, J.N.; Tatsumi, T. Dealuminated Beta zeolite as effective bifunctional catalyst for direct transformation of glucose to 5-hydroxymethylfurfural. Appl. Catal. A Gen. 2014, 470, 318–326. [Google Scholar] [CrossRef]
- Otomo, R.; Tatusmi, T.; Yokoi, T. Beta zeolite: A universally applicable catalyst for the conversion of various type of sacchrides into furfural. Catal. Sci. Technol. 2015, 5, 4001–4007. [Google Scholar] [CrossRef]
- Gebresillase, M.N.; Shavi, R.; Seo, J.G. A comprehensive investigation of the condensation of furanic platform molecules to C14–C15 fuel precursors over sulfonic acid functionalized silica supports. Green Chem. 2018, 20, 5133–5146. [Google Scholar] [CrossRef]
- Arias, K.S.; Al-Resayes, S.I.; Climent, M.J.; Corma, A.; Iborra, S. From Biomass to Chemicals: Synthesis of precusors of Biodegradable Surfactants from 5-Hydroxymethylfurfural. ChemSusChem 2013, 6, 123–131. [Google Scholar] [CrossRef]
- Galkin, K.I.; Krivodaeva, E.A.; Romashov, L.V.; Zalesskiy, S.S.; Kachala, V.V.; Burykina, J.V.; Ananikov, V.P. Critical Infulence of 5-Hydroxymethylfurfural Aging and Decomposition on the Utility of Biomass Conversion in Organic Synthesis. Angew. Chem. Int. Ed. 2016, 55, 8338–8342. [Google Scholar] [CrossRef]
- da Silva, C.X.A.; Goncalves, V.L.C.; Mota, C.J.A. Water-tolerant zeolite catalyst for the acetalisation of glycerol. Green Chem. 2009, 11, 38–41. [Google Scholar] [CrossRef]
- Zapata, P.A.; Faria, J.; Ruiz, M.P.; Jentoft, R.E.; Resasco, D.E. Hydrophobic Zeolites for Biofuel Upgrading Reactions at the Liquid-Liquid Interface in Water/Oil Emulsions. J. Am. Chem. Soc. 2012, 134, 8570–8578. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, Y.; Aoyama, J.; Miyakubo, K.; Eguchi, T.; Kamegawa, T.; Mori, K.; Yamashita, H. TiO2 photocatalyst for degradation of organic compounds in water and air supported on highly hydrophobic FAU zeolite: Structural, sorptive, and photocatalytic studies. J. Catal. 2012, 285, 223–234. [Google Scholar] [CrossRef]
- Chen, N.Y. Hydrophobic Properties of Zeolite. J. Phys. Chem. 1976, 80, 60–64. [Google Scholar] [CrossRef]






| Entry | Catalyst a | SBET b (m2 g−1) | Average Pore Size b (nm) | Acid Amount c (mmol g−1) |
|---|---|---|---|---|
| 1 | Beta (25) | 494 | 0.69 | 0.37 |
| 2 | Beta (42.2) | 639 | 0.68 | 0.42 |
| 3 | Beta (104) | 613 | 0.71 | 0.24 |
| 4 | Beta (150) | 607 | 0.73 | 0.20 |
| 5 | Beta (440) | 571 | 0.71 | 0.076 |
| 6 | Beta (1700) | 565 | 0.69 | 0.038 |
| 7 | ZSM-5 (90) | 421 | 0.60 | 0.28 |
| 8 | Mordenite (18.3) | 479 | 0.57 | >0.65 |
| 9 | Y (5.6) | 651 | 0.72 | 0.38 |
| 10 | SiO2-Al2O3 (2.1) | 305 | N/A | 0.42 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, S.; Shibata, A. Hydroxymethylation of Furfural to HMF with Aqueous Formaldehyde over Zeolite Beta Catalyst. Catalysts 2019, 9, 314. https://doi.org/10.3390/catal9040314
Nishimura S, Shibata A. Hydroxymethylation of Furfural to HMF with Aqueous Formaldehyde over Zeolite Beta Catalyst. Catalysts. 2019; 9(4):314. https://doi.org/10.3390/catal9040314
Chicago/Turabian StyleNishimura, Shun, and Atsuki Shibata. 2019. "Hydroxymethylation of Furfural to HMF with Aqueous Formaldehyde over Zeolite Beta Catalyst" Catalysts 9, no. 4: 314. https://doi.org/10.3390/catal9040314