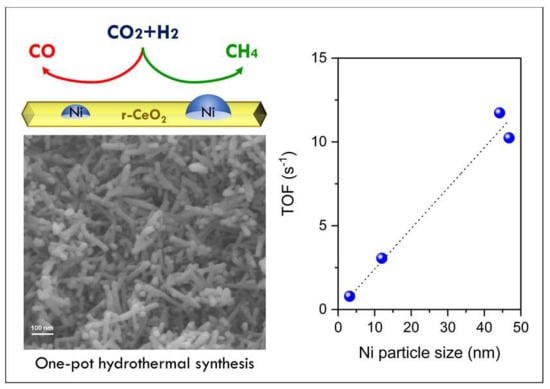
Structure-Sensitivity of CO2 Methanation over Nanostructured Ni Supported on CeO2 Nanorods
Abstract
:1. Introduction
2. Results
2.1. Catalysts’ Synthesis
2.2. X-ray Diffraction (XRD) Characterization of Calcined Samples
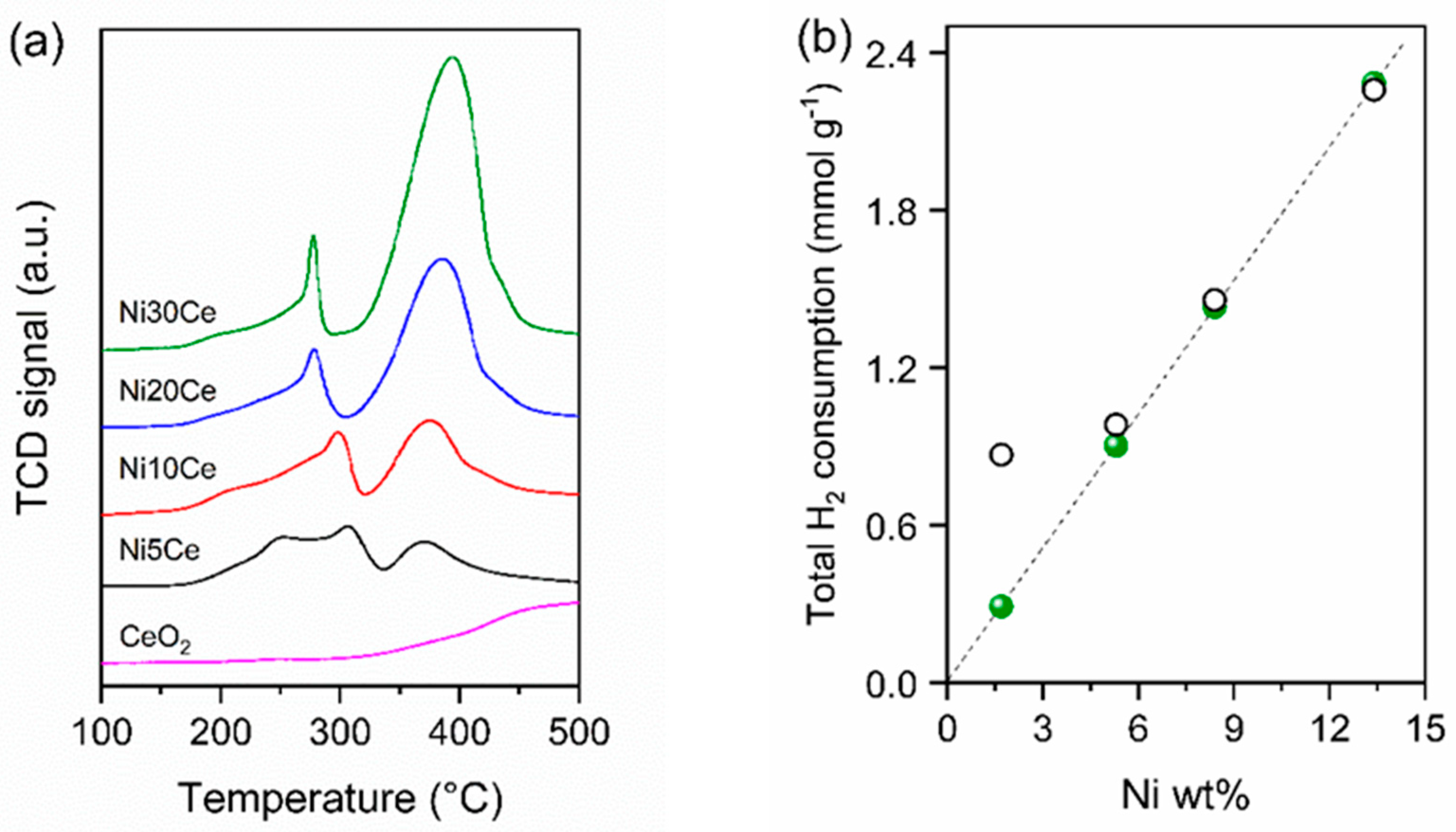
2.3. Temperature-Programmed Reduction (TPR) and H2 Chemisorption
2.4. Characterization of Reduced Samples
2.4.1. XRD Characterization
2.4.2. Brunauer–Emmet–Teller (BET) Characterization
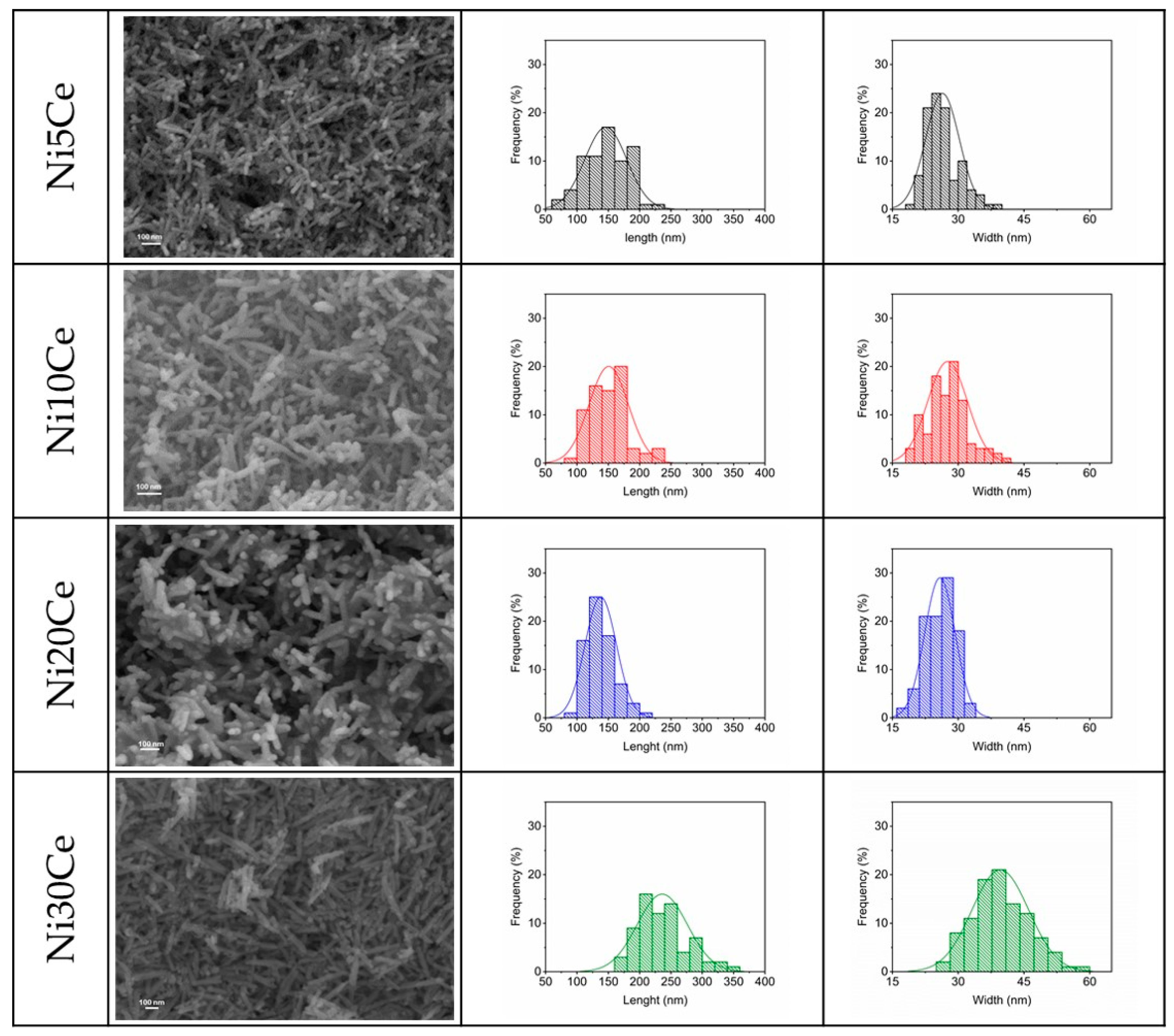
2.4.3. Field-Emission Scanning Electron Microscopy (FE-SEM) Characterization
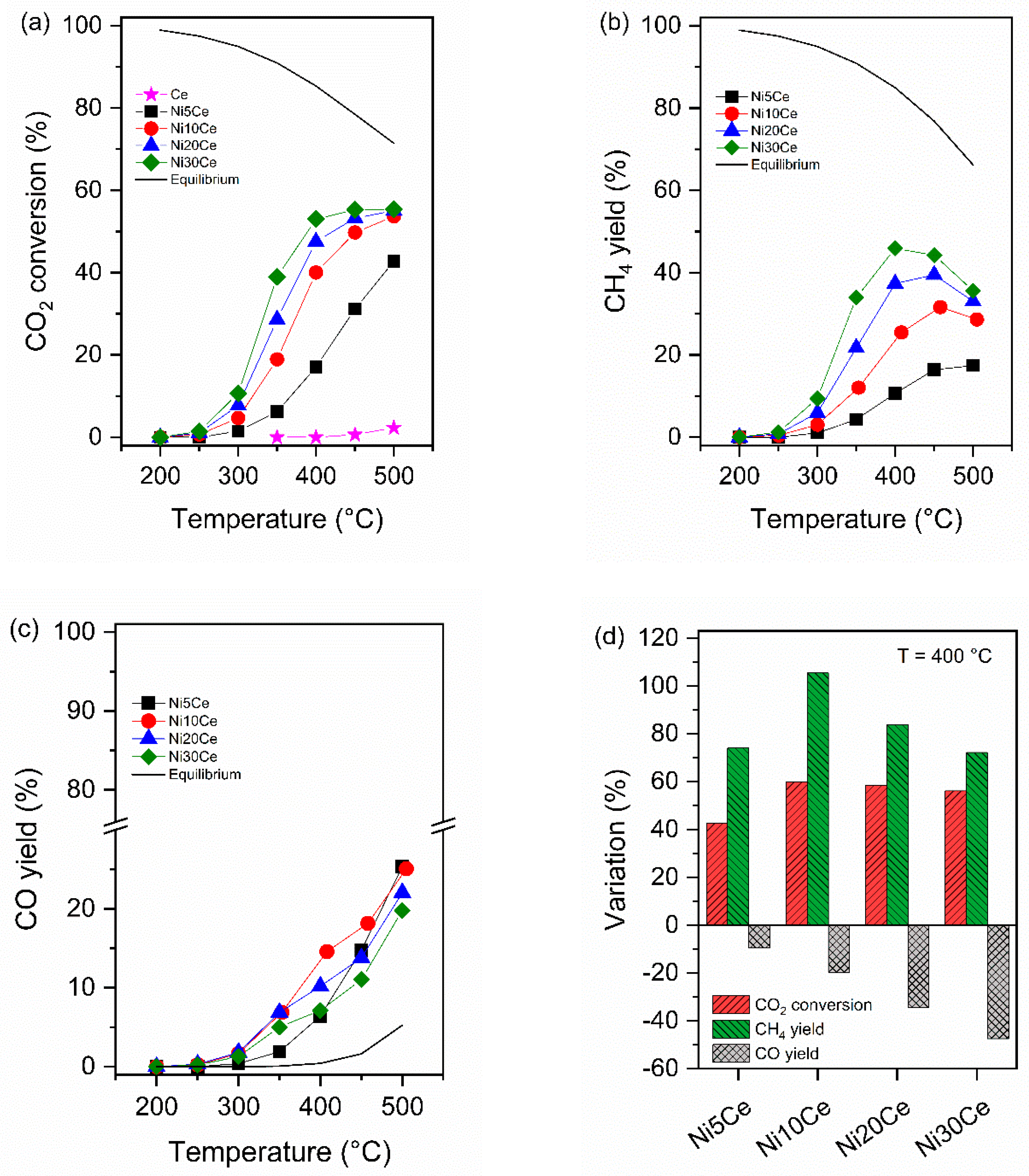
2.5. Catalytic Activity
3. Discussion
- (1)
- The inflection point observed in the CO yield, occurs at lower temperature by increasing the Ni loading. That behavior may be explained assuming that CO is produced by a different reaction mechanism on Ni-active centers with different particle size. In agreement with the literature, we assume that on small Ni0 particles, the low H2 coverage, may favor the CO formation; whereas, on large Ni0 particles, due to the high H2 surface coverage, CO2 may be directly hydrogenated to CH4 therefore the rate of CH4 production is higher than that of CO;
- (2)
- The turnover frequency for CO2 conversion increases linearly with Ni particles size. Such behavior may be explained by the dependence of CO/CH4 production rate on the metal particle size.
4. Materials and Methods
4.1. Catalysts’ Synthesis
4.2. Catalysts’ Characterization
- f = geometrical shape factor, 6 for a spherical particle
- WNi = Ni metal weight percentage in the sample
- ρNi = density of Ni: 8.9 g/cm3
- ηH2 = H2 desorbed in the H2-TPD experiment: mol/g of sample
- NA = 6.023 × 1023
- Sf = stoichiometric factor for H2 chemisorption: metal mol/gas mol = 2
- ANi = area occupied by Ni surface atom: 6.51 × 10−16 cm2
- MNi = Ni atomic mass: 58.69 g/mol
4.3. Catalytic Activity Tests
- FCO2 = molar flow of CO2: mol/s
- XCO2 = conversion of CO2: %
- m = catalyst mass: g
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Vito, C.; Ferrini, V.; Mignardi, S.; Cagnetti, M.; Leccese, F. Progress in carbon dioxide sequestration via carbonation of aqueous saline wastes. Periodico di Mineralogia 2012, 81, 333–344. [Google Scholar] [CrossRef]
- Tuti, S.; Luisetto, I.; Leccese, F.; Kesavan, J.K.; Casciardi, S. Nickel supported on Y2O3-ZrO2 as highly selective and stable CO2 methanation catalyst for in-situ propellant production on Mars. In Proceedings of the 2018 5th IEEE International Workshop on Metrology for AeroSpace (MetroAeroSpace), Rome, Italy, 20–22 June 2018; pp. 435–439. [Google Scholar] [CrossRef]
- Della Pietra, M.; Santarelli, M.; Stendardo, S.; McPhail, S.; Perez-Trujillo, J.P.; Elizalde-Blancas, F. Integration of a calcium looping process (CaL) to molten carbonate fuel cells (MCFCs), as carbon concentration system: First findings. J. CO2 Util. 2018, 25, 14–21. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef] [PubMed]
- Kesavan, J.K.; Luisetto, I.; Tuti, S.; Meneghini, C.; Battocchio, C.; Iucci, G. Ni supported on YSZ: XAS and XPS characterization and catalytic activity for CO2 methanation. J. Mater. Sci. 2017, 52, 10331–10340. [Google Scholar] [CrossRef]
- Kesavan, J.K.; Luisetto, I.; Tuti, S.; Meneghini, C.; Iucci, G.; Battocchio, C.; Mobilio, S.; Casciardi, S.; Sisto, R. Nickel supported on YSZ: The effect of Ni particle size on the catalytic activity for CO2 methanation. J. CO2 Util. 2018, 23, 200–211. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Romano, C.; Boaro, M.; Di Bartolomeo, E. Dry reforming of methane over Ni supported on doped CeO2: New insight on the role of dopants for CO2 activation. J. CO2 Util. 2019, 30, 63–78. [Google Scholar] [CrossRef]
- Luisetto, I.; Sarno, C.; De Felicis, D.; Basoli, F.; Battocchio, C.; Tuti, S.; Licoccia, S.; Di Bartolomeo, E. Ni supported on γ-Al2O3 promoted by Ru for the dry reforming of methane in packed and monolithic reactors. Fuel Process. Technol. 2017, 158, 130–140. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Battocchio, C.; Lo Mastro, S.; Sodo, A. Ni/CeO2-Al2O3 catalysts for the dry reforming of methane: The effect of CeAlO3 content and nickel crystallite size on catalytic activity and coke resistance. Appl. Catal. A Gen. 2015, 500, 12–22. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Di Bartolomeo, E. Co and Ni supported on CeO2 as selective bimetallic catalyst for dry reforming of methane. Int. J. Hydrog. Energy 2012, 37, 15992–15999. [Google Scholar] [CrossRef]
- Meylan, F.D.; Moreau, V.; Erkman, S. Material constraints related to storage of future European renewable electricity surpluses with CO2 methanation. Energy Policy 2016, 94, 366–376. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported Catalysts for CO2 Methanation: A Review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Ahmad, A. CO2 methanation over heterogeneous catalysts: Recent progress and future prospects. Green Chem. 2015, 17, 2647–2663. [Google Scholar] [CrossRef]
- Younas, M.; Loong Kong, L.; Bashir, M.J.K.; Nadeem, H.; Shehzad, A.; Sethupathi, S. Recent Advancements, Fundamental Challenges, and Opportunities in Catalytic Methanation of CO2. Energy Fuels 2016, 30, 8815–8831. [Google Scholar] [CrossRef]
- Ocampo, F.; Louis, B.; Roger, A.-C. Methanation of carbon dioxide over nickel-based Ce0.72Zr0.28O2 mixed oxide catalysts prepared by sol–gel method. Appl. Catal. A 2009, 369, 90–96. [Google Scholar] [CrossRef]
- Iglesias, I.; Quindimil, A.; Mariño, F.; De-La-Torre, U.; González-Velasco, J.R. Zr promotion effect in CO2 methanation over ceria supported nickel catalysts. Int. J. Hydrog. Energy 2019, 44, 1710–1719. [Google Scholar] [CrossRef]
- Lei, Y.; Li, W.; Liu, Q.; Lin, Q.; Zheng, X.; Huang, Q.; Guan, S.; Wang, X.; Wang, C.; Li, F. Typical crystal face effects of different morphology ceria on the activity of Pd/CeO2 catalysts for lean methane combustion. Fuel 2018, 233, 10–20. [Google Scholar] [CrossRef]
- Du, X.; Zhang, D.; Shi, L.; Gao, R.; Zhang, J. Morphology Dependence of Catalytic Properties of Ni/CeO2 Nanostructures for Carbon Dioxide Reforming of Methane. J. Chem. Chem. C 2012, 116, 10009–10016. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, L.; Wang, W.; Zhao, Y.; Zhang, G.; Ma, X.; Gong, J. Morphology control of ceria nanocrystals for catalytic conversion of CO2 with methanol. Nanoscale 2013, 5, 5582–5588. [Google Scholar] [CrossRef]
- Kwak, J.H.; Kovarik, L.; Szanyi, J. CO2 Reduction on Supported Ru/Al2O3 Catalysts: Cluster Size Dependence of Product Selectivity. ACS Catal. 2013, 3, 2449–2455. [Google Scholar] [CrossRef]
- Kwak, J.H.; Kovarik, L.; Szanyi, J. Heterogeneous Catalysis on Atomically Dispersed Supported Metals: CO2 Reduction on Multifunctional Pd Catalysts. ACS Catal. 2013, 3, 2094–2100. [Google Scholar] [CrossRef]
- Wu, H.C.; Chang, Y.C.; Wu, J.H.; Lin, J.H.; Lin, I.K.; Chen, C.S. Methanation of CO2 and reverse water gas shift reactions on Ni/SiO2 catalysts: The influence of particle size on selectivity and reaction pathway. Catal. Sci. Technol. 2015, 5, 4154–4163. [Google Scholar] [CrossRef]
- Beierlein, D.; Häussermann, D.; Pfeifer, M.; Schwarz, T.; Stöwe, K.; Traa, Y.; Klemm, E. Is the CO2 methanation on highly loaded Ni-Al2O3 catalysts really structure-sensitive? Appl. Catal. B Environ. 2019, 247, 200–219. [Google Scholar] [CrossRef]
- Takeguchi, T.; Furukawa, S.-n.; Inoue, M. Hydrogen Spillover from NiO to the Large Surface Area CeO2–ZrO2 Solid Solutions and Activity of the NiO/CeO2–ZrO2 Catalysts for Partial Oxidation of Methane. J. Catal. 2001, 202, 14–24. [Google Scholar] [CrossRef]
- Jalowiecki-Duhamel, L.; Zarrou, H.; D’Huysser, A. Hydrogen production at low temperature from methane on cerium and nickel based mixed oxides. Int. J. Hydrog. Energy 2008, 33, 5527–5534. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, B.; Tu, B.; Dong, Y.; Cheng, M. Redox cycling of Ni–YSZ anode investigated by TPR technique. Solid State Ion. 2005, 176, 2193–2199. [Google Scholar] [CrossRef]
- Perrichon, V.; Laachir, A.; Bergeret, G.; Fréty, R.; Tournayan, L.; Touret, O. Reduction of cerias with different textures by hydrogen and their reoxidation by oxygen. J. Chem. Soc. Faraday Trans. 1994, 90, 773–781. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas Solid Systems with Special Reference to the Determination of Surface–Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Ping, Y.; Hu, D.; Xu, G.; Gu, F.; Su, F. A thermodynamic analysis of methanation reactions of carbon oxides for the production of synthetic natural gas. RSC Adv. 2012, 2, 2358. [Google Scholar] [CrossRef]
- Yabe, T.; Sekine, Y. Methane conversion using carbon dioxide as an oxidizing agent: A review. Fuel Process. Technol. 2018, 181, 187–198. [Google Scholar] [CrossRef]

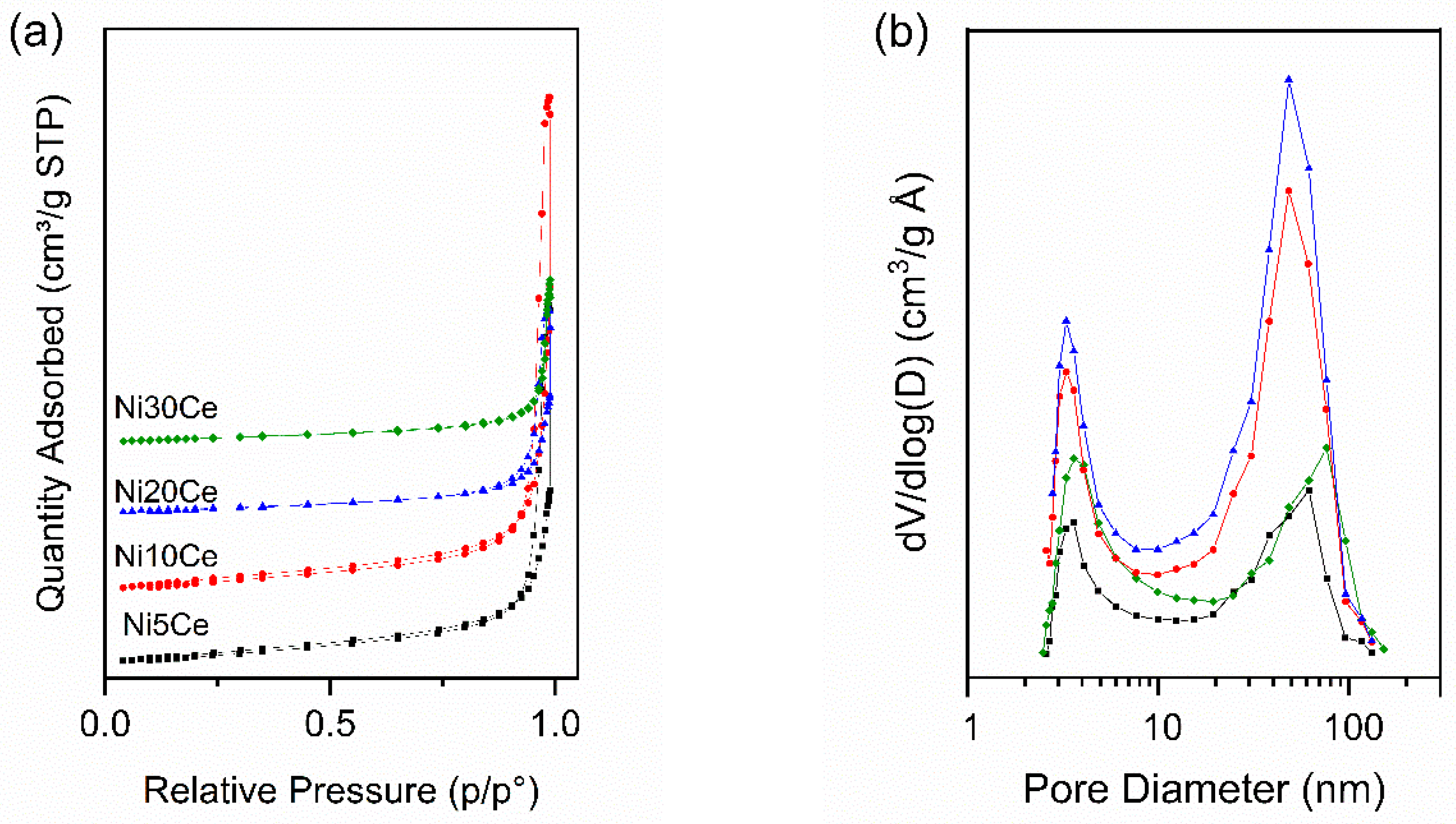

| Chemical CompositionEDAX (wt%) | S.A. (m2g−1) | S.A.norm (m2g−1) | Pore Volume (cm3g−1) | Pore Size (nm) | dCeO2XRD (nm) | dNiOXRD (nm) | dNiXRD1 (nm) | ||
|---|---|---|---|---|---|---|---|---|---|
| Ni | Ce | ||||||||
| Ni5Ce | 1.69 | 84.31 | 73 | 74 | 0.49 | 32 | 11.9 | 13.6 | 14.9 |
| Ni10Ce | 5.38 | 83.12 | 65 | 69 | 0.68 | 39 | 12.8 | 21.0 | 19.0 |
| Ni20Ce | 8.46 | 81.64 | 34 | 37 | 0.28 | 32 | 20.2 | 19.7 | 21.0 |
| Ni30Ce | 13.38 | 73.90 | 28 | 32 | 0.21 | 30 | 18.1 | 18.1 | 27.3 |
| Sample | Ni mmol/g | Ce mmol/g | Total H2 Consumption 1 mmol/g | H2/Ni 2 mmol/mmol | H2 Consumption for Ce4+ Reduction 3 (mmol g−1) | Ce3+ 4 (%) | dNiTPD (nm) | DNi (%) |
|---|---|---|---|---|---|---|---|---|
| Ni5Ce | 0.29 | 5.71 | 0.87 | 3.00 | 0.58 | 20 | 3.2 | 32.0 |
| Ni10Ce | 0.92 | 5.54 | 0.98 | 1.06 | 0.08 | 2.9 | 12.0 | 8.5 |
| Ni20Ce | 1.44 | 5.24 | 1.46 | 1.02 | 0.02 | 1.0 | 44.2 | 2.3 |
| Ni30Ce | 2.28 | 4.90 | 2.26 | 1.00 | 0.0 | 0.0 | 46.8 | 2.2 |
| Length (nm) | Width (nm) | Aspect Ratio | |
|---|---|---|---|
| Ni5Ce | 145.9 ± 33.6 | 26.4 ± 3.9 | 5.5 ± 2.1 |
| Ni10Ce | 150.5 ± 31.4 | 27.6 ± 4.6 | 5.4 ± 2.1 |
| Ni20Ce | 137.8 ± 25.0 | 25.8 ± 3.5 | 5.3 ± 1.7 |
| Ni30Ce | 236.1 ± 40.2 | 39.6 ± 6.5 | 6.0 ± 1.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marconi, E.; Tuti, S.; Luisetto, I. Structure-Sensitivity of CO2 Methanation over Nanostructured Ni Supported on CeO2 Nanorods. Catalysts 2019, 9, 375. https://doi.org/10.3390/catal9040375
Marconi E, Tuti S, Luisetto I. Structure-Sensitivity of CO2 Methanation over Nanostructured Ni Supported on CeO2 Nanorods. Catalysts. 2019; 9(4):375. https://doi.org/10.3390/catal9040375
Chicago/Turabian StyleMarconi, Eleonora, Simonetta Tuti, and Igor Luisetto. 2019. "Structure-Sensitivity of CO2 Methanation over Nanostructured Ni Supported on CeO2 Nanorods" Catalysts 9, no. 4: 375. https://doi.org/10.3390/catal9040375