CO and CO2 Co-Methanation on Ni/CeO2-ZrO2 Soft-Templated Catalysts
Abstract
:1. Introduction
2. Results
2.1. Characterization of Fresh NiO-CeO2-ZrO2 Mixed Oxides
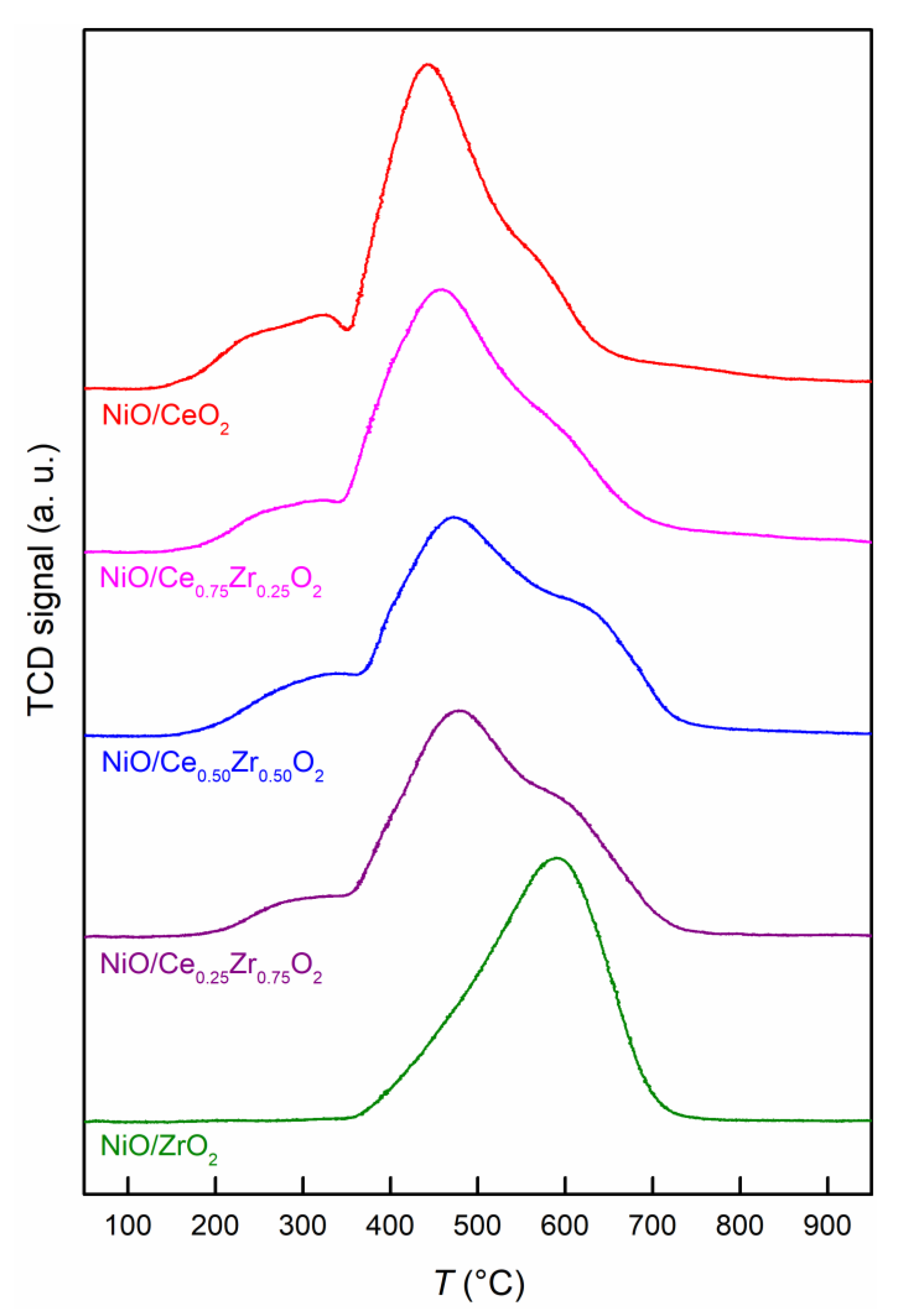
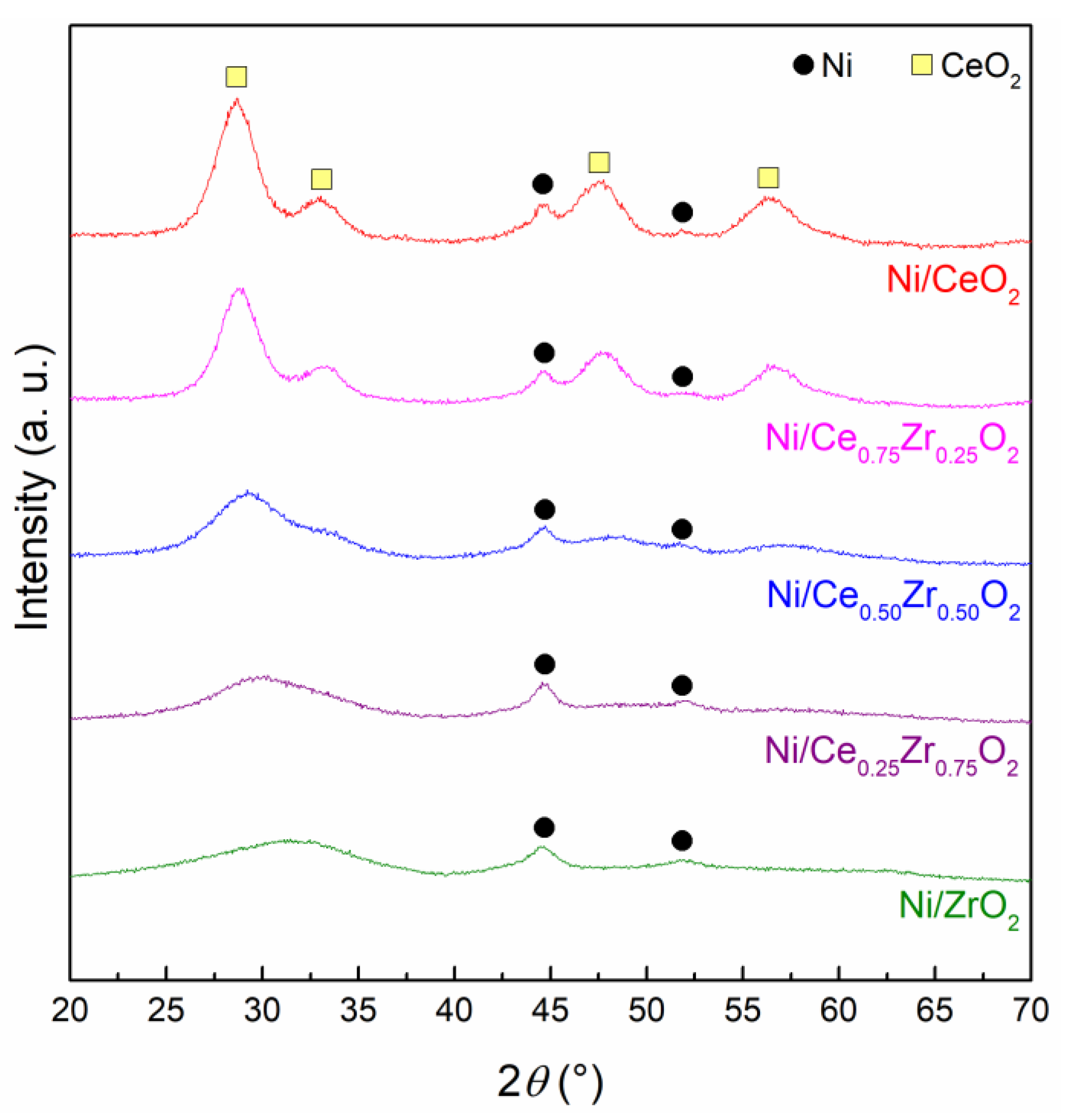
2.2. Reducibility of NiO-CeO2-ZrO2 Mixed Oxides And Characterization of the Reduced Samples
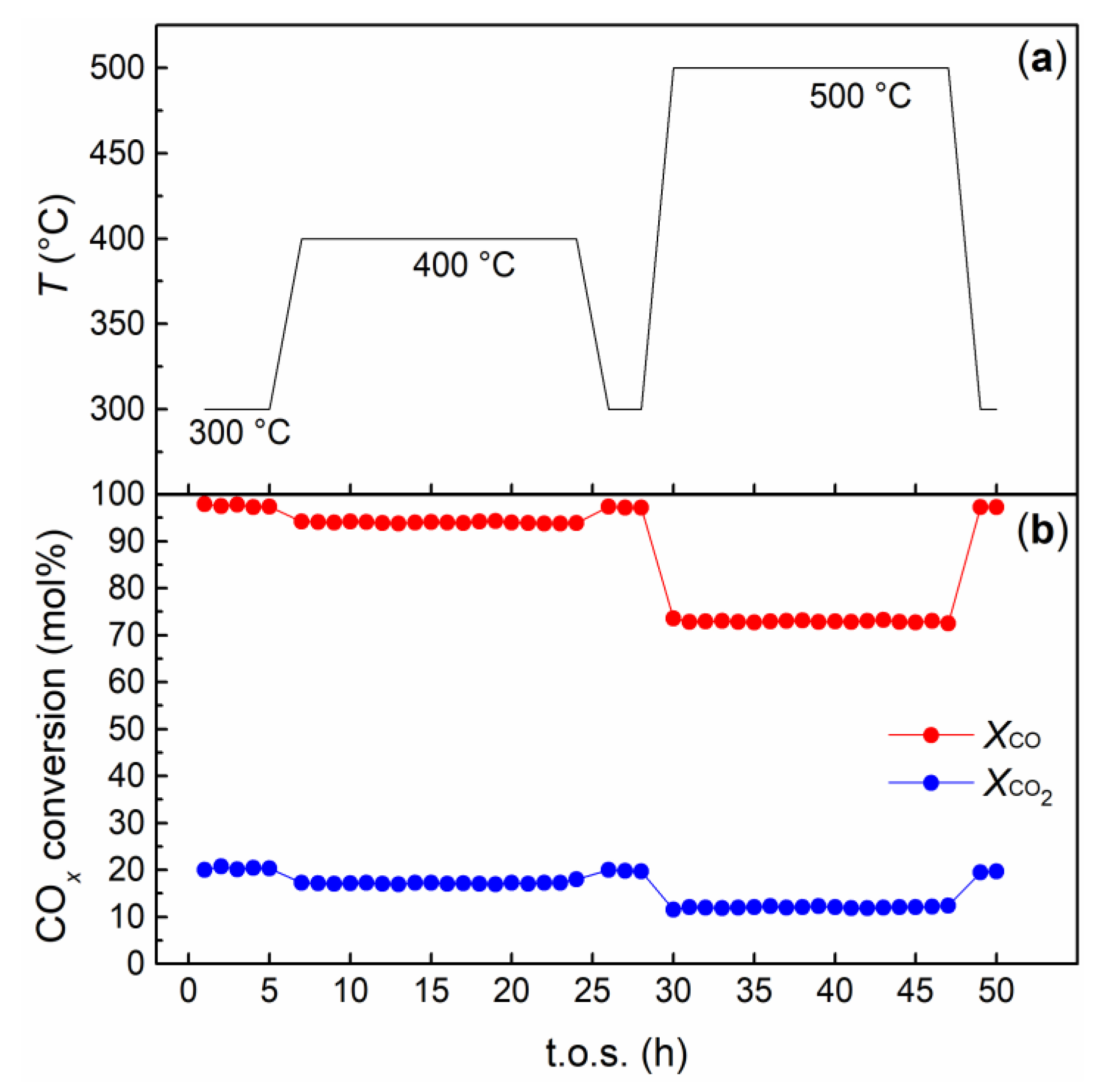
2.3. CO and CO2 Co-Methanation Catalytic Tests
3. Discussion
4. Materials and Methods
4.1. Synthesis of Materials
4.2. Characterization
4.3. CO and CO2 Co-Methanation Catalytic Tests
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, J.; Wang, Y.; Ping, Y.; Hu, D.; Xu, G.; Gu, F.; Su, F. A thermodynamic analysis of methanation reactions of carbon oxides for the production of synthetic natural gas. RSC Adv. 2012, 2, 2358–2368. [Google Scholar] [CrossRef]
- Ishihara, T.; Eguchi, K.; Arai, H. Hydrogenation of Carbon Monoxide over SiO2-Supported Fe-Co, Co-Ni and Ni-Fe Bimetallic Catalysts. Appl. Catal. 1987, 30, 225–238. [Google Scholar] [CrossRef]
- Aksoylu, A.E.; Önsan, Z.İ. Interaction between Nickel and Molybdenum in Ni-Mo/Al2O3 catalysts: II. CO hydrogenation. Appl. Catal. A Gen. 1998, 168, 399–407. [Google Scholar] [CrossRef]
- Xavier, K.O.; Sreekala, R.; Rashid, K.; Yusuff, K.; Sen, B. Doping effects of cerium oxide on Ni/Al2O3 catalysts for methanation. Catal. Today 1999, 49, 17–21. [Google Scholar] [CrossRef]
- Znak, L.; Stołecki, K.; Zieliński, J. The effect of cerium; lanthanum and zirconium on nickel/alumina catalysts for the hydrogenation of carbon oxides. Catal. Today 2005, 101, 65–71. [Google Scholar] [CrossRef]
- Kustov, A.L.; Frey, A.M.; Larsen, K.E.; Johannessen, T.; Nørskov, J.K.; Christensen, C.H. CO methanation over supported bimetallic Ni-Fe catalysts: From computational studies towards catalyst optimization. Appl. Catal. A Gen. 2007, 320, 98–104. [Google Scholar] [CrossRef]
- Jimenez, V.; Sanchez, P.; Panagiotopoulou, P.; Valverde, J.L.; Romero, A. Methanation of CO, CO2 and selective methanation of CO, in mixtures of CO and CO2, over ruthenium carbon nanofibers catalysts. Appl. Catal. A Gen. 2010, 390, 35–44. [Google Scholar] [CrossRef]
- Senanayake, S.D.; Evans, J.; Agnoli, S.; Barrio, L.; Chen, T.-L.; Hrbek, J.; Rodriguez, J.A. Water-Gas Shift and CO Methanation Reactions over Ni-CeO2(111) Catalysts. Top Catal. 2011, 54, 34–41. [Google Scholar] [CrossRef]
- Hu, D.; Gao, J.; Ping, Y.; Jia, L.; Gunawan, P.; Zhong, Z.; Xu, G.; Gu, F.; Su, F. Enhanced Investigation of CO Methanation over Ni/Al2O3 Catalysts for Synthetic Natural Gas Production. Ind. Eng. Chem. Res. 2012, 51, 4875–4886. [Google Scholar] [CrossRef]
- Zhao, A.; Ying, W.; Zhang, H.; Ma, H.; Fang, D. Ni-Al2O3 catalysts prepared by solution combustion method for syngas methanation. Catal. Commun. 2012, 17, 34–38. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, J.; Hong, U.G.; Jung, J.C.; Koh, D.J.; Lim, H.; Byun, C.; Song, I.K. Hydrogenation of carbon monoxide to methane over mesoporous nickel-M-alumina (M = Fe, Ni, Co, Ce, and La) xerogel catalysts. J. Ind. Eng. Chem. 2012, 18, 243–248. [Google Scholar] [CrossRef]
- Yan, X.; Liu, Y.; Zhao, B.; Wang, Z.; Wang, Y.; Liu, C.-J. Methanation over Ni/SiO2: Effect of the catalyst preparation methodologies. Int. J. Hydrogen Energy 2013, 38, 2283–2291. [Google Scholar] [CrossRef]
- Zyryanova, M.M.; Snytnikov, P.V.; Gulyaev, R.V.; Amosov, Y.I.; Boronin, A.I.; Sobyanin, V.A. Performance of Ni/CeO2 catalysts for selective CO methanation in hydrogen-rich gas. Chem. Eng. J. 2014, 238, 189–197. [Google Scholar] [CrossRef]
- Shinde, V.M.; Madras, G. CO Methanation Toward the Production of Synthetic Natural Gas over Highly Active Ni/TiO2 Catalyst. AIChE J. 2014, 60, 1027–1035. [Google Scholar] [CrossRef]
- Meng, F.; Li, Z.; Liu, J.; Cui, X.; Zheng, H. Effect of promoter Ce on the structure and catalytic performance of Ni/Al2O3 catalyst for CO methanation in slurry-bed reactor. J. Nat. Gas Sci. Eng. 2015, 23, 250–258. [Google Scholar] [CrossRef]
- Zheng, Y.; Ma, H.; Zhang, H.; Ying, W.; Fang, D. Ni-Ce-Al composite oxide catalysts synthesized by solution combustion method: Enhanced catalytic activity for CO methanation. Fuel 2015, 162, 16–22. [Google Scholar] [CrossRef]
- Konishcheva, M.V.; Potemkin, D.I.; Snytnikov, P.V.; Zyryanova, M.M.; Pakharukova, V.P.; Simonov, P.A.; Sobyanin, V.A. Selective CO methanation in H2-rich stream over Ni-, Co- and Fe/CeO2: Effect of metal and precursor nature. Int. J. Hydrogen Energy 2015, 40, 14058–14063. [Google Scholar] [CrossRef]
- Nematollahi, B.; Rezaei, M.; Nemati Lay, E. Preparation of highly active and stable NiO-CeO2 nanocatalysts for CO selective methanation. Int. J. Hydrogen Energy 2015, 40, 8539–8547. [Google Scholar] [CrossRef]
- Nematollahi, B.; Rezaei, M.; Nemati Lay, E. Selective methanation of carbon monoxide in hydrogen rich stream over Ni/CeO2 nanocatalysts. J. Rare Earth 2015, 33, 619–628. [Google Scholar] [CrossRef]
- Rombi, E.; Cutrufello, M.G.; Atzori, L.; Monaci, R.; Ardu, A.; Gazzoli, D.; Deiana, P.; Ferino, I. CO methanation on Ni-Ce mixed oxides prepared by hard template method. Appl. Catal. A Gen. 2016, 515, 144–153. [Google Scholar] [CrossRef]
- Yamasaki, M.; Habazaki, H.; Asami, K.; Izumiya, K.; Hashimoto, K. Effect of tetragonal ZrO2 on the catalytic activity of Ni/ZrO2 catalyst prepared from amorphous Ni-Zr alloys. Catal. Commun. 2006, 7, 24–28. [Google Scholar] [CrossRef]
- Ocampo, F.; Louis, B.; Roger, A.-C. Methanation of carbon dioxide over nickel-based Ce0.72Zr0.28O2 mixed oxide catalysts prepared by sol-gel method. Appl. Catal. A Gen. 2009, 369, 90–96. [Google Scholar] [CrossRef]
- Ocampo, F.; Louis, B.; Kiwi-Minsker, L.; Roger, A.-C. Effect of Ce/Zr composition and noble metal promotion on nickel based CexZr1−xO2 catalysts for carbon dioxide methanation. Appl. Catal. A Gen. 2011, 392, 36–44. [Google Scholar] [CrossRef]
- Takano, H.; Izumiya, K.; Kumagai, N.; Hashimoto, K. The effect of heat treatment on the performance of the Ni/(Zr-Sm oxide) catalysts for carbon dioxide methanation. Appl. Surf. Sci. 2011, 257, 8171–8176. [Google Scholar] [CrossRef]
- Tada, S.; Shimizu, T.; Kameyama, H.; Haneda, T.; Kikuchi, R. Ni/CeO2 catalysts with high CO2 methanation activity and high CH4 selectivity at low temperatures. Int. J. Hydrogen Energy 2012, 37, 5527–5531. [Google Scholar] [CrossRef]
- Cai, W.; Zhong, Q.; Zhao, Y. Fractional-hydrolysis-driven formation of non-uniform dopant concentration catalyst nanoparticles of Ni/CexZr1−xO2 and its catalysis in methanation of CO2. Catal. Commun. 2013, 39, 30–34. [Google Scholar] [CrossRef]
- Ussa Aldana, P.A.; Ocampo, F.; Kobl, K.; Louis, B.; Thibault-Starzyk, F.; Daturi, M.; Bazin, P.; Thomas, S.; Roger, A.-C. Catalytic CO2 valorization into CH4 on Ni-based ceria-zirconia. Reaction mechanism by operando IR spectroscopy. Catal. Today 2013, 215, 201–207. [Google Scholar] [CrossRef]
- Liu, J.; Li, C.; Wang, F.; He, S.; Chen, H.; Zhao, Y.; Wei, M.; Evans, D.G.; Duan, X. Enhanced low-temperature activity of CO2 methanation over highly-dispersed Ni/TiO2 catalyst. Catal. Sci. Technol. 2013, 3, 2627–2633. [Google Scholar] [CrossRef]
- Pan, Q.; Peng, J.; Sun, T.; Gao, D.; Wang, S.; Wang, S.D. CO2 methanation on Ni/Ce0.5Zr0.5O2 catalysts for the production of synthetic natural gas. Fuel Process. Technol. 2014, 123, 166–171. [Google Scholar] [CrossRef]
- Takano, H.; Shinomiya, H.; Izumiya, K.; Kumagai, N.; Habazaki, H.; Hashimoto, K. CO2 methanation of Ni catalysts supported on tetragonal ZrO2 doped with Ca2+ and Ni2+ ions. Int. J. Hydrogen Energy 2015, 40, 8347–8355. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Ahmad, A. CO2 methanation over heterogeneous catalysts: Recent progress and future prospects. Green Chem. 2015, 17, 2647–2663. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, H.; Cui, K.; Jia, A.; Hu, G.; Jiao, Z.; Liu, Y.; Zhang, X. Role of surface Ni and Ce species of Ni/CeO2 catalyst in CO2 methanation. Appl. Surf. Sci. 2016, 383, 248–252. [Google Scholar] [CrossRef]
- Danaci, S.; Protasova, L.; Lefevere, J.; Bedel, L.; Guilet, R.; Marty, P. Efficient CO2 methanation over Ni/Al2O3 coated structured catalysts. Catal. Today 2016, 273, 234–243. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P.L. Supported Catalysts for CO2 Methanation: A Review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Nizio, M.; Albarazi, A.; Cavadias, S.; Amouroux, J.; Galvez, M.E.; Da Costa, P. Hybrid plasma-catalytic methanation of CO2 at low temperature over ceria zirconia supported Ni catalysts. Int. J. Hydrogen Energy 2016, 41, 11584–11592. [Google Scholar] [CrossRef]
- Atzori, L.; Cutrufello, M.G.; Meloni, D.; Monaci, R.; Cannas, C.; Gazzoli, D.; Sini, M.F.; Deiana, P.; Rombi, E. CO2 methanation on hard-templated NiO-CeO2 mixed oxides. Int. J. Hydrogen Energy 2017, 42, 20689–20702. [Google Scholar] [CrossRef]
- Ashok, J.; Ang, M.L.; Kawi, S. Enhanced activity of CO2 methanation over Ni/CeO2-ZrO2 catalysts: Influence of preparation methods. Catal. Today 2017, 281, 304–311. [Google Scholar] [CrossRef]
- Atzori, L.; Cutrufello, M.G.; Meloni, D.; Cannas, C.; Gazzoli, D.; Monaci, R.; Sini, M.F.; Rombi, E. Highly active NiO-CeO2 catalysts for synthetic natural gas production by CO2 methanation. Catal. Today 2018, 299, 183–192. [Google Scholar] [CrossRef]
- Ratchahat, S.; Sudoh, M.; Suzuki, Y.; Kawasaki, W.; Watanabe, R.; Fukuhara, C. Development of a powerful CO2 methanation process using a structured Ni/CeO2 catalyst. J. CO2 Util. 2018, 24, 210–219. [Google Scholar] [CrossRef]
- Yu, Y.; Bian, Z.; Song, F.; Wang, J.; Zhong, Q.; Kawi, S. Influence of Calcination Temperature on Activity and Selectivity of Ni-CeO2 and Ni-Ce0.8Zr0.2O2 Catalysts for CO2 Methanation. Top. Catal. 2018, 61, 1514–1527. [Google Scholar] [CrossRef]
- Shang, X.; Deng, D.; Wang, X.; Xuan, W.; Zou, X.; Ding, W.; Lu, X. Enhanced low-temperature activity for CO2 methanation over Ru doped the Ni/CexZr(1−x)O2 catalysts prepared by one-pot hydrolysis method. Int. J. Hydrogen Energy 2018, 43, 7179–7189. [Google Scholar] [CrossRef]
- Atzori, L.; Rombi, E.; Meloni, D.; Monaci, R.; Sini, M.F.; Cutrufello, M.G. Nanostructured Ni/CeO2-ZrO2 catalysts for CO2 conversion into SNG. J. Nanosci. Nanotechnol. 2019, 19, 3269–3276. [Google Scholar] [CrossRef]
- Habazaki, H.; Yamasaki, M.; Zhang, B.P.; Kawashima, A.; Kohno, S.; Takai, T.; Hashimoto, K. Co-methanation of carbon monoxide and carbon dioxide on supported nickel and cobalt catalysts prepared from amorphous alloys. Appl. Catal. A Gen. 1998, 172, 131–140. [Google Scholar] [CrossRef]
- Gogate, M.R.; Davis, R.J. Comparative study of CO and CO2 hydrogenation over supported Rh-Fe catalysts. Catal. Commun. 2010, 11, 901–906. [Google Scholar] [CrossRef]
- Kang, S.-H.; Ryu, J.-H.; Kim, J.-H.; Seo, S.-J.; Yoo, J.-D.; Sai Prasad, P.S.; Lim, H.-J.; Byun, C.-D. Co-methanation of CO and CO2 on the NiX-Fe1−X/Al2O3 catalysts; effect of Fe contents. Korean J. Chem. Eng. 2011, 28, 2282–2286. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Wang, J.-J.; Liu, Z.-M.; Lin, G.-D.; Zhang, H.-B. Highly efficient Ni-ZrO2 catalyst doped with Yb2O3 for co-methanation of CO and CO2. Appl. Catal. A Gen. 2013, 466, 300–306. [Google Scholar] [CrossRef]
- Razzaq, R.; Zhu, H.; Jiang, L.; Muhammad, U.; Li, C.; Zhang, S. Catalytic Methanation of CO and CO2 in Coke Oven Gas over Ni-Co/ZrO2-CeO2. Ind. Eng. Chem. Res. 2013, 52, 2247–2256. [Google Scholar] [CrossRef]
- Razzaq, R.; Li, C.; Amin, N.; Zhang, S.; Suzuki, K. Co-methanation of Carbon Oxides over Nickel-Based CexZr1−xO2 Catalysts. Energy Fuels 2013, 27, 6955–6961. [Google Scholar] [CrossRef]
- Razzaq, R.; Li, C.; Usman, M.; Suzuki, K.; Zhang, S. A highly active and stable Co4N/γ-Al2O3 catalyst for CO and CO2 methanation to produce synthetic natural gas (SNG). Chem. Eng. J. 2015, 262, 1090–1098. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Chai, R.; Zhao, G.; Liu, Y.; Lu, Y. Structured Ni-CeO2-Al2O3/Ni-Foam Catalyst with Enhanced Heat Transfer for Substitute Natural Gas Production by Syngas Methanation. ChemCatChem 2015, 7, 1427–1431. [Google Scholar] [CrossRef]
- Zhao, K.; Li, Z.; Bian, L. CO2 methanation and co-methanation of CO and CO2 over Mn-promoted Ni/Al2O3 catalysts. Front. Chem. Sci. Eng. 2016, 10, 273–280. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Malara, A.; Modafferi, V.; Mascolo, M.C.; Candamano, S.; Crea, F.; Antonucci, P. CO2 and CO hydrogenation over Ni-supported materials. Funct. Mater. Lett. 2018, 11, 1850061. [Google Scholar] [CrossRef]
- Xie, Y.; Kocaefe, D.; Chen, C.; Kocaefe, Y. Review of Research on Template Methods in Preparation of Nanomaterials. J. Nanomater. 2016, 2016, 2302595. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S.W.; Llewellyn, P.; Maurin, G. Adsorption by Powders and Porous Solids, Principles, Methodology and Applications, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Groen, J.C.; Peffer, L.A.A. Perez-Ramirez, Pore size determination in modified micro- and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. J. Micropor. Mesopor. Mater. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-Ray Diffraction Procedures; Wiley: New York, NY, USA, 1962. [Google Scholar]
- Jalowiecki-Duhamel, L.; Ponchel, A.; Lamonier, C.; D’Huysser, A.; Barbaux, Y. Relationship between Structure of CeNiXOY Mixed Oxides and Catalytic Properties in Oxidative Dehydrogenation of Propane. Langmuir 2001, 17, 1511–1517. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, H.; Li, K.; Zhu, X.; Du, Y. Preparation and characterization of Ce1−xNixO2 as oxygen carrier for selective oxidation methane to syngas in absence of gaseous oxygen. J. Rare Earths 2010, 28, 357–361. [Google Scholar] [CrossRef]
- Sagar, T.V.; Sreelatha, N.; Hanmant, G.; Surendar, M.; Lingaiah, N.; Rama Rao, K.S.; Satyanarayana, C.V.V.; Reddy, I.A.K.; Sai Prasad, P.S. Influence of method of preparation on the activity of La-Ni-Ce mixed oxide catalysts for dry reforming of methane. RSC Adv. 2014, 4, 50226–50232. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.; Luo, M.; Fang, P.; He, M. Preparation of High-Surface Area Nano-CeO2 by Template-Assisted Precipitation Method. J. Rare Earths 2007, 25, 58–62. [Google Scholar]





| Sample | NiO Content (wt%) a | CeO2 Molar Fraction in (CeO2)x-(ZrO2)1−x a | SBET (m2 g−1) b | Vp (cm3 g−1) b |
|---|---|---|---|---|
| NiO/ZrO2 | 30.2 | - | 282 | 0.27 |
| NiO/Ce0.25Zr0.75O2 | 29.5 | 0.263 | 245 | 0.23 |
| NiO/Ce0.50Zr0.50O2 | 29.0 | 0.507 | 245 | 0.31 |
| NiO/Ce0.75Zr0.25O2 | 29.1 | 0.737 | 198 | 0.28 |
| NiO/CeO2 | 29.0 | - | 197 | 0.31 |
| Sample | XCO (mol%) | XCO2 (mol%) | SCH4 (mol%) |
|---|---|---|---|
| NiO/ZrO2 | 95 | 14 | >99 |
| NiO/Ce0.25Zr0.75O2 | 95 | 16 | >99 |
| NiO/Ce0.50Zr0.50O2 | 98 | 21 | >99 |
| NiO/Ce0.75Zr0.25O2 | 98 | 21 | >99 |
| NiO/CeO2 | 98 | 21 | >99 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atzori, L.; Rombi, E.; Meloni, D.; Sini, M.F.; Monaci, R.; Cutrufello, M.G. CO and CO2 Co-Methanation on Ni/CeO2-ZrO2 Soft-Templated Catalysts. Catalysts 2019, 9, 415. https://doi.org/10.3390/catal9050415
Atzori L, Rombi E, Meloni D, Sini MF, Monaci R, Cutrufello MG. CO and CO2 Co-Methanation on Ni/CeO2-ZrO2 Soft-Templated Catalysts. Catalysts. 2019; 9(5):415. https://doi.org/10.3390/catal9050415
Chicago/Turabian StyleAtzori, Luciano, Elisabetta Rombi, Daniela Meloni, Maria Franca Sini, Roberto Monaci, and Maria Giorgia Cutrufello. 2019. "CO and CO2 Co-Methanation on Ni/CeO2-ZrO2 Soft-Templated Catalysts" Catalysts 9, no. 5: 415. https://doi.org/10.3390/catal9050415






