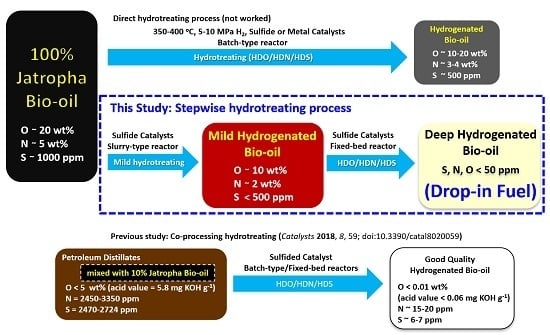
Hydrotreating of Jatropha-derived Bio-oil over Mesoporous Sulfide Catalysts to Produce Drop-in Transportation Fuels
Abstract
:1. Introduction
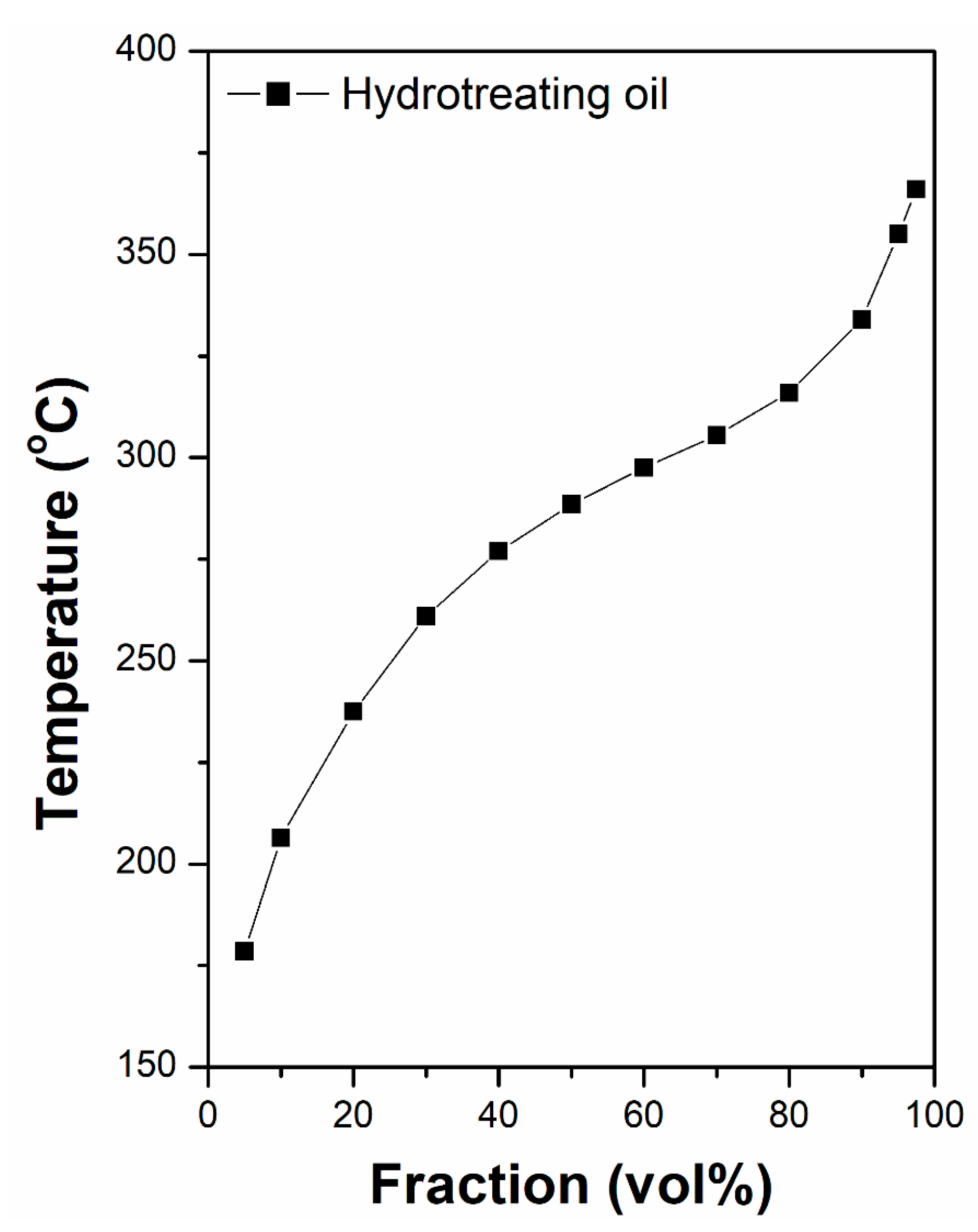
2. Results
3. Material and Methods
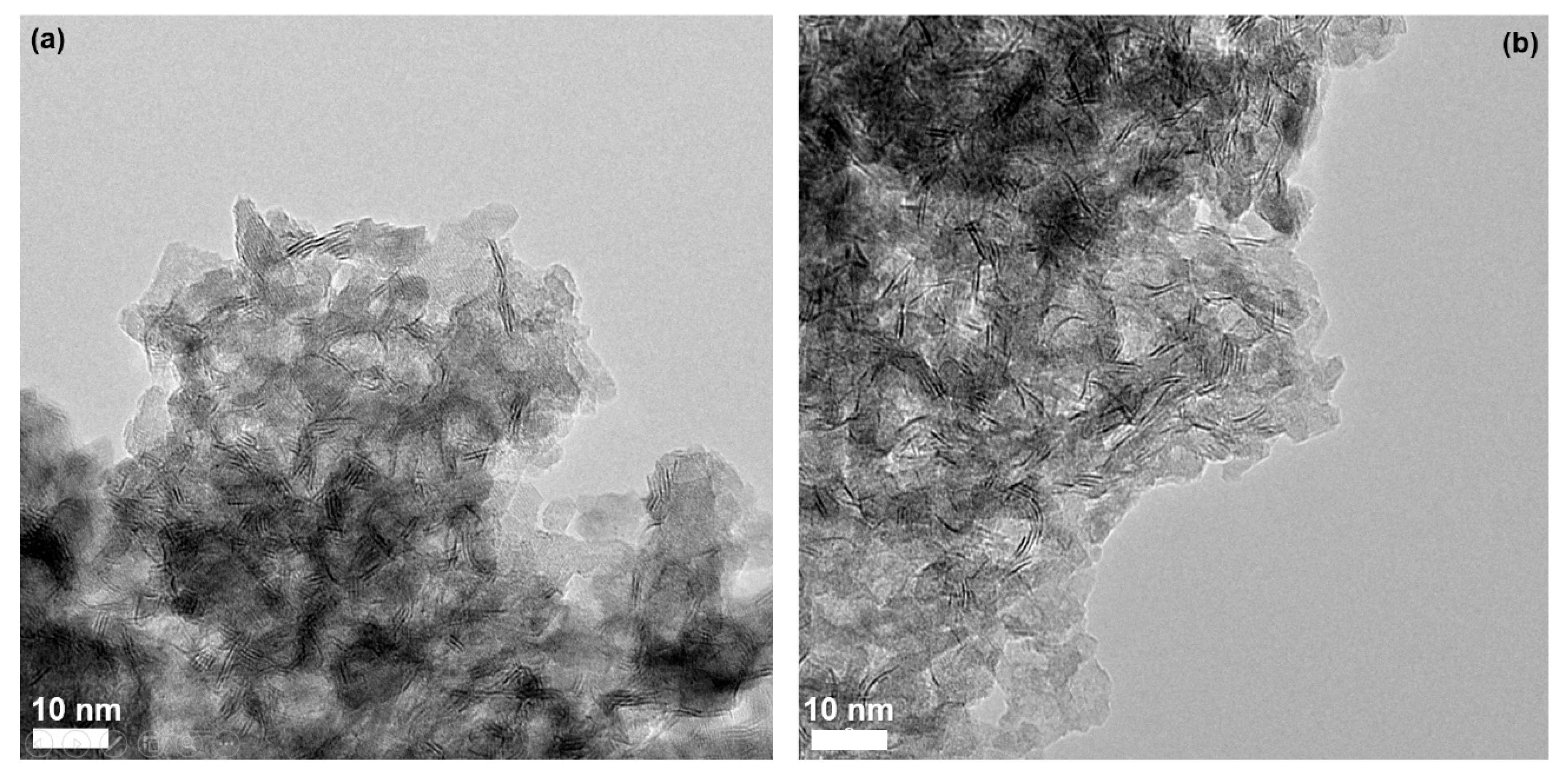
3.1. Preparation and Characterization of Sulfide Catalysts
3.2. Stepwise Hydrotreating of Jatropha Bio-Oil
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kohl, M.; Neupane, P.R.; Lotfiomran, N. The impact of tree age on biomass growth and carbon accumulation capacity: A retrospective analysis using tree ring data of three tropical tree species grown in natural forests of Suriname. PLoS ONE 2017, 12, e0181187. [Google Scholar] [CrossRef]
- 2010 Survey of Energy Resources. World Energy Council. Available online: https://www.worldenergy.org/wp-content/uploads/2012/09/ser_2010_report_1.pdf (accessed on 26 November 2010).
- Biomass First Results from an Integrated Assessment; Production, Supply, Uses and Flows in the European Union. JRC Science for Policy Report. Available online: http://publications.jrc.ec.europa.eu/repository/bitstream/JRC109869/jrc109869_biomass_report_final2pdf2.pdf (accessed on 21 February 2018).
- United Nations Framework Convention on Climate Change (UNFCCC). Adoption of the Paris Agreement. Available online: http://unfccc.int/resource/docs/2015/cop21/eng/l09r01.pdf (accessed on 21 February 2018).
- Schneier, T.; Kaul, C.M.; Pressel, K.G. Possible climate transitions from breakup of stratocumulus decks under greenhouse warming. Nature 2019, 12, 163–167. [Google Scholar]
- Lee, A.F.; Bennett, J.A.; Manayil, J.C.; Wilson, K. Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification. Chem. Soc. Rev. 2014, 43, 7887–7916. [Google Scholar] [CrossRef] [PubMed]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Dumesic, J.A. Catalytic routes for the conversion of biomass into liquid hydrocarbon transportation fuels. Energy Environ. Sci. 2011, 4, 83–99. [Google Scholar] [CrossRef]
- Carlson, T.R.; Vispute, T.P.; Huber, G.W. Green Gasoline by Catalytic Fast Pyrolysis of Solid Biomass Derived Compounds. ChemSusChem 2008, 1, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Kabir, G.; Hameed, B.H. Recent progress on catalytic pyrolysis of lignocellulosic biomass to high-grade bio-oil and bio-chemicals. Renew. Sustain. Energy Rev. 2017, 70, 945–967. [Google Scholar] [CrossRef]
- Chen, S.Y.; Mochizuki, T.; Abe, Y.; Toba, M.; Yoshimura, Y. Ti-incorporated SBA-15 mesoporous silica as an efficient and robustLewis solid acid catalyst for the production of high-qualitybiodiesel fuels. Appl. Catal. B Environ. 2014, 148–149, 344–356. [Google Scholar] [CrossRef]
- Chen, S.Y.; Lao-ubol, S.; Mochizuki, T.; Abe, Y.; Toba, M.; Yoshimura, Y. Transformation of non-edible vegetable oils into biodiesel fuels catalyzed by unconventional sulfonic acid-functionalized SBA-15. Appl. Catal. A Gen. 2014, 485, 28–39. [Google Scholar] [CrossRef]
- Chen, S.Y.; Mochizuki, T.; Abe, Y.; Toba, M.; Yoshimura, Y.; Somwongsa, P.; Lau-ubol, S. Carbonaceous Ti-incorporated SBA-15 with enhanced activity and durability for high-quality biodiesel production: Synthesis and utilization of the P123 template as carbon source. Appl. Catal. B Environ. 2016, 181, 800–809. [Google Scholar] [CrossRef]
- Chen, S.Y.; Attanatho, L.; Chang, A.; Laosombut, T.; Nishi, M.; Mochizuki, T.; Takagi, H.; Yang, C.M.; Abe, Y.; Toba, M.; et al. Profiling and catalytic upgrading of commercial palm oil-derived biodiesel fuels for high-blend fuels. Catal. Today 2018, in press. [Google Scholar] [CrossRef]
- Mansir, N.; Taufiq-Yap, Y.H.; Rashid, U.; Lokman, I.M. Investigation of heterogeneous solid acid catalyst performance on low grade feedstocks for biodiesel production: A review. Energy Convers. Manag. 2017, 141, 171–182. [Google Scholar] [CrossRef]
- Masoumeh Hajjari, M.; Tabatabaei, M.; Aghbashlo, M.; Ghanavati, H. A review on the prospects of sustainable biodiesel production: A global scenario with an emphasis on waste-oil biodiesel utilization. Renew. Sustain. Energy Rev. 2017, 72, 445–464. [Google Scholar] [CrossRef]
- Luterbacher, J.S.; Alonso, D.M.; Dumesic, J.A. Targeted chemical upgrading of lignocellulosic biomass to platform molecules. Green Chem. 2014, 16, 4816–4838. [Google Scholar] [CrossRef]
- Mochizuki, T.; Toba, M.; Yoshimura, Y. Fast pyrolysis of Jatropha residues over zeolite catalysts. J. Jpn. Petrol. Inst. 2012, 55, 69–70. [Google Scholar] [CrossRef]
- Mochizuki, T.; Toba, M.; Yoshimura, Y. Effect of electrostatic precipitator on collection efficiency of bio-oil in fast pyrolysis of biomass. J. Jpn. Petrol. Inst. 2013, 56, 401–405. [Google Scholar] [CrossRef]
- Mochizuki, T.; Chen, S.Y.; Toba, M.; Yoshimura, Y. Pyrolyzer–GC/MS system-based analysis of the effects of zeolite catalysts on the fast pyrolysis of Jatropha husk. Appl. Catal. A Gen. 2013, 456, 174–181. [Google Scholar] [CrossRef]
- Mochizuki, T.; Chen, S.Y.; Toba, M.; Yoshimura, Y. Deoxygenation of guaiacol and woody tar over reduced catalysts. Appl. Catal. B Environ. 2014, 146, 237–243. [Google Scholar] [CrossRef]
- Mortensena, P.M.; Grunwaldt, J.D.; Jensena, P.A.; Knudsenc, K.G.; Jensena, A.D. A review of catalytic upgrading of bio-oil to engine fuels. Appl. Catal. A Gen. 2011, 407, 1–19. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Sato, T.; Shimada, H.; Matsubayashi, N.; Nishijima, A. Influences of oxygen-containing substances on deactivation of sulfided molybdate catalysts. Appl. Catal. 1991, 73, 55–63. [Google Scholar] [CrossRef]
- Laurent, E.; Delmon, B. Study of the hydrodeoxygenation of carbonyl, carboxylic and guaiacyl groups over sulfide CoMo/γ-Al2O3 and NiMo/γ-Al2O3 catalyst. II. Influence of water, ammonia and hydrogen sulfide. Appl. Catal. A Gen. 1994, 109, 97–115. [Google Scholar] [CrossRef]
- Hrabar, A.; Hein, J.; Gutiérrez, O.Y.; Lercher, J.A. Selective poisoning of the direct denitrogenation route in o-propylaniline HDN by DBT on Mo and NiMo/γ-Al2O3 sulfide catalysts. J. Catal. 2011, 281, 325–338. [Google Scholar] [CrossRef]
- Prins, R.; Egorova, M.; Röthlisberger, A.; Zhao, Y.; Sivasankar, N.; Kukula, P. Mechanisms of hydrodesulfurization and hydrodenitrogenation. Catal. Today 2006, 111, 84–93. [Google Scholar] [CrossRef]
- Hiroshima, K.; Mochizuki, T.; Honma, T.; Shimizu, T.; Yamada, M. High HDS activity of Co-Mo/Al2O3 modified by some chelates and their surface fine structures. Appl. Sur. Sci. 1997, 121/122, 433–436. [Google Scholar] [CrossRef]
- Prins, R.; Zhao, Y.; Sivasankar, N.; Kukula, P. Mechanism of CN bond breaking in hydrodenitrogenation. J. Catal. 2005, 234, 509–512. [Google Scholar] [CrossRef]
- Egorova, M.; Prins, R. The role of Ni and Co promoters in the simultaneous HDS of dibenzothiophene and HDN of amines over Mo/γ-Al2O3 catalysts. J. Catal. 2006, 241, 162–172. [Google Scholar] [CrossRef]
- Chen, S.Y.; Nishi, M.; Mochizuki, T.; Takagi, H.; Takatsuki, A.; Roschat, W.; Toba, M.; Yoshimura, Y. Co-processing of Jatropha-derived bio-oil with petroleum distillates over mesoporous CoMo and NiMo sulfide catalysts. Catalysts 2018, 8, 59. [Google Scholar] [CrossRef]
- Toba, M.; Abe, Y.; Kuramochi, H.; Osako, M.; Mochizuki, T.; Yoshimura, Y. Hydrodeoxygenation of waste vegetable oil over sulfide catalysts. Catal. Today 2011, 164, 533–537. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Matsubayashi, N.; Sato, T.; Shimada, H.; Nishijima, A. Molybdate catalysts prepared by a novel impregnation method: Effect of citric acid as a ligand on the catalytic activities. Appl. Catal. A. Gen. 1991, 79, 145–159. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Toba, M.; Matsubayashi, N.; Matsui, T. Hydrotreating Catalyst of Catalytic Cracking Gasoline. U.S. Patent US7393807 B2, 1 July 2008. [Google Scholar]
- Kwon, D.; Ko, M.S.; Yang, J.S.; Kwon, M.J.; Lee, S.-W.; Lee, S. Identification of refined petroleum products in contaminated soils using an identification index for GC chromatograms. Environ. Sci. Pollut. Res. 2015, 22, 12029–12034. [Google Scholar] [CrossRef]
- The JIS K2249 Method, Petroleum Products—Determination of Distillation Characteristics. Available online: http://kikakurui.com/k2/K2254-2018-01.html (accessed on 12 December 2018).
- The K2280-5 Method, Petroleum Products—Fuels—Determination of Octane Number, Cetane Number and Calculation of Cetane Index. Available online: http://kikakurui.com/k2/K2280-5-2013-01.html (accessed on 20 November 2013).
- Kouzu, M.; Kuriki, Y.; Uchida, K.; Sakanishi, K.; Sugimoto, Y.; Saito, I.; Fujii, D.; Hirona, K. Catalytic hydrotreating of petroleum residue over carbon-supported nickel-molybdenum sulfides. Energy Fuels 2005, 19, 725–730. [Google Scholar] [CrossRef]





| Samples | S (ppm) | N (ppm) | Octane Number | H/C | Density (g/cm3) | n-Paraffin (vol.%) | iso-Paraffin (vol.%) | Olefin (vol.%) | Naphthene (vol.%) | Aromatic (vol.%) |
|---|---|---|---|---|---|---|---|---|---|---|
| This work | 0 | 49 | 44.5 | 1.77 | 0.71 | 29.5 | 14.4 | 4.99 | 21.4 | 29.6 |
| Gasoline b | max. 10 | - | min. 89 | - | max. 0.783 | - | - | - | - | min. 1 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-Y.; Mochizuki, T.; Nishi, M.; Takagi, H.; Yoshimura, Y.; Toba, M. Hydrotreating of Jatropha-derived Bio-oil over Mesoporous Sulfide Catalysts to Produce Drop-in Transportation Fuels. Catalysts 2019, 9, 392. https://doi.org/10.3390/catal9050392
Chen S-Y, Mochizuki T, Nishi M, Takagi H, Yoshimura Y, Toba M. Hydrotreating of Jatropha-derived Bio-oil over Mesoporous Sulfide Catalysts to Produce Drop-in Transportation Fuels. Catalysts. 2019; 9(5):392. https://doi.org/10.3390/catal9050392
Chicago/Turabian StyleChen, Shih-Yuan, Takehisa Mochizuki, Masayasu Nishi, Hideyuki Takagi, Yuji Yoshimura, and Makoto Toba. 2019. "Hydrotreating of Jatropha-derived Bio-oil over Mesoporous Sulfide Catalysts to Produce Drop-in Transportation Fuels" Catalysts 9, no. 5: 392. https://doi.org/10.3390/catal9050392
APA StyleChen, S.-Y., Mochizuki, T., Nishi, M., Takagi, H., Yoshimura, Y., & Toba, M. (2019). Hydrotreating of Jatropha-derived Bio-oil over Mesoporous Sulfide Catalysts to Produce Drop-in Transportation Fuels. Catalysts, 9(5), 392. https://doi.org/10.3390/catal9050392