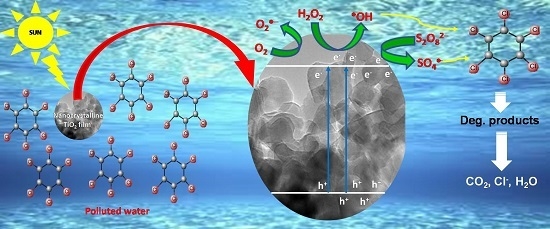
Exhaustive Photocatalytic Lindane Degradation by Combined Simulated Solar Light-Activated Nanocrystalline TiO2 and Inorganic Oxidants
Abstract
:1. Introduction
2. Results and Discussion
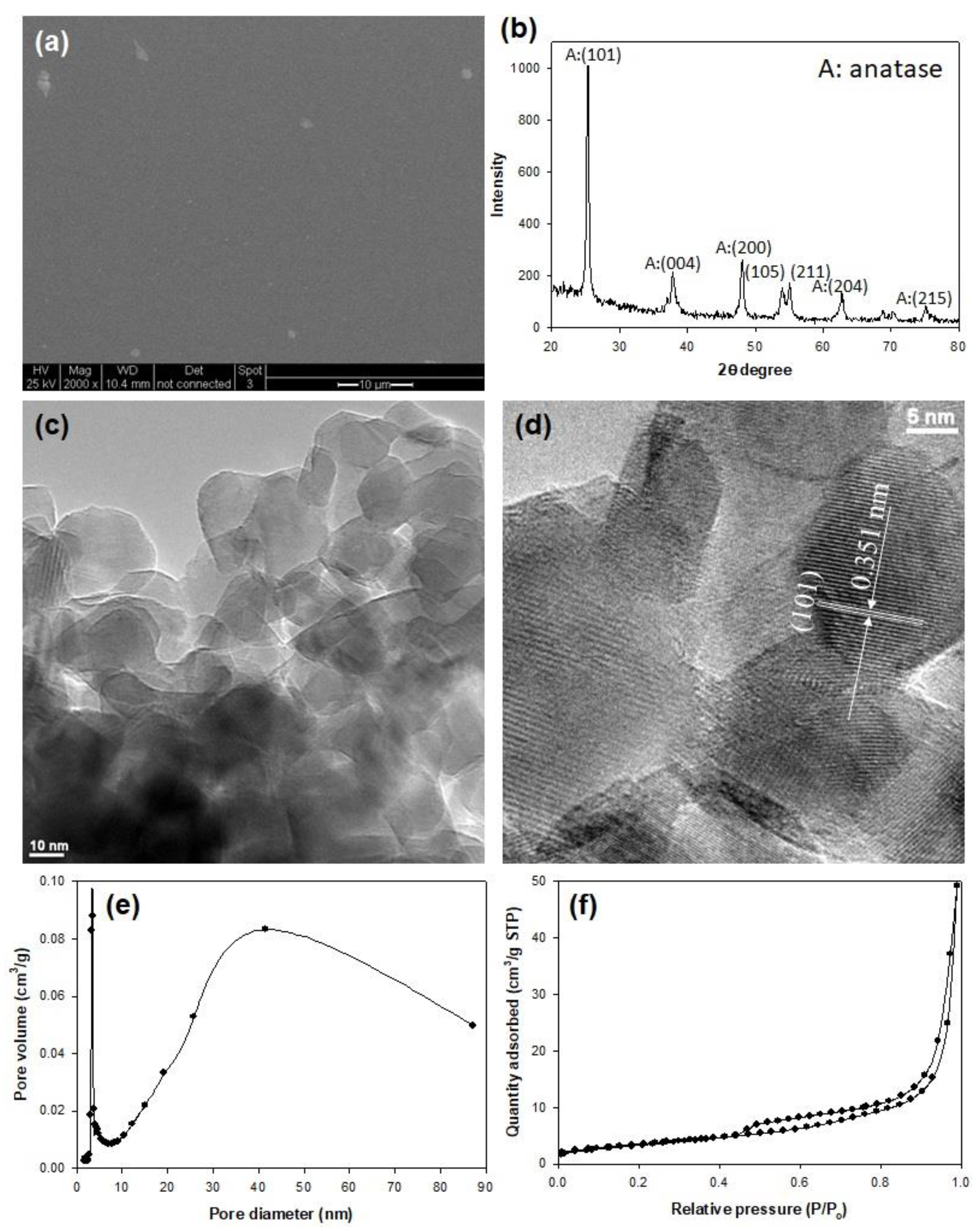
2.1. Characteristics of Synthesized TiO2 Films
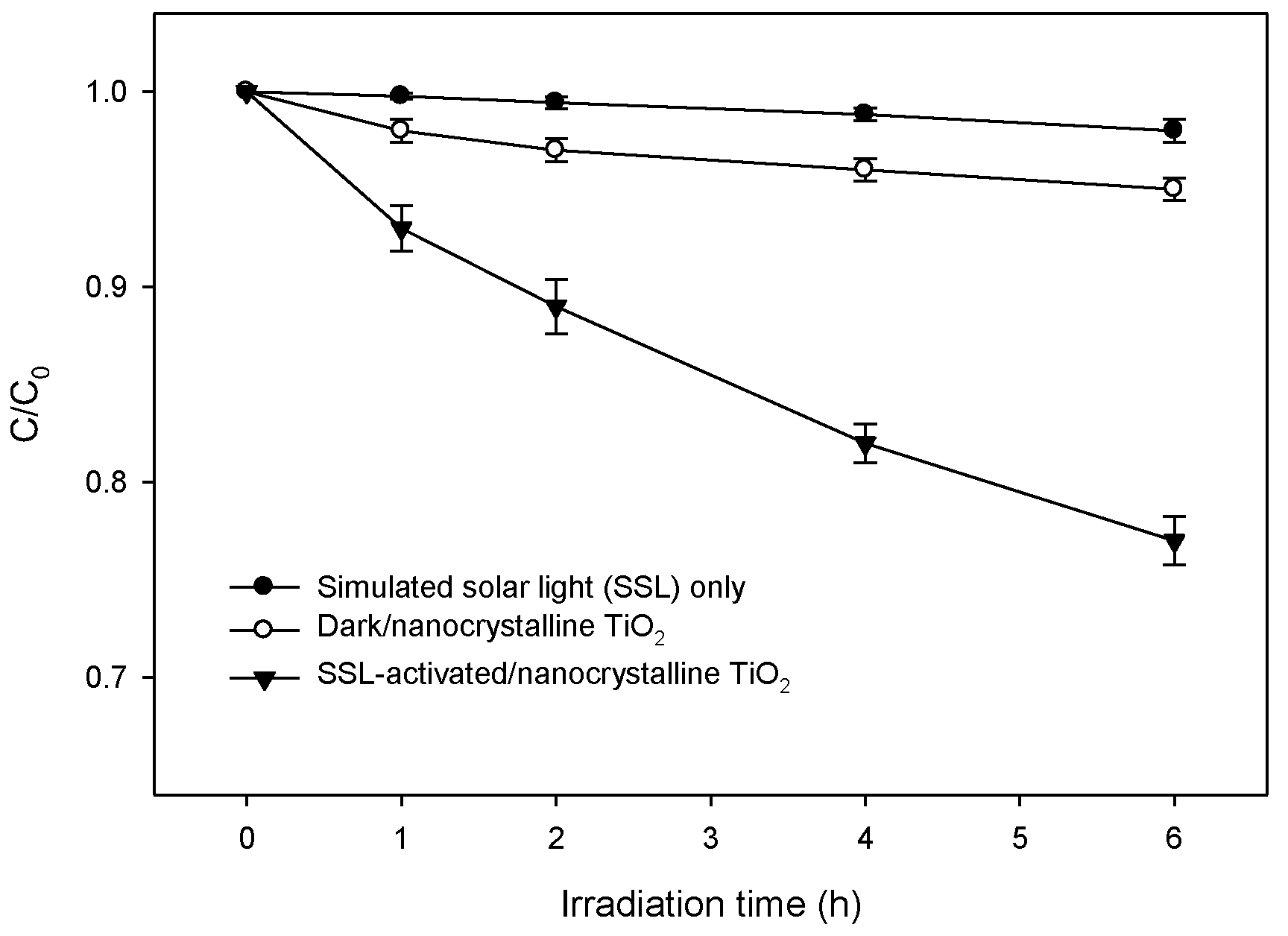
2.2. SSLA-TiO2 Photocatalysis of Lindane
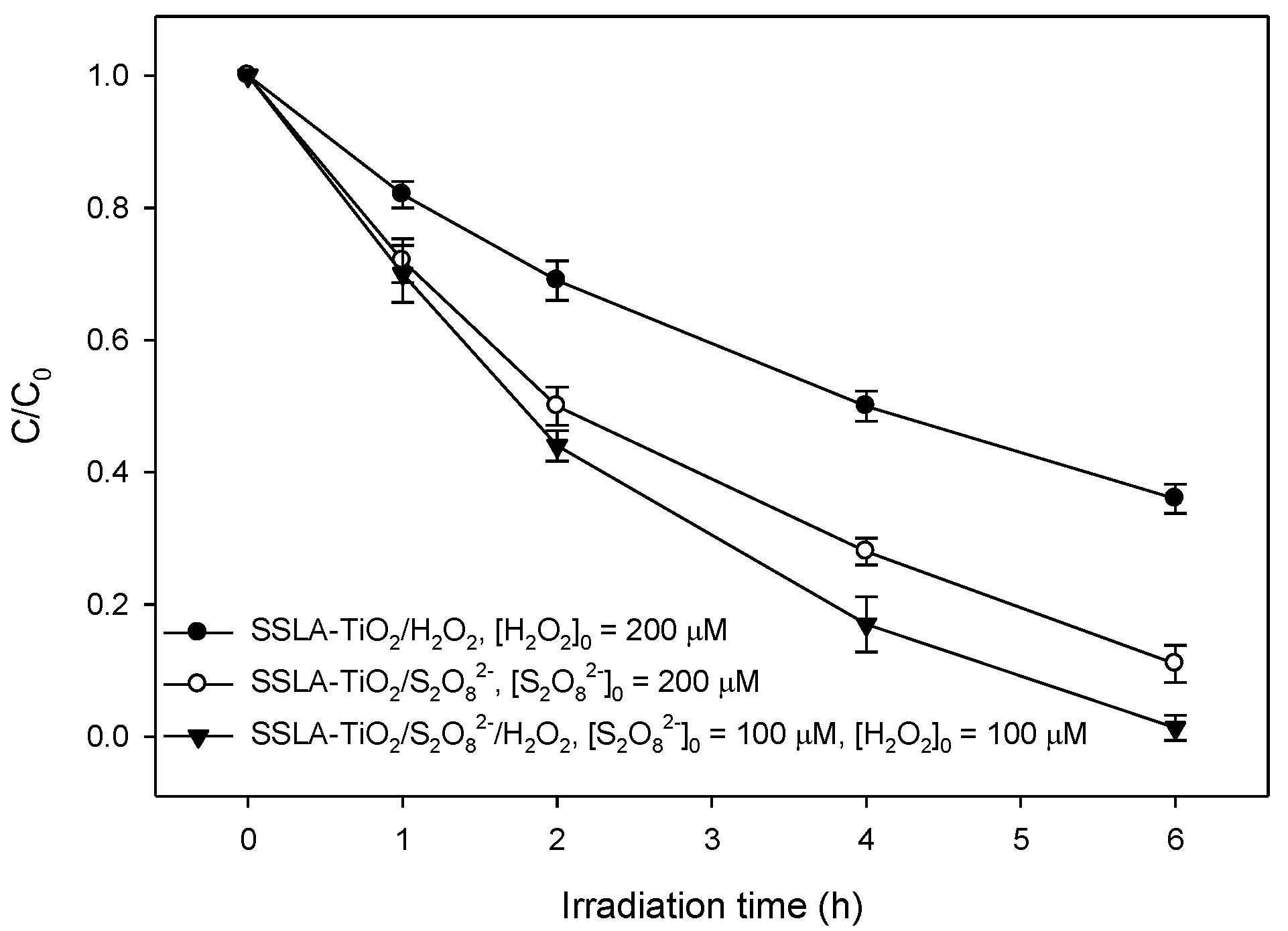
2.3. Effect of S2O82− and H2O2 on Lindane Degradation by SSLA-TiO2 Photocatalysis
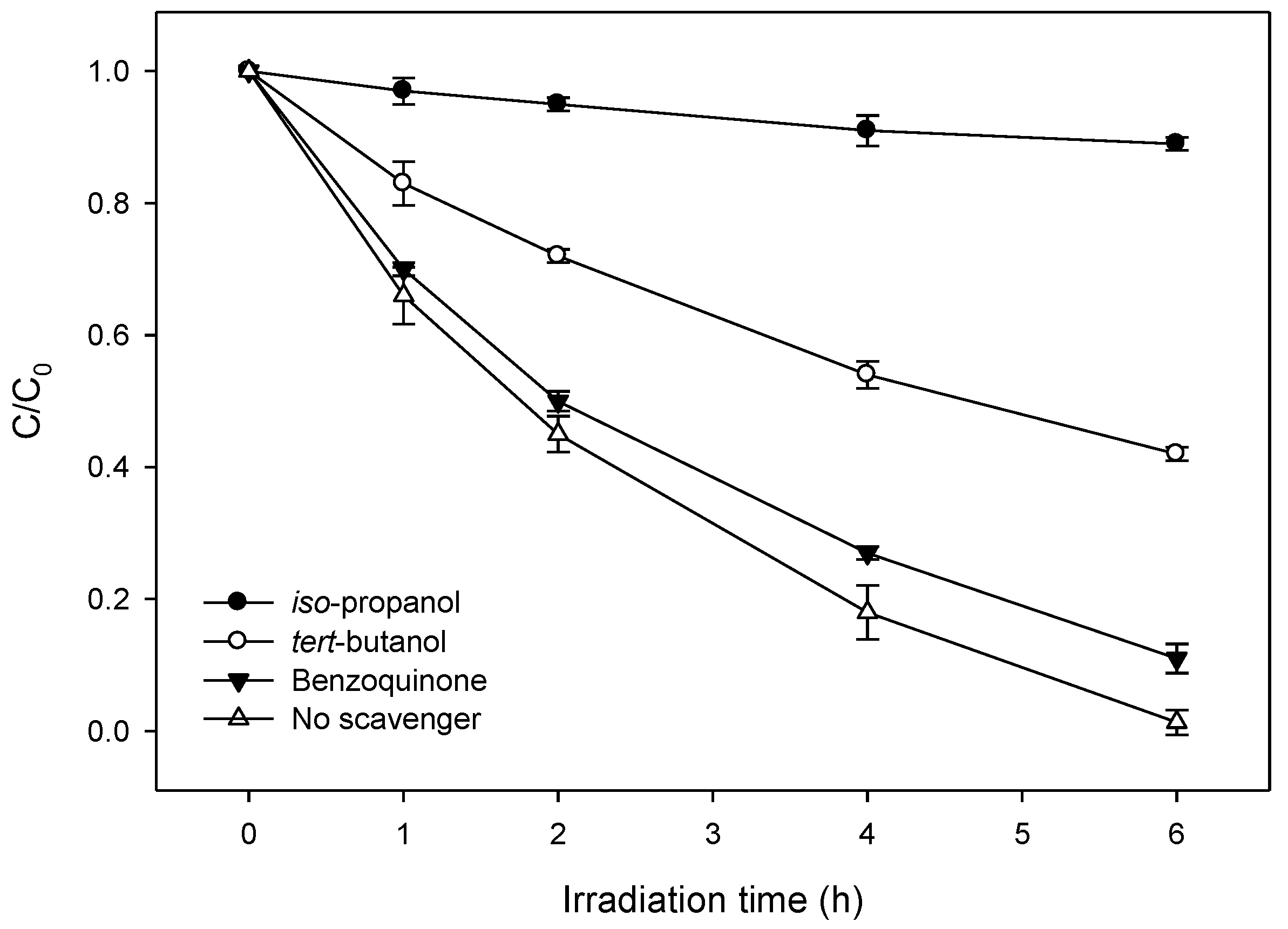
2.4. Radical Scavenger Studies and Role of the Reactive Species
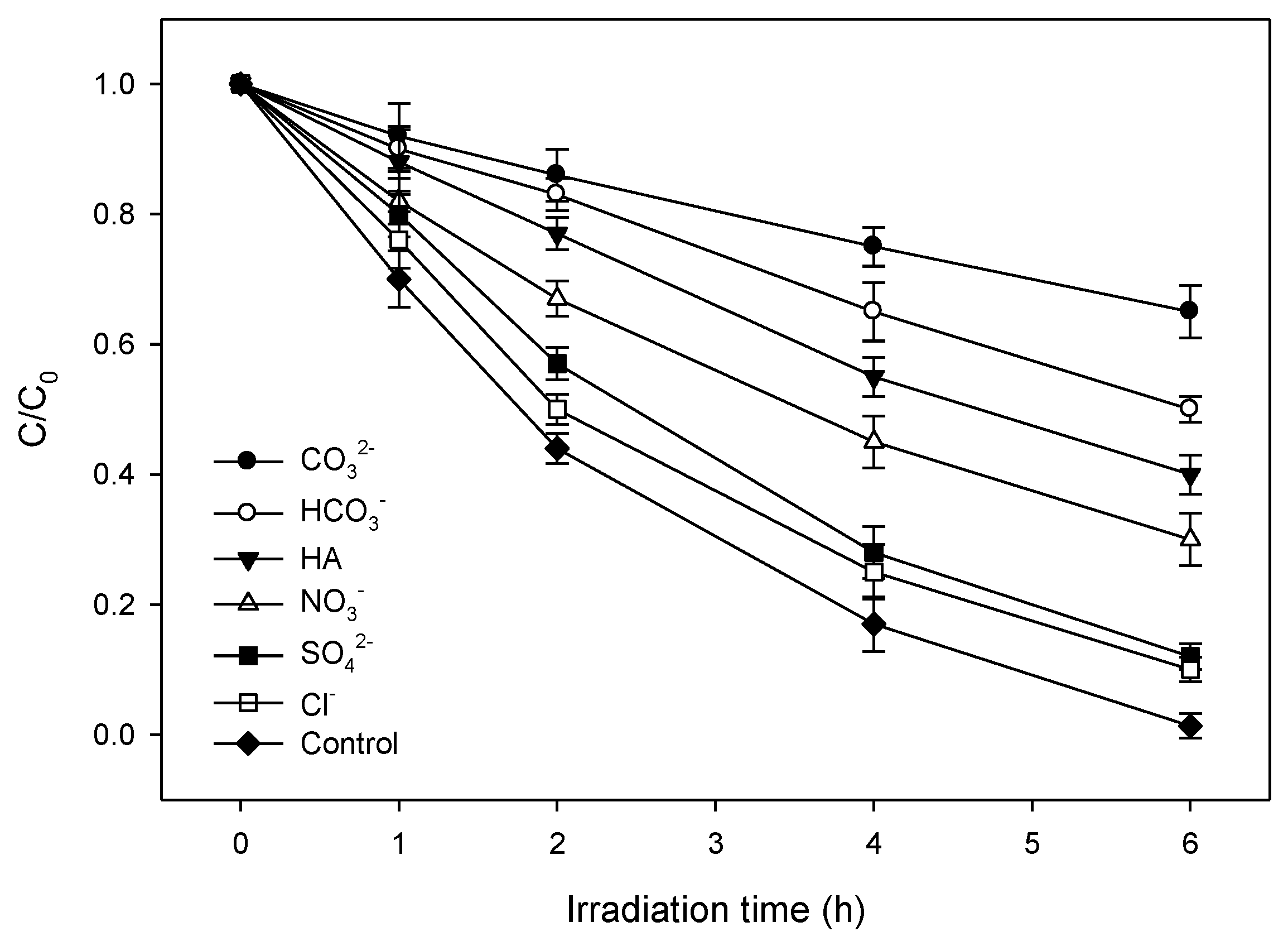
2.5. Influence of Natural Water Constituents
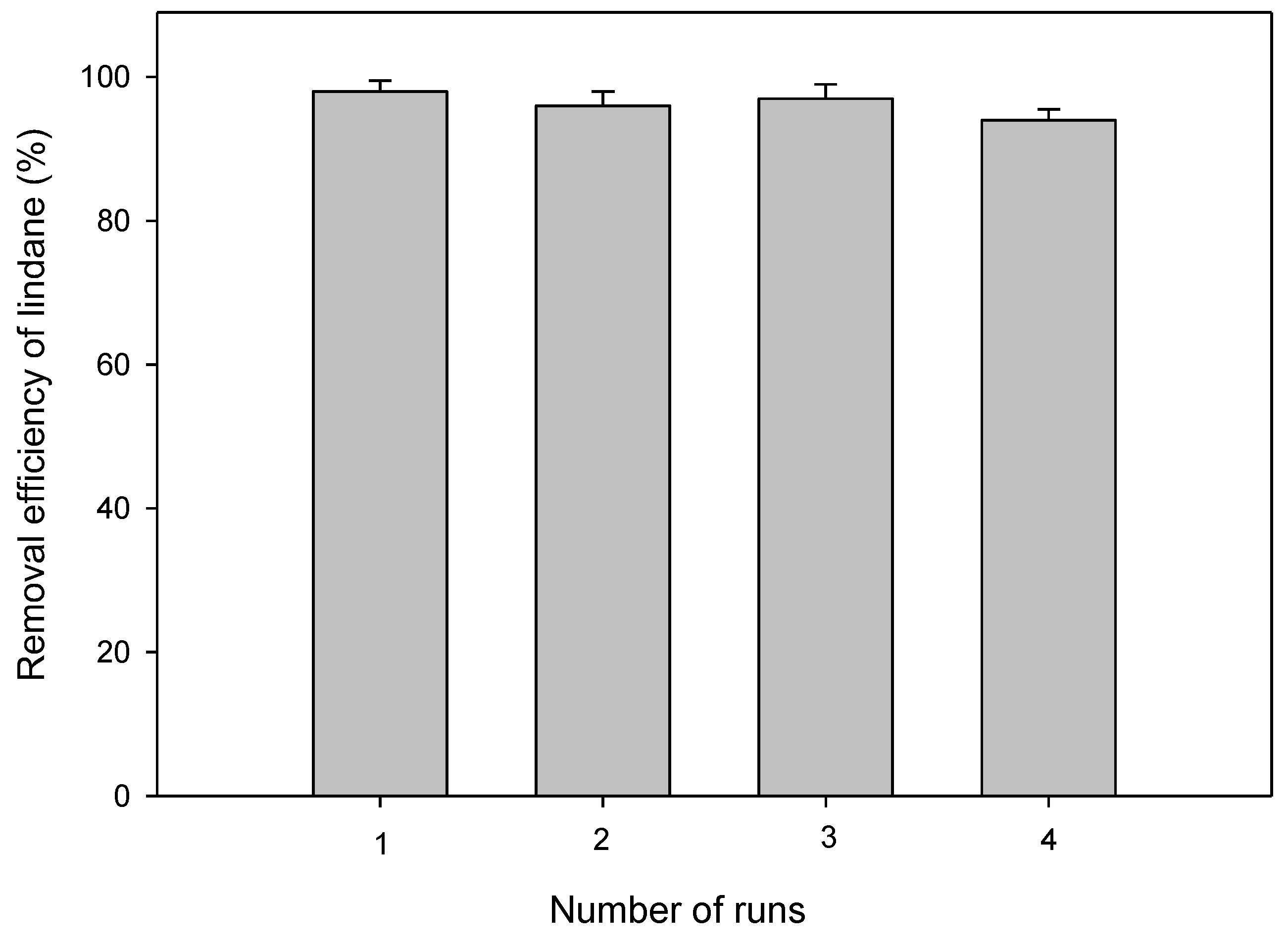
2.6. Performance Sustainability of the Synthesized TiO2 Film
3. Materials and Methods
3.1. Materials
3.2. TiO2 Film Preparation
3.3. Characterization of Synthesized TiO2 Films
3.4. Photocatalytic Experiments
3.5. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, S.; Su, G.; Zheng, M.; Jia, M.; Qi, C.; Li, W. The degradation of 1,2,4-trichlorobenzene using synthesized Co3O4 and the hypothesized mechanism. J. Hazard. Mater. 2011, 192, 1697–1704. [Google Scholar] [CrossRef]
- Movahedyan, H.; Mohammadi, A.S.; Assadi, A. Comparison of different advanced oxidation processes degrading p-chlorophenol in aqueous solution. J. Environ. Health Sci. Eng. 2009, 6, 153–160. [Google Scholar]
- Li, S.; Elliott, D.W.; Spear, S.T.; Ma, L.; Zhang, W.-X. Hexachlorocyclohexanes in the Environment: Mechanisms of Dechlorination. Crit. Rev. Environ. Sci. Technol. 2011, 41, 1747–1792. [Google Scholar] [CrossRef]
- Okeke, B.C.; Siddique, T.; Arbestain, M.C.; Frankenberger, W.T. Biodegradation of gamma-hexachlorocyclohexane (lindane) and alpha-hexachlorocyclohexane in water and a soil slurry by a Pandoraea species. J. Agric. Food Chem. 2002, 50, 2548–2555. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Kumar, M.S.; Chakrabarti, T. Assessment of bioremediation possibilities of technical grade hexachlorocyclohexane (tech-HCH) contaminated soils. J. Hazard. Mater. 2007, 143, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.W.F. Benzene hexachloride. In Encyclopedia of Chemical Technology, 2nd ed.; Kirk, R.E., Othmer, D.F., Eds.; John Wiley & Sons: New York, NY, USA, 1964; Volume 5, pp. 267–281. [Google Scholar]
- Begum, A.; Gautam, S.K. Dechlorination of endocrine disrupting chemicals using Mg0/ZnCl2 bimetallic system. Water Res. 2011, 45, 2383–2391. [Google Scholar] [CrossRef]
- Pant, N.; Shukla, M.; Upadhyay, A.; Chaturvedi, P.; Saxena, D.; Gupta, Y. Association between environmental exposure to p, p′-DDE and lindane and semen quality. Environ. Sci. Pollut. Res. 2014, 21, 11009–11016. [Google Scholar] [CrossRef] [PubMed]
- Evgenidou, E.; Konstantinou, I.; Fytianos, K.; Poulios, I. Oxidation of two organophosphorous insecticides by the photo-assisted Fenton reaction. Water Res. 2007, 41, 2015–2027. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Byrne, J.A.; Fernandez-Ibanez, P.A.; Dunlop, P.S.; Alrousan, D.; Hamilton, J.W. Photocatalytic Enhancement for Solar Disinfection of Water: A Review. Int. J. Photoenergy 2011, 2011, 798051. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, C.M.; Choi, J.W.; Park, G.C.; Joo, J. Enhanced photocatalytic activity of porous single crystal TiO2/CNT composites by annealing process. Ceram. Int. 2018, 44, 1641–1645. [Google Scholar] [CrossRef]
- Chandran, K.; Kait, C.F.; Zaid, H.F.M. Morphology of titanium dioxide synthesized via precipitation technique: Effect of calcination and reflux on the surface morphology. J. Phys. Conf. Ser. 2018, 1123, 012059. [Google Scholar] [CrossRef]
- Ghosh, M.; Liu, J.; Chuang, S.S.; Jana, S.C. Fabrication of hierarchical V2O5 nanorods on TiO2 nanofibers and their enhanced photocatalytic activity under visible light. ChemCatChem 2018, 10, 3305–3318. [Google Scholar] [CrossRef]
- Shah, N.S.; He, X.; Khan, H.M.; Khan, J.A.; O’Shea, K.E.; Boccelli, D.L.; Dionysiou, D.D. Efficient removal of endosulfan from aqueous solution by UV-C/peroxides: A comparative study. J. Hazard. Mater. 2013, 263 Pt 2, 584–592. [Google Scholar] [CrossRef]
- Khan, J.A.; He, X.; Khan, H.M.; Shah, N.S.; Dionysiou, D.D. Dionysiou, Oxidative degradation of atrazine in aqueous solution by UV/H2O2/Fe2+, UV/S2O82−/Fe2+ and UV/HSO5−/Fe2+ processes: A comparative study. Chem. Eng. J. 2013, 218, 376–383. [Google Scholar] [CrossRef]
- Boczkaj, G.; Fernandes, A. Wastewater treatment by means of Advanced Oxidation Processes at basic pH conditions: A review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Sayed, M.; Ismail, M.; Khan, S.; Tabassum, S.; Khan, H.M. Degradation of ciprofloxacin in water by advanced oxidation process: Kinetics study, influencing parameters and degradation pathways. Environ. Technol. 2016, 37, 590–602. [Google Scholar] [CrossRef]
- Khan, S.; He, X.; Khan, J.A.; Khan, H.M.; Boccelli, D.L.; Dionysiou, D.D. Kinetics and mechanism of sulfate radical- and hydroxyl radical-induced degradation of highly chlorinated pesticide lindane in UV/peroxymonosulfate system. Chem. Eng. J. 2017, 318, 135–142. [Google Scholar] [CrossRef]
- Dai, S.; Wu, Y.; Sakai, T.; Du, Z.; Sakai, H.; Abe, M. Preparation of Highly Crystalline TiO2 Nanostructures by Acid-assisted Hydrothermal Treatment of Hexagonal-structured Nanocrystalline Titania/Cetyltrimethylammonium Bromide Nanoskeleton. Nanoscale Res. Lett. 2010, 5, 1829–1835. [Google Scholar] [CrossRef]
- Helali, S.; Polo-López, M.I.; Fernández-Ibáñez, P.; Ohtani, B.; Amano, F.; Malato, S.; Guillard, C. Solar photocatalysis: A green technology for E. coli contaminated water disinfection. Effect of concentration and different types of suspended catalyst. J. Photochem. Photobiol. A Chem. 2014, 276, 31–40. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Lin, H.; Huang, C.P.; Li, W.; Ni, C.; Shah, S.I.; Tseng, Y.H. Size dependency of nanocrystalline TiO2 on its optical property and photocatalytic reactivity exemplified by 2-chlorophenol. Appl. Catal. B Environ. 2006, 68. [Google Scholar] [CrossRef]
- Alalm, M.G.; Tawfik, A.; Ookawara, S. Comparison of solar TiO2 photocatalysis and solar photo-Fenton for treatment of pesticides industry wastewater: Operational conditions, kinetics, and costs. J. Water Process Eng. 2015, 8, 55–63. [Google Scholar] [CrossRef]
- Adishkumar, S.; Kanmani, S. Treatment of phenolic wastewaters in single baffle reactor by Solar/TiO2/H2O2 process. Desalin. Water Treat. 2010, 24, 67–73. [Google Scholar] [CrossRef]
- Pelizzetti, E.; Borgarello, M.; Minero, C.; Pramauro, E.; Borgarello, E.; Serpone, N. Photocatalytic degradation of polychlorinated dioxins and polychlorinated biphenyls in aqueous suspensions of semiconductors irradiated with simulated solar light. Chemosphere 1988, 17, 499–510. [Google Scholar] [CrossRef]
- Robert, D.; Malato, S. Solar photocatalysis: A clean process for water detoxification. Sci. Total Environ. 2002, 291, 85–97. [Google Scholar] [CrossRef]
- Oller, I.; Gernjak, W.; Maldonado, M.; Pérez-Estrada, L.; Sánchez-Pérez, J.; Malato, S. Solar photocatalytic degradation of some hazardous water-soluble pesticides at pilot-plant scale. J. Hazard. Mater. 2006, 138, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Han, C.; Khan, H.M.; Boccelli, D.L.; Nadagouda, M.N.; Dionysiou, D.D. Efficient degradation of lindane by visible and simulated solar light-assisted S-TiO2/peroxymonosulfate process: Kinetics and mechanistic investigations. J. Mol. Catal. A Chem. 2017, 428, 9–16. [Google Scholar] [CrossRef]
- Koltsakidou, A.; Antonopoulou, M.; Evgenidou, E.; Konstantinou, I.; Lambropoulou, D. Cytarabine degradation by simulated solar assisted photocatalysis using TiO2. Chem. Eng. J. 2017, 316, 823–831. [Google Scholar] [CrossRef]
- Borges, M.; Sierra, M.; Méndez-Ramos, J.; Acosta-Mora, P.; Ruiz-Morales, J.; Esparza, P. Solar degradation of contaminants in water: TiO2 solar photocatalysis assisted by up-conversion luminescent materials. Sol. Energy Mater. Sol. Cells 2016, 155, 194–201. [Google Scholar] [CrossRef]
- De la Cruz, N.; Dantas, R.; Giménez, J.; Esplugas, S. Photolysis and TiO2 photocatalysis of the pharmaceutical propranolol: Solar and artificial light. Appl. Catal. B Environ. 2013, 130, 249–256. [Google Scholar] [CrossRef]
- Bhatkhande, D.S.; Pangarkar, V.G.; Beenackers, A.A.C.M. Photocatalytic degradation for environmental applications—A review. J. Chem. Technol. Biotechnol. 2002, 77, 102–116. [Google Scholar] [CrossRef]
- Parra, S.; Stanca, S.E.; Guasaquillo, I.; Thampi, K.R. Photocatalytic degradation of atrazine using suspended and supported TiO2. Appl. Catal. B Environ. 2004, 51, 107–116. [Google Scholar] [CrossRef]
- Khan, S.; Sayed, M.; Sohail, M.; Shah, L.A.; Raja, M.A. Advanced Oxidation and Reduction Processes. In Advances in Water Purification Techniques; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–164. [Google Scholar] [CrossRef]
- Getoff, N. Factors influencing the efficiency of radiation-induced degradation of water pollutants. Radiat. Phys. Chem. 2002, 65, 437–446. [Google Scholar] [CrossRef]
- Khataee, A.; Kasiri, M.B. Photocatalytic degradation of organic dyes in the presence of nanostructured titanium dioxide: Influence of the chemical structure of dyes. J. Mol. Catal. A Chem. 2010, 328, 8–26. [Google Scholar] [CrossRef]
- Zaleska, A.; Hupka, J.; Wiergowski, M.; Biziuk, M. Photocatalytic degradation of lindane, p,p’-DDT and methoxychlor in an aqueous environment. J. Photochem. Photobiol. A Chem. 2000, 135, 213–220. [Google Scholar] [CrossRef]
- Koltsakidou, A.; Antonopoulou, M.; Evgenidou, E.; Konstantinou, I.; Giannakas, A.; Papadaki, M.; Bikiaris, D.; Lambropoulou, D.A. Photocatalytical removal of fluorouracil using TiO2-P25 and N/S doped TiO2 catalysts: A kinetic and mechanistic study. Sci. Total Environ. 2017, 578, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Khandarkhaeva, M.; Batoeva, A.; Aseev, D.; Sizykh, M.; Tsydenova, O. Oxidation of atrazine in aqueous media by solar- enhanced Fenton-like process involving persulfate and ferrous ion. Ecotoxicol. Environ. Saf. 2017, 137, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Anipsitakis, G.P.; Dionysiou, D.D. Transition metal/UV-based advanced oxidation technologies for water decontamination. Appl. Catal. B Environ. 2004, 54, 155–163. [Google Scholar] [CrossRef]
- Kositzi, M.; Poulios, I.; Malato, S.; Caceres, J.; Campos, A. Campos, Solar photocatalytic treatment of synthetic municipal wastewater. Water Res. 2004, 38, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Saien, J.; Ojaghloo, Z.; Soleymani, A.; Rasoulifard, M. Homogeneous and heterogeneous AOPs for rapid degradation of Triton X-100 in aqueous media via UV light, nanotitania hydrogen peroxide and potassium persulfate. Chem. Eng. J. 2011, 167, 172–182. [Google Scholar] [CrossRef]
- Olmez-Hanci, T.; Dursun, D.; Aydin, E.; Arslan-Alaton, I.; Girit, B.; Mita, L.; Diano, N.; Mita, D.G.; Guida, M. S2O82−/UV-C and H2O2/UV-C treatment of Bisphenol A: Assessment of toxicity, estrogenic activity, degradation products and results in real water. Chemosphere 2015, 119, S115–S123. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, M.; Konstantinou, I. Effect of oxidants in the photocatalytic degradation of DEET under simulated solar irradiation in aqueous TiO2 suspensions. Glob. NEST J. 2014, 16, 507–515. [Google Scholar] [CrossRef]
- Vela, N.; Calín, M.; Yáñez-Gascón, M.J.; Garrido, I.; Pérez-Lucas, G.; Fenoll, J.; Navarro, S. Photocatalytic oxidation of six pesticides listed as endocrine disruptor chemicals from wastewater using two different TiO2 samples at pilot plant scale under sunlight irradiation. J. Photochem. Photobiol. A Chem. 2018, 353, 271–278. [Google Scholar] [CrossRef]
- Burrows, H.D.; Santaballa, J.; Steenken, S. Reaction pathways and mechanisms of photodegradation of pesticides. J. Photochem. Photobiol. B Biol. 2002, 67, 71–108. [Google Scholar] [CrossRef] [Green Version]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Antonaraki, S.; Triantis, T.; Papaconstantinou, E.; Hiskia, A. Photocatalytic degradation of lindane by polyoxometalates: Intermediates and mechanistic aspects. Catal. Today 2010, 151, 119–124. [Google Scholar] [CrossRef]
- Tan, C.; Gao, N.; Deng, Y.; Zhang, Y.; Sui, M.; Deng, J.; Zhou, S. Degradation of antipyrine by UV, UV/H2O2 and UV/PS. J. Hazard. Mater. 2013, 260, 1008–1016. [Google Scholar] [CrossRef]
- Kwon, M.; Kim, S.; Yoon, Y.; Jung, Y.; Hwang, T.-M.; Lee, J.; Kang, J.-W. Comparative evaluation of ibuprofen removal by UV/H2O2 and UV/S2O82− processes for wastewater treatment. Chem. Eng. J. 2015, 269, 379–390. [Google Scholar] [CrossRef]
- Bekkouche, S.; Merouani, S.; Hamdaoui, O.; Bouhelassa, M. Efficient photocatalytic degradation of Safranin O by integrating solar-UV/TiO2/persulfate treatment: Implication of sulfate radical in the oxidation process and effect of various water matrix components. J. Photochem. Photobiol. A Chem. 2017, 345, 80–91. [Google Scholar] [CrossRef]
- Wu, X.; Gu, X.; Lu, S.; Xu, M.; Zang, X.; Miao, Z.; Qiu, Z.; Sui, Q. Degradation of trichloroethylene in aqueous solution by persulfate activated with citric acid chelated ferrous ion. Chem. Eng. J. 2014, 255, 585–592. [Google Scholar] [CrossRef]
- Han, C.; Pelaez, M.; Likodimos, V.; Kontos, A.G.; Falaras, P.; O’Shea, K.; Dionysiou, D.D. Innovative visible light-activated sulfur doped TiO2 films for water treatment. Appl. Catal. B Environ. 2011, 107, 77–87. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Motti, C.A.; Glass, B.D.; Oelgemöller, M. TiO2 photocatalysis of naproxen: Effect of the water matrix, anions and diclofenac on degradation rates. Chemosphere 2015, 139, 579–588. [Google Scholar] [CrossRef]
- Liang, H.-C.; Li, X.-Z.; Yang, Y.-H.; Sze, K.-H. Effects of dissolved oxygen, pH, and anions on the 2, 3-dichlorophenol degradation by photocatalytic reaction with anodic TiO2 nanotube films. Chemosphere 2008, 73, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Simmons, M.S.; Zepp, R.G. Influence of humic substances on photolysis of nitroaromatic compounds in aqueous systems. Water Res. 1986, 20, 899–904. [Google Scholar] [CrossRef]
- Zhu, X.; Nanny, M.A.; Butler, E.C. Effect of inorganic anions on the titanium dioxide-based photocatalytic oxidation of aqueous ammonia and nitrite. J. Photochem. Photobiol. A Chem. 2007, 185, 289–294. [Google Scholar] [CrossRef]
- Niessner, R. Photocatalytic Atrazine Degradation by Synthetic Minerals, Atmospheric Aerosols, and Soil Particles. Environ. Sci. Technol. 2002, 36, 5342–5347. [Google Scholar] [CrossRef]
- Xia, X.-H.; Xu, J.-L.; Yun, Y. Effects of common inorganic anions on the rates of photocatalytic degradation of sodium dodecylbenzenesulfonate over illuminated titanium dioxide. J. Environ. Sci. 2002, 14, 188–194. [Google Scholar]
- Muruganandham, M.; Swaminathan, M. TiO2–UV photocatalytic oxidation of Reactive Yellow 14: Effect of operational parameters. J. Hazard. Mater. 2006, 135, 78–86. [Google Scholar] [CrossRef]
- Khan, S.; He, X.; Khan, H.M.; Boccelli, D.; Dionysiou, D.D. Efficient degradation of lindane in aqueous solution by iron (II) and/or UV activated peroxymonosulfate. J. Photochem. Photobiol. A Chem. 2016, 316, 37–43. [Google Scholar] [CrossRef]
- Huie, R.E.; Clifton, C.L. Temperature dependence of the rate constants for reactions of the sulfate radical, SO4●−, with anions. J. Phys. Chem. 1990, 94, 8561–8567. [Google Scholar] [CrossRef]
- Padmaja, S.; Neta, P.; Huie, R. Rate constants for some reactions of inorganic radicals with inorganic ions. Temperature and solvent dependence. Int. J. Chem. Kinet. 1993, 25, 445–455. [Google Scholar] [CrossRef]
- Westerhoff, P.; Mezyk, S.P.; Cooper, W.J.; Minakata, D. Electron pulse radiolysis determination of hydroxyl radical rate constants with Suwannee River fulvic acid and other dissolved organic matter isolates. Environ. Sci. Technol. 2007, 41, 4640–4646. [Google Scholar] [CrossRef]
- Gara, P.M.D.; Bosio, G.N.; Gonzalez, M.C.; Russo, N.; del Carmen Michelini, M.; Diez, R.P.; Mártire, D.O. A combined theoretical and experimental study on the oxidation of fulvic acid by the sulfate radical anion. Photochem. Photobiol. Sci. 2009, 8, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Epling, G.A.; Lin, C. Investigation of retardation effects on the titanium dioxide photodegradation system. Chemosphere 2002, 46, 937–944. [Google Scholar] [CrossRef]
- Van Doorslaer, X.; Dewulf, J.; De Maerschalk, J.; Van Langenhove, H.; Demeestere, K. Heterogeneous photocatalysis of moxifloxacin in hospital effluent: Effect of selected matrix constituents. Chem. Eng. J. 2015, 261, 9–16. [Google Scholar] [CrossRef]
- Pelaez, M.; Falaras, P.; Likodimos, V.; Kontos, A.G.; Armah, A.; O’shea, K.; Dionysiou, D.D. Synthesis, structural characterization and evaluation of sol–gel-based NF-TiO2 films with visible light-photoactivation for the removal of microcystin-LR. Appl. Catal. B Environ. 2010, 99, 378–387. [Google Scholar] [CrossRef]
- Hung, C.-H.; Yuan, C.; Li, H.-W. Photodegradation of diethyl phthalate with PANi/CNT/TiO2 immobilized on glass plate irradiated with visible light and simulated sunlight-effect of synthesized method and pH. J. Hazard. Mater. 2017, 322, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Huang, C.-F.; Mohanty, N.; Kurakalva, R.M. A rapid spectrophotometric determination of persulfate anion in ISCO. Chemosphere 2008, 73, 1540–1543. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.O.; Hochanadel, C.; Ghormley, J.; Davis, T. Decomposition of water and aqueous solutions under mixed fast neutron and γ-radiation. J. Phys. Chem. 1952, 56, 575–586. [Google Scholar] [CrossRef]
- Huang, Y.; Han, C.; Liu, Y.; Nadagouda, M.N.; Machala, L.; O’Shea, K.E.; Sharma, V.K.; Dionysiou, D.D. Degradation of atrazine by ZnxCu1−xFe2O4 nanomaterial-catalyzed sulfite under UV–vis light irradiation: Green strategy to generate SO4•−. Appl. Catal. B Environ. 2018, 221, 380–392. [Google Scholar] [CrossRef]
- Lepentsiotis, V.; van Eldik, R.; Prinsloo, F.F.; Pienaar, J.J. A kinetic study of the redox behaviour of Fe-III(TPPS) TPPS=5,10,15,20-tetrakis(p-sulfonato)porphyrinate in the presence of peroxymonosulfate, hydrogen peroxide, and sulfite/oxygen. Direct evidence for multiple redox cycling and suggested mechanisms. J. Chem. Soc.-Dalton Trans. 1999, 2759–2767. [Google Scholar] [CrossRef]


| Pesticides | kobs(h−1) | Reaction Type | Reference |
|---|---|---|---|
| Vinclozoline | 0.408 | a | [48] |
| Quinalphos | 0.426 | a | [48] |
| Malathion | 4.266 | a | [48] |
| Fenarimol | 0.168 | a | [48] |
| Fenitrothion | 0.300 | a | [48] |
| Dimethoate | 0.666 | a | [48] |
| Lambda-Cyhalothrin | 0.504 | b | [25] |
| Chlorpyrifos | 0.498 | b | [25] |
| Diazinon | 0.270 | b | [25] |
| Lindane | 0.550 | c | this study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.; Han, C.; Sayed, M.; Sohail, M.; Jan, S.; Sultana, S.; Khan, H.M.; Dionysiou, D.D. Exhaustive Photocatalytic Lindane Degradation by Combined Simulated Solar Light-Activated Nanocrystalline TiO2 and Inorganic Oxidants. Catalysts 2019, 9, 425. https://doi.org/10.3390/catal9050425
Khan S, Han C, Sayed M, Sohail M, Jan S, Sultana S, Khan HM, Dionysiou DD. Exhaustive Photocatalytic Lindane Degradation by Combined Simulated Solar Light-Activated Nanocrystalline TiO2 and Inorganic Oxidants. Catalysts. 2019; 9(5):425. https://doi.org/10.3390/catal9050425
Chicago/Turabian StyleKhan, Sanaullah, Changseok Han, Murtaza Sayed, Mohammad Sohail, Safeer Jan, Sabiha Sultana, Hasan M. Khan, and Dionysios D. Dionysiou. 2019. "Exhaustive Photocatalytic Lindane Degradation by Combined Simulated Solar Light-Activated Nanocrystalline TiO2 and Inorganic Oxidants" Catalysts 9, no. 5: 425. https://doi.org/10.3390/catal9050425





_Dionysiou.jpg)