Selective Hydrogenation of 3-Nitrostyrene over a Co-promoted Pt Catalyst Supported on P-containing Activated Charcoal
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalytic Performance
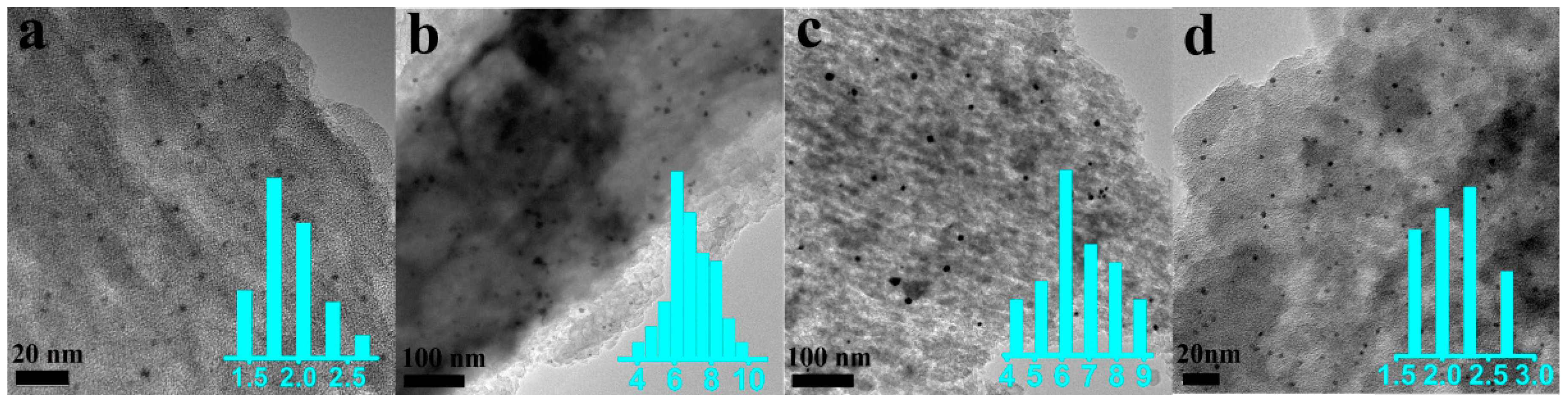
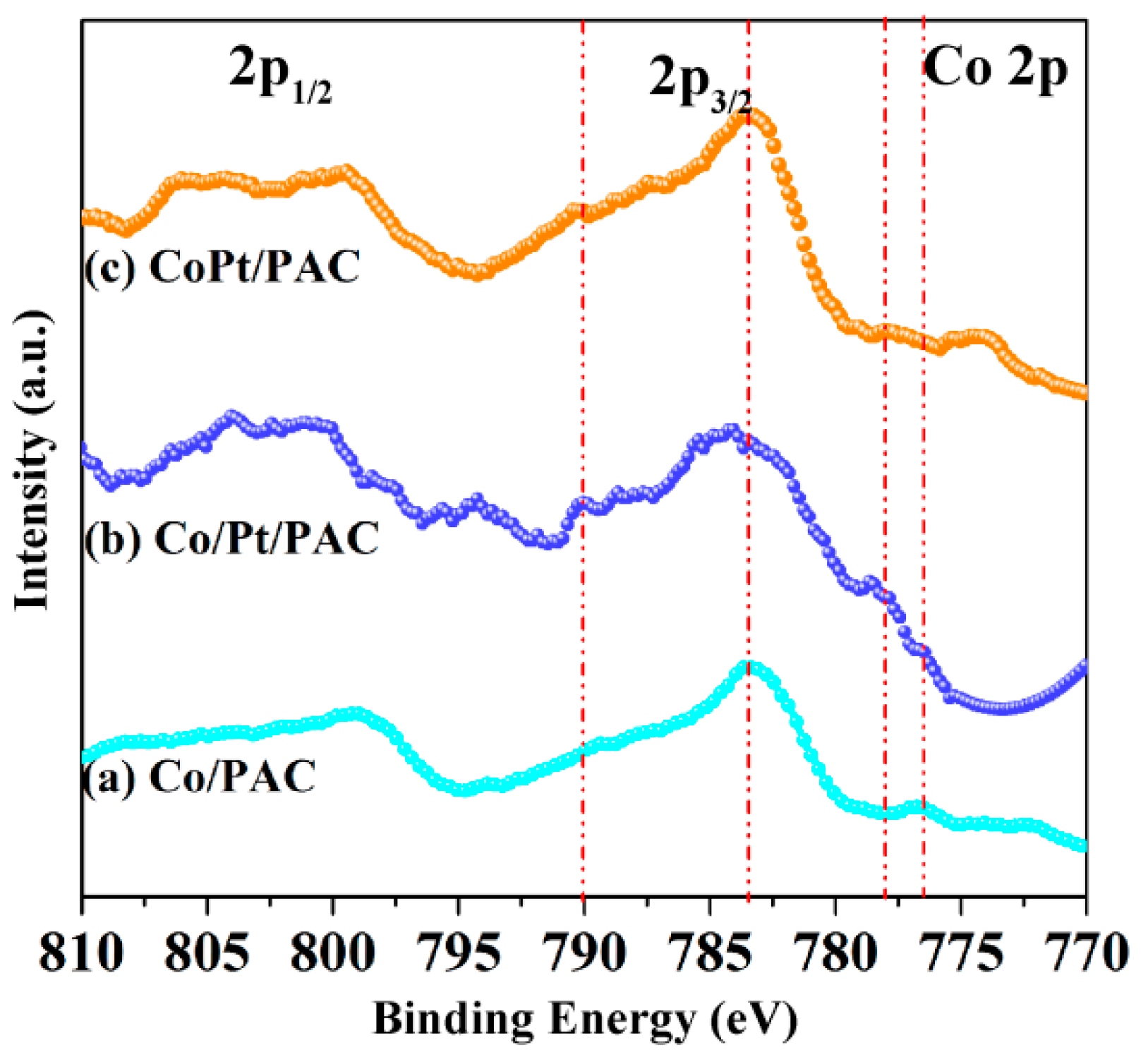
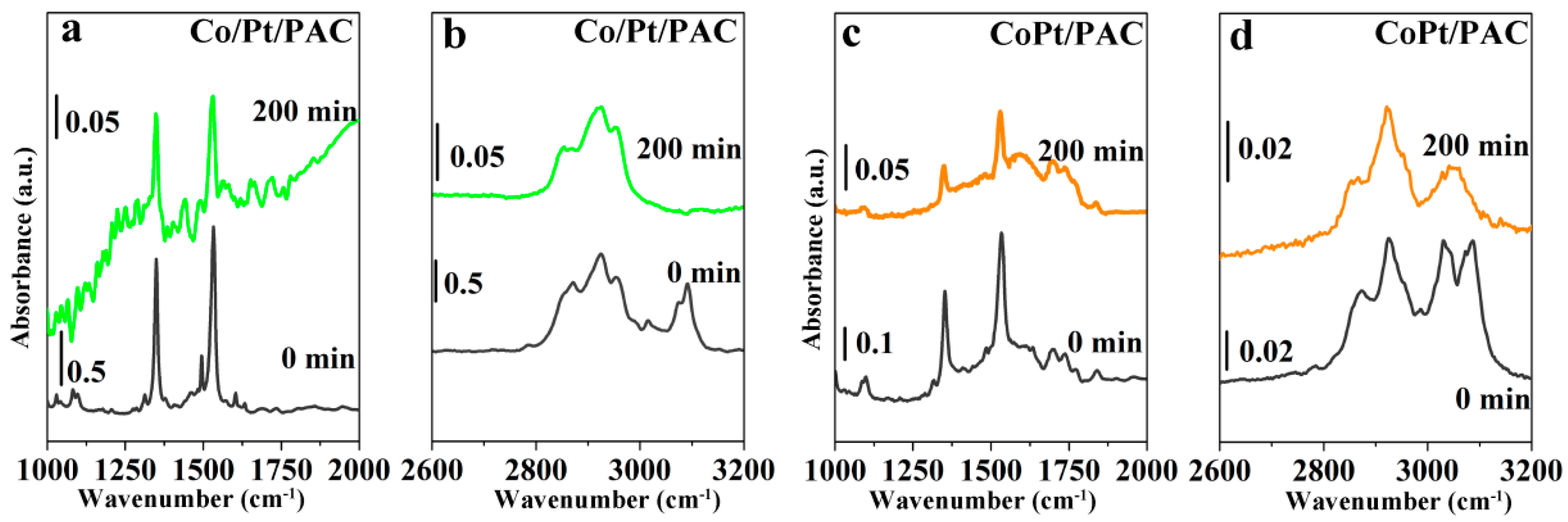
2.2. Catalyst Characterization
3. Roles of Co Additive and Surface P-containing Species
4. Experimental Section
4.1. Materials
4.2. Catalyst Preparation
4.3. Characterization
4.4. Hydrogenation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cao, Y.; Mao, S.; Li, M.; Chen, Y.; Wang, Y. Metal/porous carbon composites for heterogeneous catalysis: Old catalysts with improved performance promoted by N-doping. ACS Catal. 2017, 7, 8090–8112. [Google Scholar] [CrossRef]
- Lin, R.; Albani, D.; Fako, E.; Kaiser, S.K.; Safonova, O.V.; López, N.; Pérez-Ramírez, J. Design of single gold atoms on nitrogen-doped carbon for molecular recognition in alkyne semi-hydrogenation. Angew. Chem. Int. Ed. 2019, 58, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tu, D.; Li, Y.; Wang, W.; Yu, Q.; Yang, J.; Lu, J. Co-N-doped carbon nanotubes supported on diatomite for highly efficient catalysis oxidative carbonylation of amines with CO and air. Appl. Catal. A Gen. 2018, 549, 112–116. [Google Scholar] [CrossRef]
- Hu, X.; Long, Y.; Fan, M.; Yuan, M.; Zhao, H.; Ma, J.; Dong, Z. Two-dimensional covalent organic frameworks as self-template derived nitrogen-doped carbon nanosheets for eco-friendly metal-free catalysis. Appl. Catal. B Environ. 2019, 244, 25–35. [Google Scholar] [CrossRef]
- Ruiz-García, C.; Heras, F.; Calvo, L.; Alonso-Morales, N.; Rodriguez, J.J.; Gilarranz, M.A. Platinum and N-doped carbon nanostructures as catalysts in hydrodechlorination reactions. Appl. Catal. B Environ. 2018, 238, 609–617. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, H.; Chen, D.; Chen, C.; Ciucci, F. In situ synthesis of mesoporous manganese oxide/sulfur-doped graphitized carbon as a bifunctional catalyst for oxygen evolution/reduction reactions. Carbon 2015, 94, 1028–1036. [Google Scholar] [CrossRef]
- Yang, S.; Peng, L.; Oveisi, E.; Bulut, S.; Sun, D.T.; Asgari, M.; Trukhina, O.; Queen, W.L. MOF-derived cobalt phosphide/carbon nanocubes for selective hydrogenation of nitroarenes to anilines. Chem. Eur. J. 2018, 24, 4234–4238. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, A.; Sankaranarayanan, T.M.; Gómez, G.; Moreno, I.; Coronado, J.M.; Pizarro, P.; Serrano, D.P. Evaluation of transition metal phosphides supported on ordered mesoporous materials as catalysts for phenol hydrodeoxygenation. Green Chem. 2016, 18, 1938–1951. [Google Scholar] [CrossRef]
- Sweeny, N.P.; Rohrer, C.S.; Brown, O.W. Dinickel phosphide as a heterogeneous catalyst for the vapor phase reduction of nitrobenzene with hydrogen to aniline and water. J. Am. Chem. Soc. 1958, 80, 799–800. [Google Scholar] [CrossRef]
- Zhang, P.; Gong, Y.; Li, H.; Chen, Z.; Wang, Y. Solvent-free aerobic oxidation of hydrocarbons and alcohols with Pd@N-doped carbon from glucose. Nat. Commun. 2013, 4, 1593. [Google Scholar] [CrossRef] [Green Version]
- Chao, S.; Zou, F.; Wan, F.; Dong, X.; Wang, Y.; Wang, Y.; Guan, Q.; Wang, G.; Li, W. Nitrogen-doped carbon derived from ZIF-8 as a high-performance metal-free catalyst for acetylene hydrochlorination. Sci. Rep. 2017, 7, 39789. [Google Scholar] [CrossRef]
- Duan, X.; O’Donnell, K.; Sun, H.; Wang, Y.; Wang, S. Sulfur and nitrogen Co-doped graphene for metal-free catalytic oxidation reactions. Small 2015, 11, 3036–3044. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhang, B.; Lin, Y.; Wang, Q.; Zhang, Q.; Su, D.S. Enhanced chemoselective hydrogenation through tuning the interaction between Pt nanoparticles and carbon supports: Insights from identical location transmission electron microscopy and X-ray photoelectron spectroscopy. ACS Catal. 2016, 7844–7854. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, N.; Geng, L.; Fu, J.; Hu, H.; Zhang, D.; Zhu, B.; Carozza, J.; Han, H. Facile synthesis of ultrafine cobalt oxides embedded into N-doped carbon with superior activity in hydrogenation of 4-nitrophenol. J. Colloid Interface Sci. 2018, 512, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yu, G.; Yang, J.; Yuan, M.; Xu, D.; Dong, Z. Biowaste soybean curd residue-derived Pd/nitrogen-doped porous carbon with excellent catalytic performance for phenol hydrogenation. J. Colloid Interface Sci. 2019, 533, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Fiorio, J.L.; Gonçalves, R.V.; Teixeira-Neto, E.; López, N.; Rossi, L.M. Accessing frustrated lewis pair chemistry through robust gold@N-doped carbon for selective hydrogenation of alkynes. ACS Catal. 2018, 8, 3516–3524. [Google Scholar] [CrossRef]
- Pisduangdaw, S.; Mekasuwandumrong, O.; Fujita, S.I.; Arai, M.; Yoshida, H.; Panpranot, J. One step synthesis of Pt–Co/TiO2 catalysts by flame spray pyrolysis for the hydrogenation of 3-nitrostyrene. Catal. Commun. 2015, 61, 11–15. [Google Scholar] [CrossRef]
- Chu, K.; Wang, F.; Tian, Y.; Wei, Z. Phosphorus doped and defects engineered graphene for improved electrochemical sensing: Synergistic effect of dopants and defects. Electrochim. Acta 2017, 231, 557–564. [Google Scholar] [CrossRef]
- Ayusheev, A.B.; Taran, O.P.; Seryak, I.A.; Podyacheva, O.Y.; Descorme, C.; Besson, M.; Kibis, L.S.; Boronin, A.I.; Romanenko, A.I.; Ismagilov, Z.R.; et al. Ruthenium nanoparticles supported on nitrogen-doped carbon nanofibers for the catalytic wet air oxidation of phenol. Appl. Catal. B Environ. 2014, 146, 177–185. [Google Scholar] [CrossRef]
- Zhao, L. Natural phosphorus-doped honeycomb carbon materials as oxygen reduction catalysts derived from pulsatilla chinensis (bunge) regel. RSC Adv. 2017, 7, 13904–13910. [Google Scholar] [CrossRef]
- Lu, C.; Wang, M.; Feng, Z.; Qi, Y.; Feng, F.; Ma, L.; Zhang, Q.; Li, X. A phosphorus–carbon framework over activated carbon supported palladium nanoparticles for the chemoselective hydrogenation of para-chloronitrobenzene. Catal. Sci. Technol. 2017, 7, 1581–1589. [Google Scholar] [CrossRef]
- Hita, I.; Cordero-Lanzac, T.; Gallardo, A.; Arandes, J.M.; Rodríguez-Mirasol, J.; Bilbao, J.; Cordero, T.; Castaño, P. Phosphorus-containing activated carbon as acid support in a bifunctional Pt–Pd catalyst for tire oil hydrocracking. Catal. Commun. 2016, 78, 48–51. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, Q.; Zhang, R.; Wang, Q.; Kang, G.; Peng, F. Phosphorus-doped carbon nanotubes supported low Pt loading catalyst for the oxygen reduction reaction in acidic fuel cells. J. Power Sources 2014, 268, 171–175. [Google Scholar] [CrossRef]
- Wang, B.; Yu, L.; Zhang, J.; Pu, Y.; Zhang, H.; Li, W. Phosphorus-doped carbon supports enhance gold-based catalysts for acetylene hydrochlorination. RSC Adv. 2014, 4, 15877. [Google Scholar] [CrossRef]
- Zhuang, M.; Ou, X.; Dou, Y.; Zhang, L.; Zhang, Q.; Wu, R.; Ding, Y.; Shao, M.; Luo, Z. Polymer-embedded fabrication of Co2P nanoparticles encapsulated in N,P-doped graphene for hydrogen generation. Nano Lett. 2016, 16, 4691–4698. [Google Scholar] [CrossRef]
- Song, P.; Zhu, L.; Bo, X.; Wang, A.; Wang, G.; Guo, L. Pt nanoparticles incorporated into phosphorus-doped ordered mesoporous carbons: Enhanced catalytic activity for methanol electrooxidation. Electrochim. Acta 2014, 127, 307–314. [Google Scholar] [CrossRef]
- Xue, X.; Ge, J.; Liu, C.; Xing, W.; Lu, T. Novel chemical synthesis of Pt–Ru–P electrocatalysts by hypophosphite deposition for enhanced methanol oxidation and CO tolerance in direct methanol fuel cell. Electrochem. Commun. 2006, 8, 1280–1286. [Google Scholar] [CrossRef]
- Xue, X.; Ge, J.; Tian, T.; Liu, C.; Xing, W.; Lu, T. Enhancement of the electrooxidation of ethanol on Pt–Sn–P/C catalysts prepared by chemical deposition process. J. Power Sources 2007, 172, 560–569. [Google Scholar] [CrossRef]
- Tong, Y.C.; Zhang, X.Y.; Wang, Q.Y.; Xu, X.J. The adsorption mechanism of platinum on phosphorus-doped single walled carbon nanotube. Comput. Theor. Chem. 2015, 1059, 1–6. [Google Scholar] [CrossRef]
- Paradies, J. Metal-free hydrogenation of unsaturated hydrocarbons employing molecular hydrogen. Angew. Chem. Int. Ed. 2014, 53, 3552–3557. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Zhang, J.; Liu, X.; Wang, H.; Zhang, W.; Yang, Q.; Ma, J.; Dong, X.; Yoo, S.J.; et al. Selective hydrogenation of CO2 to ethanol over cobalt catalysts. Angew. Chem. Int. Ed. 2018, 57, 6104–6108. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Zhang, J.; Wang, H.; Lewis, J.P.; Xiao, F. Product selectivity controlled by zeolite crystals in biomass hydrogenation over a palladium catalyst. J. Am. Chem. Soc. 2016, 138, 7880–7883. [Google Scholar] [CrossRef]
- Albani, D.; Shahrokhi, M.; Chen, Z.; Mitchell, S.; Hauert, R.; López, N.; Pérez-Ramírez, J. Selective ensembles in supported palladium sulfide nanoparticles for alkyne semi-hydrogenation. Nat. Commun. 2018, 9, 2634. [Google Scholar] [CrossRef] [PubMed]
- Durndell, L.J.; Parlett, C.M.A.; Hondow, N.S.; Isaacs, M.A.; Wilson, K.; Lee, A.F. Selectivity control in Pt-catalyzed cinnamaldehyde hydrogenation. Sci. Rep. 2015, 5, 9425. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo-Carrillo, J.; Sebti, J.; Marinas, A.; Marinas, J.M.; Sebti, S.; Urbano, F.J. XPS evidence for structure–performance relationship in selective hydrogenation of crotonaldehyde to crotyl alcohol on platinum systems supported on natural phosphates. J. Colloid Interface Sci. 2012, 382, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Bornschein, C.; Werkmeister, S.; Wendt, B.; Jiao, H.; Alberico, E.; Baumann, W.; Junge, H.; Junge, K.; Beller, M. Mild and selective hydrogenation of aromatic and aliphatic (di)nitriles with a well-defined iron pincer complex. Nat. Commun. 2014, 5, 4111. [Google Scholar] [CrossRef]
- Lu, X.; He, J.; Jing, R.; Tao, P.; Nie, R.; Zhou, D.; Xia, Q. Microwave-activated Ni/carbon catalysts for highly selective hydrogenation of nitrobenzene to cyclohexylamine. Sci. Rep. 2017, 7, 2676. [Google Scholar] [CrossRef]
- Chatterjee, M.; Chatterjee, A.; Kawanami, H.; Ishizaka, T.; Suzuki, T.; Suzuki, A. Rapid hydrogenation of aromatic nitro compounds in supercritical carbon dioxide: Mechanistic implications via experimental and theoretical investigations. Adv. Synth. Catal. 2012, 354, 2009–2018. [Google Scholar] [CrossRef]
- Wang, K.; Gao, W.; Jiang, P.; Lan, K.; Yang, M.; Huang, X.; Ma, L.; Niu, F.; Li, R. Bi-functional catalyst of porous N-doped carbon with bimetallic FeCu for solvent-free resultant imines and hydrogenation of nitroarenes. Mole. Catal. 2019, 465, 43–53. [Google Scholar] [CrossRef]
- Budi, C.S.; Saikia, D.; Chen, C.-S.; Kao, H.-M. Catalytic evaluation of tunable Ni nanoparticles embedded in organic functionalized 2D and 3D ordered mesoporous silicas from the hydrogenation of nitroarenes. J. Catal. 2019, 370, 274–288. [Google Scholar] [CrossRef]
- Sreedhar, B.; Devi, D.K.; Yada, D. Selective hydrogenation of nitroarenes using gum acacia supported Pt colloid an effective reusable catalyst in aqueous medium. Catal. Commun. 2011, 12, 1009–1014. [Google Scholar] [CrossRef]
- Veerakumar, P.; Thanasekaran, P.; Lin, K.-C.; Liu, S.-B. Well-dispersed rhenium nanoparticles on three-dimensional carbon nanostructures: Efficient catalysts for the reduction of aromatic nitro compounds. J. Colloid Interface Sci. 2017, 506, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, Y.; Liu, Y.; Park, J.; Liang, X. Atomic layer deposited Pt-Co bimetallic catalysts for selective hydrogenation of α, β-unsaturated aldehydes to unsaturated alcohols. J. Catal. 2018, 366, 61–69. [Google Scholar] [CrossRef]
- Han, X.; Zhou, R.; Yue, B.; Zheng, X. Selective hydrogenation of cinnamaldehyde over Pt/ZrO2 catalyst modified by Cr, Mn, Fe, Co and Ni. Catal. Lett. 2006, 109, 157–161. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, P.-F.; Zhou, R.-X. Selective hydrogenation of cinnamaldehyde to cinnamyl alcohol with carbon nanotubes supported Pt-Co catalysts. Appl. Surf. Sci. 2008, 254, 2609–2614. [Google Scholar] [CrossRef]
- Tian, Z.; Li, Q.; Hou, J.; Pei, L.; Li, Y.; Ai, S. Platinum nanocrystals supported on CoAl mixed metal oxide nanosheets derived from layered double hydroxides as catalysts for selective hydrogenation of cinnamaldehyde. J. Catal. 2015, 331, 193–202. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, C.; Li, Q.; Hou, J.; Li, Y.; Ai, S. Nitrogen- and oxygen-functionalized carbon nanotubes supported Pt-based catalyst for the selective hydrogenation of cinnamaldehyde. Appl. Catal. A Gen. 2015, 506, 134–142. [Google Scholar] [CrossRef]
- Chen, T.; Rodionov, V.O. Controllable catalysis with nanoparticles: Bimetallic alloy systems and surface adsorbates. ACS Catal. 2016, 6, 4025–4033. [Google Scholar] [CrossRef]
- Wang, H.; Bai, S.; Pi, Y.; Shao, Q.; Tan, Y.; Huang, X. A strongly coupled ultrasmall Pt3Co nanoparticle-ultrathin Co(OH)2 nanosheet architecture enhances selective hydrogenation of α,β-unsaturated aldehydes. ACS Catal. 2019, 9, 154–159. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, B.; Zhang, C.; Meng, X.; Su, X.; Jiang, S.; Shi, R.; Li, Y.; Lin, W.; Arai, M.; et al. Significance of surface oxygen-containing groups and heteroatom P species in switching the selectivity of Pt/C catalyst in hydrogenation of 3-nitrostyrene. J. Catal. 2018, 364, 297–307. [Google Scholar] [CrossRef]
- Lu, X.H.; Shen, Y.; He, J.; Jing, R.; Tao, P.P.; Hu, A.; Nie, R.F.; Zhou, D.; Xia, Q.H. Selective hydrogenation of benzoic acid to cyclohexane carboxylic acid over microwave-activated Ni/carbon catalysts. Mol. Catal. 2018, 444, 53–61. [Google Scholar] [CrossRef]
- Musci, J.J.; Merlo, A.B.; Casella, M.L. Aqueous phase hydrogenation of furfural using carbon-supported Ru and RuSn catalysts. Catal. Today 2017, 296, 43–50. [Google Scholar] [CrossRef]
- Chang, J.; Xiao, Y.; Xiao, M.; Ge, J.; Liu, C.; Xing, W. Surface oxidized cobalt-phosphide nanorods as an advanced oxygen evolution catalyst in alkaline solution. ACS Catal. 2015, 5, 6874–6878. [Google Scholar] [CrossRef]
- Prins, R.; Bussell, M.E. Metal phosphides: Preparation, characterization and catalytic reactivity. Catal. Lett. 2012, 142, 1413–1436. [Google Scholar] [CrossRef]
- Infantes-Molina, A.; Cecilia, J.A.; Pawelec, B.; Fierro, J.L.G.; Rodríguez-Castellón, E.; Jiménez-López, A. Ni2P and CoP catalysts prepared from phosphite-type precursors for HDS–HDN competitive reactions. Appl. Catal. A Gen. 2010, 390, 253–263. [Google Scholar] [CrossRef]
- Sennu, P.; Kim, H.S.; An, J.Y.; Aravindan, V.; Lee, Y.S. Synthesis of 2D/2D structured mesoporous Co3O4 nanosheet/N-doped reduced graphene oxide composites as a highly stable negative electrode for lithium battery applications. Chem. Asian J. 2015, 10, 1776–1783. [Google Scholar] [CrossRef]
- Hasegawa, G.; Deguchi, T.; Kanamori, K.; Kobayashi, Y.; Kageyama, H.; Abe, T.; Nakanishi, K. High-level doping of nitrogen, phosphorus, and sulfur into activated carbon monoliths and their electrochemical capacitances. Chem. Mater. 2015, 27, 4703–4712. [Google Scholar] [CrossRef]
- Patel, M.A.; Luo, F.; Khoshi, M.R.; Rabie, E.; Zhang, Q.; Flach, C.R.; Mendelsohn, R.; Garfunkel, E.; Szostak, M.; He, H. P-doped porous carbon as metal free catalysts for selective aerobic oxidation with an unexpected mechanism. ACS Nano 2016, 10, 2305–2315. [Google Scholar] [CrossRef]
- Shimizu, K.-i.; Miyamoto, Y.; Kawasaki, T.; Tanji, T.; Tai, Y.; Satsuma, A. Chemoselective hydrogenation of nitroaromatics by supported gold catalysts: Mechanistic reasons of size- and support-dependent activity and selectivity. J. Phys. Chem. C 2009, 113, 17803–17810. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, X.Y.; Zhang, L.; Wang, A.; Li, L.; Pan, X.; Miao, S.; Haruta, M.; Wei, H.; Wang, H.; et al. ZnAl-hydrotalcite-supported Au25 nanoclusters as precatalysts for chemoselective hydrogenation of 3-nitrostyrene. Angew. Chem. Int. Ed. 2017, 56, 2709–2713. [Google Scholar] [CrossRef]
- Shimizu, K.-i.; Miyamoto, Y.; Satsuma, A. Size- and support-dependent silver cluster catalysis for chemoselective hydrogenation of nitroaromatics. J. Catal. 2010, 270, 86–94. [Google Scholar] [CrossRef]
- Yoshida, H.; Kato, K.; Wang, J.; Meng, X.; Narisawa, S.; Fujita, S.I.; Wu, Z.; Zhao, F.; Arai, M. Hydrogenation of nitrostyrene with a Pt/TiO2 catalyst in CO2-dissolved expanded polar and nonpolar organic liquids: Their macroscopic and microscopic features. J. Phys. Chem. C 2011, 115, 2257–2267. [Google Scholar] [CrossRef]
- Minejima, C.; Ebata, T.; Mikami, N. C-H stretching vibrations of benzene and toluene in their S1 states observed by double resonance vibrational spectroscopy in supersonic jets. PCCP 2002, 4, 1537–1541. [Google Scholar] [CrossRef]
- Supriya, P.; Srinivas, B.T.V.; Chowdeswari, K.; Naidu, N.V.S.; Sreedhar, B. Biomimetic synthesis of gum acacia mediated Pd-ZnO and Pd-TiO2–promising nanocatalysts for selective hydrogenation of nitroarenes. Mater. Chem. Phys. 2018, 204, 27–36. [Google Scholar] [CrossRef]
- Nan, J.; Yu, H.B.; Xue, Q.S.; Li, Y.D. In situ FT-IR study on hydrogenation of olefin and aromatic on Pd-based metal catalysts. Chem. Eng. Oil Gas 2009, 38, 183–188. [Google Scholar]
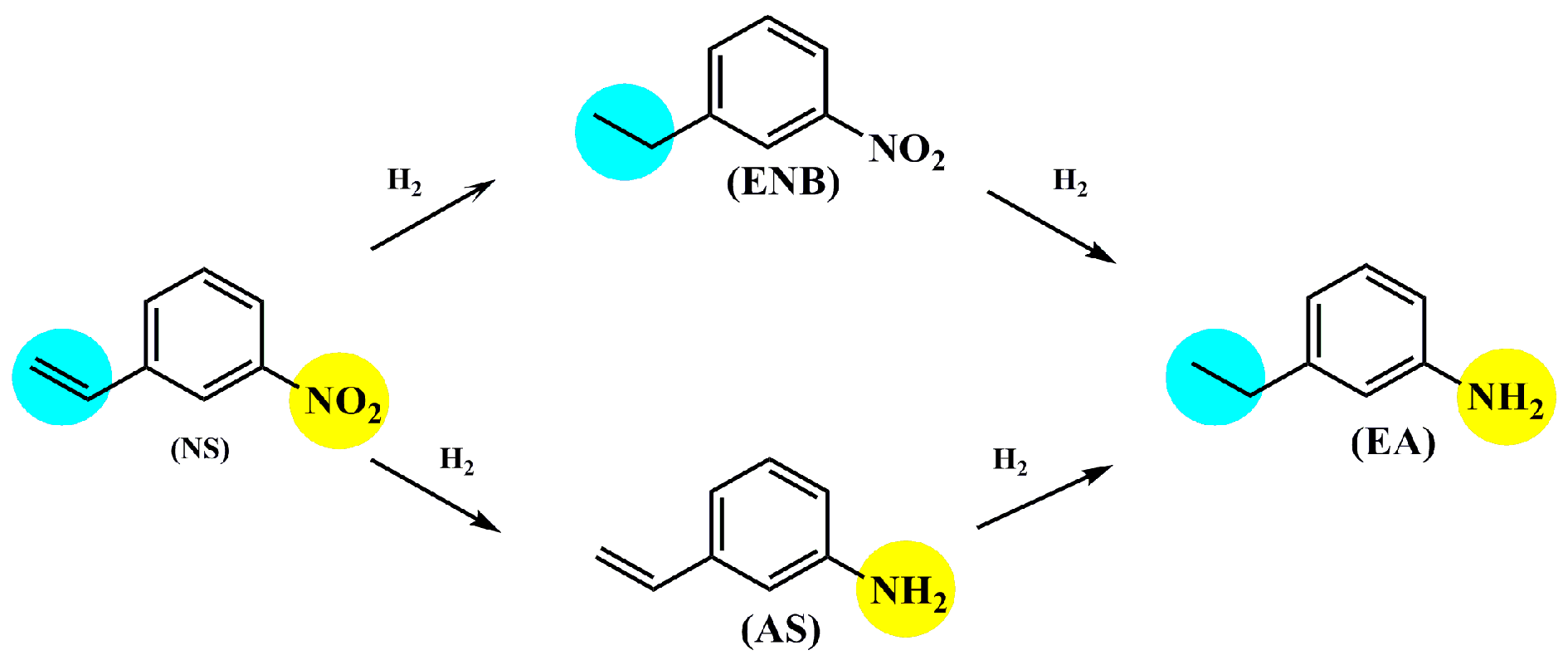




| Entry | Catalysts | Time (h) | Conversion (%) | Selectivity (%) | |||
|---|---|---|---|---|---|---|---|
| AS | ENB | EA | Others a | ||||
| 1 | Co/PAC | 6 | 0 | 0 | 0 | 0 | 0 |
| 2 | Pt/PAC | 8 | 94 | 81 | 2 | 15 | 2 |
| 3 | Co/Pt/PAC | 10 | 84 | 94 | 0 | 2 | 4 |
| 4 | Co/Pt/PAC | 14 | 97 | 94 | 1 | 2 | 3 |
| 5 | Co/Pt/AC | 1 | 95 | 15 | 24 | 46 | 15 |
| 6 | CoPt/PAC | 1 | 88 | 12 | 35 | 40 | 13 |
| Entry | Content of Co (wt.-%) | Co/Pt | Time (h) | Conversion (%) | Selectivity (%) | Average Reaction Rate (mmol·g−1·h−1) b | |||
|---|---|---|---|---|---|---|---|---|---|
| AS | ENB | EA | Others a | ||||||
| 1 | 0 | 0 | 8 | 94 | 81 | 2 | 15 | 2 | 1.5 |
| 2 | 0.075 | 0.5 | 16 | 81 | 95 | 0 | 1 | 4 | 0.7 |
| 3 | 0.15 | 1 | 10 | 84 | 94 | 0 | 2 | 4 | 1.1 |
| 4 | 0.30 | 2 | 8 | 81 | 92 | 0 | 4 | 4 | 1.5 |
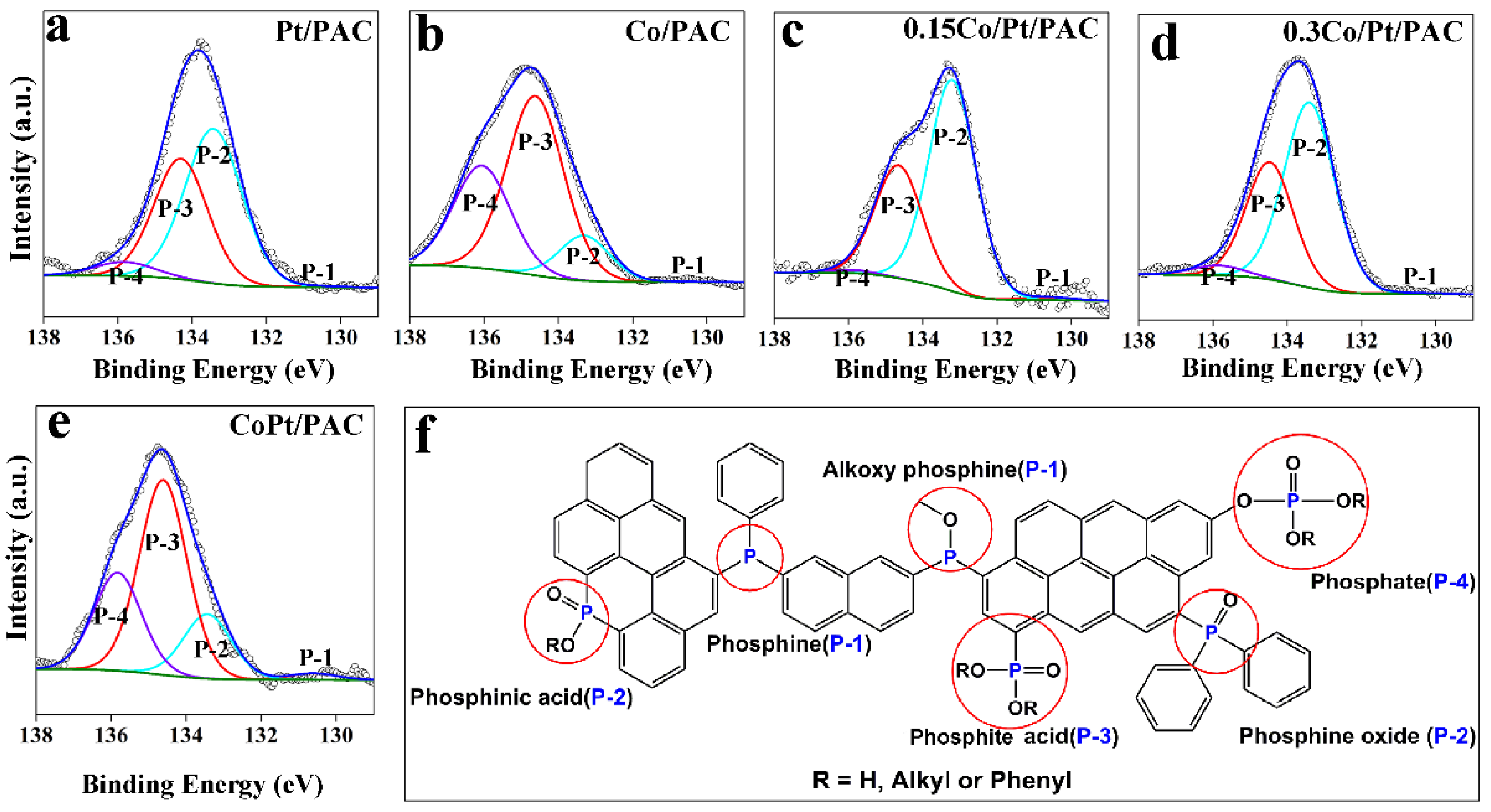
| Entry | Catalysts a | Co/Pt Molar Ratio | Content of P-containing Functional Groups (%) | |||
|---|---|---|---|---|---|---|
| Phosphine (P-1) | Phosphinic (P-2) | Phosphite (P-3) | Phosphate (P-4) | |||
| 1 | Pt/PAC | 0 | 0.3 | 54.4 | 40.1 | 5.2 |
| 2 | 0.15Co/PAC | ∞ | 0.1 | 12.4 | 55.6 | 31.9 |
| 3 | 0.15Co/Pt/PAC | 1 | 0.5 | 64.3 | 34.3 | 0.9 |
| 4 | 0.30Co/Pt/PAC | 2 | 0.7 | 60.2 | 35.8 | 3.3 |
| 5 | 0.15CoPt/PAC | 1 | 0.2 | 18.0 | 54.4 | 27.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Zhang, C.; Lin, W.; Cheng, H.; Arai, M.; Zhao, F. Selective Hydrogenation of 3-Nitrostyrene over a Co-promoted Pt Catalyst Supported on P-containing Activated Charcoal. Catalysts 2019, 9, 428. https://doi.org/10.3390/catal9050428
Wu Q, Zhang C, Lin W, Cheng H, Arai M, Zhao F. Selective Hydrogenation of 3-Nitrostyrene over a Co-promoted Pt Catalyst Supported on P-containing Activated Charcoal. Catalysts. 2019; 9(5):428. https://doi.org/10.3390/catal9050428
Chicago/Turabian StyleWu, Qifan, Chao Zhang, Weiwei Lin, Haiyang Cheng, Masahiko Arai, and Fengyu Zhao. 2019. "Selective Hydrogenation of 3-Nitrostyrene over a Co-promoted Pt Catalyst Supported on P-containing Activated Charcoal" Catalysts 9, no. 5: 428. https://doi.org/10.3390/catal9050428