Development of Titanium Dioxide-Supported Pd Catalysts for Ligand-Free Suzuki–Miyaura Coupling of Aryl Chlorides
Abstract
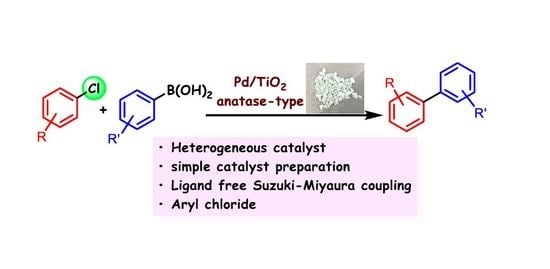
:1. Introduction
2. Results and Discussion



3. Materials and Methods
3.1. General
3.2. Experimentals
3.2.1. Preparation of 5% Pd/TiO2 (Cat. D, anatase-type)
3.2.2. Preparation of 5% Pd(red)/TiO2 (Cat. A, Anatase-Type)
3.2.3. Typical Procedure for the Coupling Reaction between Aryl Chlorides and Arylboronic Acids
3.2.4. Procedure for Reuse Test of Recovered 5% Pd/TiO2 (Cat. D, anatase-type) (Equation (2))
3.2.5. Procedure for the Confirmation Experiment of Pd Leaching in the Reaction Medium and Pd Contamination of the Coupling Product after Purification Processes (Equatiopn (3))
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Hassan, J.; Sèvignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl-Aryl Bond Formation One Century after the Discovery of the Ullmann Reaction. Chem. Rev. 2002, 102, 1359–1470. [Google Scholar] [PubMed]
- Ackermann, L. Modern Arylation Methods; Wiley: Weinheim, Germany, 2009. [Google Scholar]
- Bringmann, G.; Gulder, T.; Gulder, T.A.M.; Breuning, M. Atroposelective Total Synthesis of Axially Chiral Biaryl Natural Products. Chem. Rev. 2011, 111, 563–639. [Google Scholar] [CrossRef]
- Wu, J.-S.; Cheng, S.-W.; Cheng, Y.-J.; Hsu, C.-S. Donor–acceptor conjugated polymers based on multifused ladder-type arenes for organic solar cells. Chem. Soc. Rev. 2015, 44, 1113–1154. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lan, J.; You, J. Oxidative C−H/C−H Coupling Reactions between Two (Hetero)arenes. Chem. Rev. 2017, 117, 8787–8863. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Liebscher, J. Carbon-Carbon Coupling Reactions Catalyzed by Heterogeneous Palladium Catalysts. Chem. Rev. 2007, 107, 133–173. [Google Scholar] [CrossRef] [PubMed]
- Fihri, A.; Bouhrara, M.; Nekoueishahraki, B.; Basset, J.-M.; Polshettiwar, V. Nanocatalysts for Suzuki cross-coupling reactions. Chem. Soc. Rev. 2011, 40, 5181–5203. [Google Scholar] [CrossRef]
- Monguchi, Y.; Fujita, Y.; Hashimoto, S.; Ina, M.; Takahashi, T.; Ito, R.; Nozaki, K.; Maegawa, T.; Sajiki, H. Palladium on carbon-catalyzed solvent-free and solid-phase hydrogenation and Suzuki–Miyaura reaction. Tetrahedron 2011, 67, 8628–8634. [Google Scholar]
- Kitamura, Y.; Sako, S.; Udzu, T.; Tsutsui, A.; Maegawa, T.; Monguchi, Y.; Sajiki, H. Ligand-free Pd/C-catalyzed Suzuki–Miyaura coupling reaction for the synthesis of heterobiaryl derivatives. Chem. Commun. 2007, 5069–5071. [Google Scholar] [CrossRef] [PubMed]
- Maegawa, T.; Kitamura, Y.; Sako, S.; Udzu, T.; Sakurai, A.; Tanaka, A.; Kobayashi, Y.; Endo, K.; Bora, U.; Kurita, T.; et al. Heterogeneous Pd/C-Catalyzed Ligand-Free, Room-Temperature Suzuki–Miyaura Coupling Reactions in Aqueous Media. Chem. Eur. J. 2007, 13, 5937–5943. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Sakurai, A.; Udzu, T.; Maegawa, T.; Monguchi, Y.; Sajiki, H. Heterogeneous Pd/C-catalyzed ligand-free Suzuki–Miyaura coupling reaction using aryl boronic esters. Tetrahedron 2007, 6, 10596–10602. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Asl, M.S.; Mazinani, B. Modulated large-pore mesoporous silica as an efficient base catalyst for the Henry reaction. Res. Chem. Intermed. 2018, 44, 1617–1626. [Google Scholar] [CrossRef]
- Shokouhimehr, M. Magnetically Separable and Sustainable Nanostructured Catalysts for Heterogeneous Reduction of Nitroaromatics. Catalysts 2015, 5, 534–560. [Google Scholar] [CrossRef]
- Zhang, K.; Hong, K.; Suh, J.M.; Lee, T.H.; Kwon, O.; Shokouhimehr, M.; Jang, H.W. Facile synthesis of monodispersed Pd nanocatalysts decorated on graphene oxide for reduction of nitroaromatics in aqueous solution. Res. Chem. Intermediat. 2019, 45, 599–611. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Hong, K.; Lee, T.H.; Moon, C.W.; Hong, S.P.; Zhang, K.; Suh, J.M.; Choi, K.S.; Varma, R.S.; Jang, H.W. Magnetically retrievable nanocomposite adorned with Pd nanocatalysts: Efficient reduction of nitroaromatics in aqueous media. Green Chem. 2018, 20, 3809–3817. [Google Scholar] [CrossRef]
- Choi, K.-H.; Shokouhimehr, M.; Sung, Y.-E. Heterogeneous Suzuki Cross-Coupling Reaction Catalyzed by Magnetically Recyclable Nanocatalyst. Bull. Korean Chem. Soc. 2013, 34, 1477–1480. [Google Scholar] [CrossRef]
- Kim, A.; Rafiaei, S.M.; Abolhosseini, S.; Shokouhimehr, M. Palladium Nanocatalysts Confined in Mesoporous Silica for Heterogeneous Reduction of Nitroaromatics. Energy Environ. Focus 2015, 4, 18–23. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Lee, J.E.; Han, S.I.; Hyeon, T. Magnetically recyclable hollow nanocomposite catalysts for heterogeneous reduction of nitroarenes and Suzuki reactions. Chem. Commun. 2013, 49, 4779–4781. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Kim, T.; Jun, S.W.; Shin, K.; Jang, Y.; Kim, B.H.; Kim, J.; Hyeon, T. Magnetically separable carbon nanocomposite catalysts for efficient nitroarene reduction and Suzuki reactions. Appl. Catal. A General 2014, 476, 133–139. [Google Scholar] [CrossRef]
- Li, G.; Yang, H.; Li, W.; Zhang, G. Rationally designed palladium complexes on a bulky N-heterocyclic carbene-functionalized organosilica: An efficient solid catalyst for the Suzuki–Miyaura coupling of challenging aryl chlorides. Green Chem. 2011, 13, 2939–2947. [Google Scholar] [CrossRef]
- Karimi, B.; Akhavan, P.F. A Study on Applications of N-Substituted Main-Chain NHC-Palladium Polymers as Recyclable Self-Supported Catalysts for the Suzuki–Miyaura Coupling of Aryl Chlorides in Water. Inorg. Chem. 2011, 50, 6063–6072. [Google Scholar] [CrossRef]
- Das, P.; Sharma, D.; Shil, A.K.; Kumari, A. Solid-supported palladium nano and microparticles: An efficient heterogeneous catalyst for ligand-free Suzuki–Miyaura cross coupling reaction. Tetrahedron Lett. 2011, 52, 1176–1178. [Google Scholar] [CrossRef]
- Yamada, Y.M.A.; Sarkar, S.M.; Uozumi, Y. Self-Assembled Poly(imidazole-palladium): Highly Active, Reusable Catalyst at Parts per Million to Parts per Billion Levels. J. Am. Chem. Soc. 2012, 134, 3190–3198. [Google Scholar] [CrossRef]
- Wu, L.; Li, B.-O.; Huang, Y.-Y.; Zhou, H.-F.; He, Y.-M.; Fan, Q.-H. Phosphine Dendrimer-Stabilized Palladium Nanoparticles, a Highly Active and Recyclable Catalyst for the Suzuki−Miyaura Reaction and Hydrogenation. Org. Lett. 2006, 8, 3605–3608. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, S.; Becht, J.-M.; Drian, C.L. Highly Efficient and Reusable Supported Pd Catalysts for Suzuki−Miyaura Reactions of Aryl Chlorides. Org. Lett. 2007, 9, 3777–3780. [Google Scholar] [CrossRef] [PubMed]
- Sayah, R.; Glegoła, K.; Framery, E.; Dufaud, V. Suzuki–Miyaura Reactions of Aryl Chloride Derivatives with Arylboronic Acids using Mesoporous Silica-Supported Aryldicyclohexylphosphine. Adv. Synth. Catal. 2007, 349, 373–381. [Google Scholar] [CrossRef]
- Pandarus, V.; Desplantier-Giscard, D.; Gingras, G.; Béland, F.; Ciriminna, R.; Pagliaro, M. Greening the Valsartan Synthesis: Scale-up of Key Suzuki–Miyaura Coupling over SiliaCat DPP-Pd. Org. Process Res. Dev. 2013, 17, 1492–1497. [Google Scholar] [CrossRef]
- Lee, D.-H.; Choi, M.; Yu, B.-W.; Ryoo, R.; Taher, A.; Hossain, S.; Jin, M.-J. Expanded Heterogeneous Suzuki–Miyaura Coupling Reactions of Aryl and Heteroaryl Chlorides under Mild Conditions. Adv. Synth. Catal. 2009, 351, 2912–2920. [Google Scholar] [CrossRef]
- Jin, M.-J.; Lee, D.-H. A Practical Heterogeneous Catalyst for the Suzuki, Sonogashira, and Stille Coupling Reactions of Unreactive Aryl Chlorides. Angew. Chem. Int. Ed. 2010, 49, 1119–1122. [Google Scholar] [CrossRef]
- LeBlond, C.R.; Andrews, A.T.; Sun, Y.; Sowa, J.R., Jr. Activation of Aryl Chlorides for Suzuki Cross-Coupling by Ligandless, Heterogeneous Palladium. Org. Lett. 2001, 3, 1555–1557. [Google Scholar] [CrossRef]
- Tagata, T.; Nishida, M. Palladium Charcoal-Catalyzed Suzuki–Miyaura Coupling to Obtain Arylpyridines and Arylquinolines. J. Org. Chem. 2003, 68, 9412–9415. [Google Scholar] [CrossRef] [PubMed]
- Arvela, R.K.; Leadbeater, N.E. Suzuki Coupling of Aryl Chlorides with Phenylboronic Acid in Water, Using Microwave Heating with Simultaneous Cooling. Org. Lett. 2005, 7, 2101–2104. [Google Scholar] [CrossRef] [PubMed]
- Ohtaka, A.; Sakaguchi, E.; Yamaguchi, T.; Hanasaka, G.; Uozumi, Y.; Shimomura, O.; Nomura, R. A Recyclable “Boomerang” Linear Polystyrene-Stabilized Pd Nanoparticles for the Suzuki Coupling Reaction of Aryl Chlorides in Water. ChemCatChem 2013, 5, 2167–2169. [Google Scholar] [CrossRef]
- Yuan, B.; Pan, Y.; Li, Y.; Yin, B.; Jiang, H.A. Highly Active Heterogeneous Palladium Catalyst for the Suzuki–Miyaura and Ullmann Coupling Reactions of Aryl Chlorides in Aqueous Media. Angew. Chem. Int. Ed. 2010, 49, 4054–4058. [Google Scholar] [CrossRef] [PubMed]
- Kogan, V.; Aizenshtat, Z.; Popovitz-Biro, R.; Newmann, R. Carbon-Carbon and Carbon-Nitrogen Coupling Reactions Catalyzed by Palladium Nanoparticles Derived from a Palladium Substituted Keggin-Type Polyoxometalate. Org. Lett. 2002, 4, 3529–3532. [Google Scholar] [CrossRef] [PubMed]
- Choudary, B.M.; Madhi, S.; Chowdari, N.S.; Kantam, M.L.; Sreedhar, B. Layered Double Hydroxide Supported Nanopalladium Catalyst for Heck-, Suzuki-, Sonogashira-, and Stille-Type Coupling Reactions of Chloroarenes. J. Am. Chem. Soc. 2002, 124, 14127–14136. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Pandarus, V.; Gingras, G.; Béland, F.; Demma, P.; Pagliaro, M. Heterogeneously catalyzed Suzuki–Miyaura conversion of broad scope. RSC Adv. 2012, 2, 10798–10804. [Google Scholar] [CrossRef]
- Monguchi, Y.; Ichikawa, T.; Netsu, M.; Hattori, T.; Mizusaki, T.; Sawama, Y.; Sajiki, H. Tertiary-Amino-Functionalized Resin-Supported Palladium Catalyst for the Heterogeneous Suzuki–Miyaura Reaction of Aryl Chlorides. Synlett 2015, 26, 2014–2018. [Google Scholar] [CrossRef]
- Ichikawa, T.; Netsu, M.; Mizuno, M.; Mizusaki, T.; Takagi, Y.; Sawama, Y.; Monguchi, Y.; Sajiki, H. Development of a Unique Heterogeneous Palladium Catalyst for the Suzuki–Miyaura Reaction using (Hetero)aryl Chlorides and Chemoselective Hydrogenation. Adv. Synth. Catal. 2017, 359, 2269–2279. [Google Scholar] [CrossRef]
- Ichikawa, T.; Mizuno, M.; Ueda, S.; Ohneda, N.; Odajima, H.; Sawama, Y.; Monguchi, Y.; Sajiki, H. A practical method for heterogeneously-catalyzed Mizoroki–Heck reaction: Flow system with adjustment of microwave resonance as an energy source. Tetrahedron 2018, 74, 1810–1816. [Google Scholar] [CrossRef]
- Sreedhar, B.; Yada, D.; Reddy, P.S. Nanocrystalline Titania-Supported Palladium(0) Nanoparticles for Suzuki–Miyaura Cross-Coupling of Aryl and Heteroaryl Halides. Adv. Synth. Catal. 2011, 353, 2823–2836. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M. Green synthesis, characterization and catalytic activity of the Pd/TiO2 nanoparticles for the ligand-free Suzuki–Miyaura coupling reaction. J. Colloid Interf. Sci. 2016, 465, 121–127. [Google Scholar] [CrossRef]
- Mondal, P.; Khatun, R.; Bhanja, P.; Bhaumik, A.; Das, D.; Islam, S.M. Palladium nanoparticles embedded on mesoporous TiO2 material (Pd@MTiO2) as an efficient heterogeneous catalyst for Suzuki-Coupling reactions in water medium. J. Colloid Interf. Sci. 2017, 508, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Niu, Y.; Li, Y.; Yang, F.; Guo, J.; Wang, Q.; Jing, P.; Zhang, J.; Yun, G. A mesoporous “shell-in-shell” structured nanocatalyst with large surface area, enhanced synergy, and improved catalytic performance for Suzuki–Miyaura coupling reaction. Chem. Commun. 2014, 50, 12356–12359. [Google Scholar] [CrossRef]
- Kamari, Y.; Ghiaci, M. Incorporation of TiO2 coating on a palladium heterogeneous nanocatalyst. A new method to improve reusability of a catalyst. Catal. Commun. 2016, 84, 16–20. [Google Scholar] [CrossRef]
- Koohgard, M.; Hosseini-Sarvari, M. Enhancement of Suzuki–Miyaura coupling reaction by photocatalytic palladium nanoparticles anchored to TiO2 under visible light irradiation. Catal. Commun. 2018, 111, 10–15. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Takeda, Y.; Sugano, Y.; Ichikawa, S.; Tanaka, S.; Hirai, T. Highly efficient photocatalytic dehalogenation of organic halides on TiO2 loaded with bimetallic Pd–Pt alloy nanoparticles. Chem. Commun. 2011, 47, 7863–7865. [Google Scholar] [CrossRef]
- Yoshida, H.; Fujimura, Y.; Yuzawa, H.; Kumagai, J.; Yoshida, M. A heterogeneous palladium catalyst hybridised with a titanium dioxide photocatalyst for direct C–C bond formation between an aromatic ring and acetonitrile. Chem. Commun. 2013, 49, 3793–3795. [Google Scholar] [CrossRef] [PubMed]
- Sikhwivhilu, L.M.; Coville, N.J.; Naresh, D.; Chary, K.V.R.; Vishwanathan, V. Nanotubular titanate supported palladium catalysts: The influence of structure and morphology on phenol hydrogenation activity. Appl. Catal. A General 2007, 324, 52–61. [Google Scholar] [CrossRef]
- Yang, J.; Cao, L.-X.; Wang, G.-C. Acetylene hydrogenation on anatase TiO2(101) supported Pd4 cluster: Oxygen deficiency effect. J. Mol. Model. 2012, 18, 3329–3339. [Google Scholar] [CrossRef] [PubMed]
- Ortel, E.; Sokolov, S.; Zielke, C.; Lauermann, I.; Selve, S.; Weh, K.; Paul, B.; Polte, J.; Kraehnert, R. Supported Mesoporous and Hierarchical Porous Pd/TiO2 Catalytic Coatings with Controlled Particle Size and Pore Structure. Chem. Mater. 2012, 24, 3828–3838. [Google Scholar] [CrossRef]
- Khatun, R.; Bhanja, P.; Mondal, P.; Bhaumik, A.; Das, D.; Islam, S.M. Palladium nanoparticles embedded over mesoporous TiO2 for chemical fixation of CO2 under atmospheric pressure and solvent-free conditions. New J. Chem. 2017, 41, 12937–12946. [Google Scholar] [CrossRef]
- Melchionna, M.; Beltram, A.; Montini, T.; Monai, M.; Nasi, L.; Fornasiero, P.; Prato, M. Highly efficient hydrogen production through ethanol photoreforming by a carbon nanocone/ Pd@TiO2 hybrid catalyst. Chem. Commun. 2016, 52, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Sayed, F.N.; Jayakumar, O.D.; Sasikala, R.; Kadam, R.M.; Bharadwaj, S.R.; Kienle, L.; Schürmann, U.; Kaps, S.; Adelung, R.; Mittal, J.P.; Tyagi, A.K. Photochemical Hydrogen Generation Using Nitrogen-Doped TiO2−Pd Nanoparticles: Facile Synthesis and Effect of Ti3+ Incorporation. J. Phys. Chem. C 2012, 116, 12462–12467. [Google Scholar] [CrossRef]
- Bahruji, H.; Bowkera, M.; Brookes, C.; Davies, P.R.; Wawata, I. The adsorption and reaction of alcohols on TiO2 and Pd/TiO2 catalysts. Appl. Catal. A General 2013, 454, 66–73. [Google Scholar] [CrossRef]
- Cao, C.; Yan, Y.; Yu, Y.; Yang, X.; Liu, W.; Cao, Y. Modification of Pd and Mn on the Surface of TiO2 with Enhanced Photocatalytic Activity for Photoreduction of CO2 into CH4. J. Phys. Chem. C 2017, 121, 270–277. [Google Scholar] [CrossRef]
- Sayed, F.N.; Sasikala, R.; Jayakumar, O.D.; Rao, R.; Betty, C.A.; Chokkalingam, A.; Kadam, R.M.; Jagannath; Bharadwaj, S.R.; Vinuc, A.; Tyagi, A.K. Photocatalytic hydrogen generation from water using a hybrid of graphene nanoplatelets and self doped TiO2–Pd. RSC Adv. 2014, 4, 13469–13476. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Azarian, A.; Ehsani, A.; Khalaj, M. Preparation, optical properties and catalytic activity of TiO2@Pd nanoparticles as heterogeneous and reusable catalysts for ligand-free Heck coupling reaction. J. Mol. Catal. A Chem. 2014, 394, 205–210. [Google Scholar] [CrossRef]
- Dodson, J.J.; Hagelin-Weaver, H.E. Effect of titania structure on palladium oxide catalysts in the oxidativecoupling of 4-methylpyridine. J. Mol. Catal. A Chem. 2015, 410, 271–279. [Google Scholar] [CrossRef]
- Sá, J.; Bernardi, J.; Anderson, J.A. Imaging of low temperature induced SMSI on Pd/TiO2 catalysts. Catal. Lett. 2007, 114, 91–95. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Magdziarz, A.; Aramendia, M.A.; Marinas, A.; Marinas, J.M.; Urbano, F.J.; Navio, J.A. Influence of the strong metal support interaction effect (SMSI) of Pt/TiO2 and Pd/TiO2 systems in the photocatalytic biohydrogen production from glucose solution. Catal. Commun. 2011, 16, 1–6. [Google Scholar] [CrossRef]
- Pan, C.-J.; Tsai, M.-C.; Su, W.-N.; Rick, J.; Akalework, N.G.; Agegnehu, A.K.; Cheng, S.-Y.; Hwang, B.-J. Tuning/exploiting Strong Metal-Support Interaction (SMSI) in Heterogeneous Catalysis. J. Taiwan Inst. Chem. E 2017, 74, 154–186. [Google Scholar] [CrossRef]
- O’Brien, C.J.; Kantchev, E.A.B.; Valente, C.; Hadei, N.; Chass, G.A.; Lough, A.; Hopkinson, A.C.; Organ, M.G. Easily Prepared Air-and Moisture-Stable Pd–NHC (NHC=N-Heterocyclic Carbene) Complexes: A Reliable, User-Friendly, Highly Active Palladium Precatalyst for the Suzuki–Miyaura Reaction. Chem. Eur. J. 2006, 12, 4743–4748. [Google Scholar] [CrossRef]
- Valente, C.; Çalimsiz, S.; Hoi, K.H.; Mallik, D.; Sayah, M.; Organ, M.G. The Development of Bulky Palladium NHC Complexes for the Most-Challenging Cross-Coupling Reactions. Angew. Chem. Int. Ed. 2012, 51, 3314–3332. [Google Scholar] [CrossRef] [PubMed]
- Petrassi, H.M.; Johnson, S.M.; Purkey, H.E.; Chiang, K.P.; Walkup, T.; Jiang, X.; Powers, E.T.; Kelly, J.W. Potent and Selective Structure-Based Dibenzofuran Inhibitors of Transthyretin Amyloidogenesis: Kinetic Stabilization of the Native State. J. Am. Chem. Soc. 2005, 127, 6662–6671. [Google Scholar] [CrossRef]
- Oliveira, A.M.A.G.; Raposo, M.M.M.; Oliveira-Campos, A.M.F.; Machado, A.E.H.; Puapairoj, P.; Pedro, M.; Nascimento, M.S.J.; Portela, C.; Afonso, C.; Pinto, M. Psoralen analogues: Synthesis, inhibitory activity of growth of human tumor cell lines and computational studies. Eur. J. Med. Chem. 2006, 41, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Lusic, H.; Uprety, R.; Deiters, A. Improved Synthesis of the Two-Photon Caging Group 3-Nitro-2-Ethyldibenzofuran and Its Application to a Caged Thymidine Phosphoramidite. Org. Lett. 2010, 12, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Love, B.E. Isolation and synthesis of polyoxygenated dibenzofurans possessing biological activity. Eur. J. Med. Chem. 2015, 97, 377–387. [Google Scholar] [CrossRef]
- Huang, W.; Xu, J.; Liu, C.; Chen, Z.; Gu, Y. Lewis Acid-Catalyzed Synthesis of Benzofurans and 4,5,6,7-Tetrahydrobenzofurans from Acrolein Dimer and 1,3-Dicarbonyl Compounds. J. Org. Chem. 2019, 84, 2941–2950. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Jain, P.; Lin, B.; Ferrer, M.; Hu, Z.; Southall, N.; Hu, X.; Zheng, W.; Neuenswander, B.; Cho, C.-H.; et al. High-Throughput Screening, Discovery, and Optimization To Develop a Benzofuran Class of Hepatitis C Virus Inhibitors. ACS Comb. Sci. 2015, 17, 641–652. [Google Scholar] [CrossRef]
- Chong, P.Y.; Shotwell, J.B.; Miller, J.; Price, D.J.; Maynard, A.; Voitenleitner, C.; Mathis, A.; Williams, S.; Pouliot, J.; Creech, K.; et al. Design of N-Benzoxaborole Benzofuran GSK8175–Optimization of Human PK Inspired by Metabolites of a Failed Clinical HCV Inhibitor. J. Med. Chem. 2019, 62, 3254–3267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.-X.; Hu, Y.; Shi, X.-B.; Jiang, Z.-Q.; Wang, Z.-K.; Liao, L.-S. Highly Efficient Blue Phosphorescent Organic Light-Emitting Diodes Employing a Host Material with Small Bandgap. ACS Appl. Mater. Interfaces 2016, 8, 16186–16191. [Google Scholar] [CrossRef] [PubMed]
- Solórzano, P.C.; Brigante, F.; Pierini, A.B.; Jimenez, L.B. Photoinduced Synthesis of Dibenzofurans: Intramolecular and Intermolecular Comparative Methodologies. J. Org. Chem. 2018, 83, 7867–7877. [Google Scholar] [CrossRef] [PubMed]





| Entry | Solvent | Reductant of Pd Catalyst | Pd Catalyst | Color |
|---|---|---|---|---|
| 1 | MeCN | NH2NH2•H2O a | 5% Pd(red)/TiO2 (Cat. A, anatase-type) | Grayish white |
| 2 | MeCN | NaBH4 a | 5% Pd(red)/TiO2 (Cat. B, anatase-type) | Grayish white |
| 3 | MeCN | H2 b | 5% Pd(red)/TiO2 (Cat. C, anatase-type) | Grayish white |
| 4 | MeCN | – | 5% Pd/TiO2 (Cat. D, anatase-type) | Light yellow |
| 5 | EtOAc | – | 4% Pd/TiO2 (Cat. E, anatase-type) | Light yellow |
| 6 | MeCN | – | 5% Pd/TiO2 (Cat. F, rutile-type) | Light yellow |
| 7 | EtOAc | – | 5% Pd/TiO2 (Cat. G, rutile-type) | Light yellow |
| 8 | MeCN | – | 5% Pd/TiO2 (Cat. H, brookite-type) | Light yellow |
| 9 | EtOAc | – | 5% Pd/TiO2 (Cat. I, brookite-type) | Light yellow |

| Entry | Catalyst | Ratio of 1a and 3a b |
|---|---|---|
| 1 | 5% Pd(red)/TiO2 (Cat. A, anatase-type) | 70:30 |
| 2 | 5% Pd(red)/TiO2 (Cat. B, anatase-type) | 96:4 |
| 3 | 5% Pd(red)/TiO2 (Cat. C, anatase-type) | 89:11 |
| 4 | 5% Pd/TiO2 (Cat. D, anatase-type) | 35:65 |
| 5 | 4% Pd/TiO2 (Cat. E, anatase-type) | 38:62 |
| 6 | 5% Pd/TiO2 (Cat. F, rutile-type) | 63:37 |
| 7 | 5% Pd/TiO2 (Cat. G, rutile-type) | 56:44 |
| 8 | 5% Pd/TiO2 (Cat. H, brookite-type) | 49:51 |
| 9 | 5% Pd/TiO2 (Cat. I, brookite-type) | 35:65 |
| 10 | 5% Pd/TiO2 (Cat. D, anatase-type) | 0:100 (99) c,d |
| 11 | 5% Pd/TiO2 (Cat. I, brookite-type) | 32:68 c |

| Entry | Catalyst | Ratio of 1a and 3a |
|---|---|---|
| 1 | 5% Pd/TiO2 (Cat. D, anatase-type) | 0:100 (99) b,c,d |
| 2 | 7% Pd/WA30 | 0:100 e [35] |
| 3 | 10% Pd/C | 97:3 e [35] |
| 4 | Pd(OAc)2 | 57:43 a,b,c |

| Entry | Gas/Reaction Vessel | Ratio of 1a and 3a a |
|---|---|---|
| 1 | Ar/sealed vessel | 0:100 (99) b |
| 2 | Ar/sealed vessel | 100:0 c |
| 3 | Air/sealed vessel | 90:10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, T.; Masuda, H.; Park, K.; Tachikawa, T.; Ito, N.; Ichikawa, T.; Yoshimura, M.; Takagi, Y.; Sawama, Y.; Ohya, Y.; et al. Development of Titanium Dioxide-Supported Pd Catalysts for Ligand-Free Suzuki–Miyaura Coupling of Aryl Chlorides. Catalysts 2019, 9, 461. https://doi.org/10.3390/catal9050461
Yamada T, Masuda H, Park K, Tachikawa T, Ito N, Ichikawa T, Yoshimura M, Takagi Y, Sawama Y, Ohya Y, et al. Development of Titanium Dioxide-Supported Pd Catalysts for Ligand-Free Suzuki–Miyaura Coupling of Aryl Chlorides. Catalysts. 2019; 9(5):461. https://doi.org/10.3390/catal9050461
Chicago/Turabian StyleYamada, Tsuyoshi, Hayato Masuda, Kwihwan Park, Takumu Tachikawa, Naoya Ito, Tomohiro Ichikawa, Masatoshi Yoshimura, Yukio Takagi, Yoshinari Sawama, Yutaka Ohya, and et al. 2019. "Development of Titanium Dioxide-Supported Pd Catalysts for Ligand-Free Suzuki–Miyaura Coupling of Aryl Chlorides" Catalysts 9, no. 5: 461. https://doi.org/10.3390/catal9050461