One-Pot Synthesized Pd@N-Doped Graphene: An Efficient Catalyst for Suzuki–Miyaura Couplings
Abstract
:1. Introduction
2. Results and Discussions
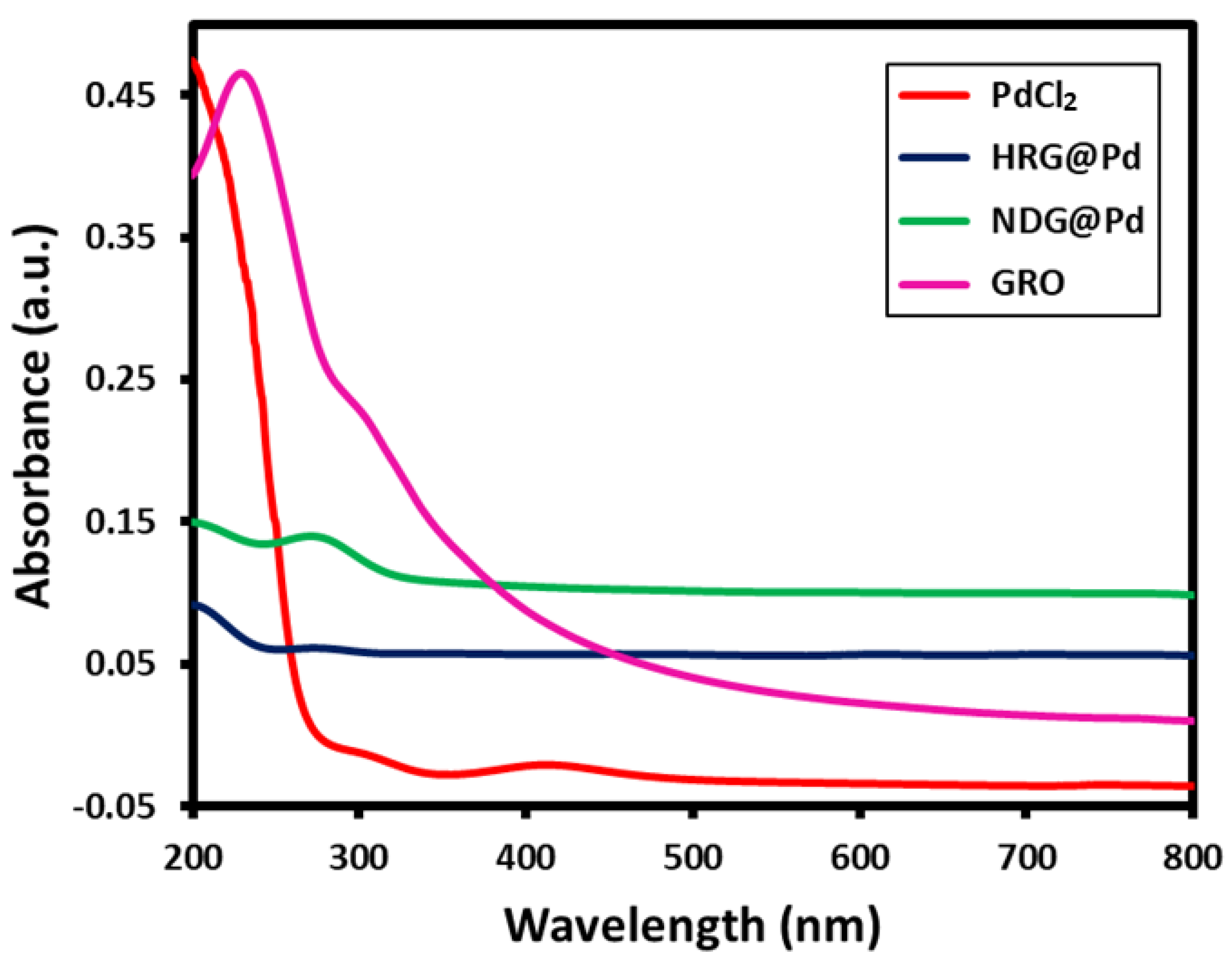
2.1. UV Analysis
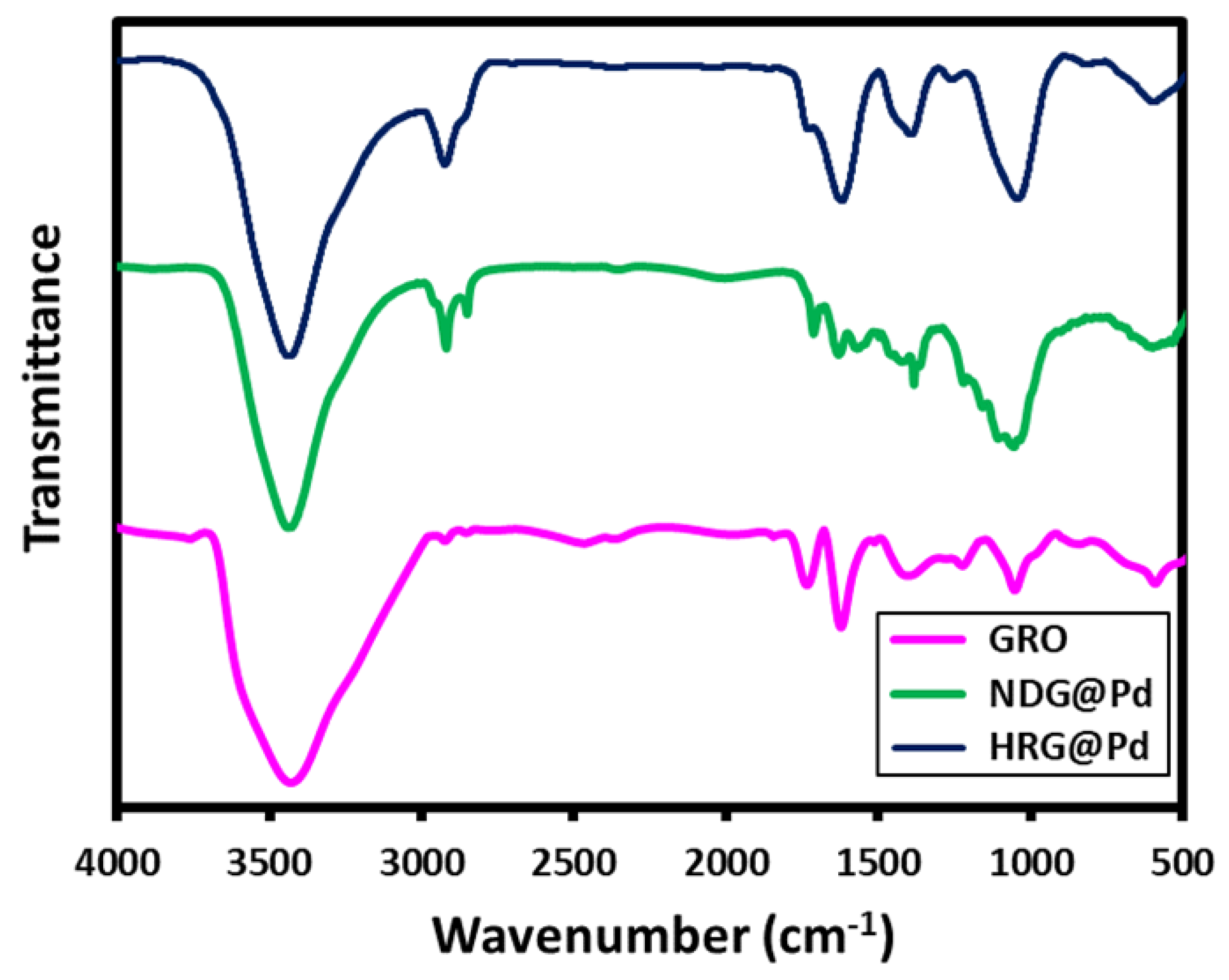
2.2. FT-IR Analysis
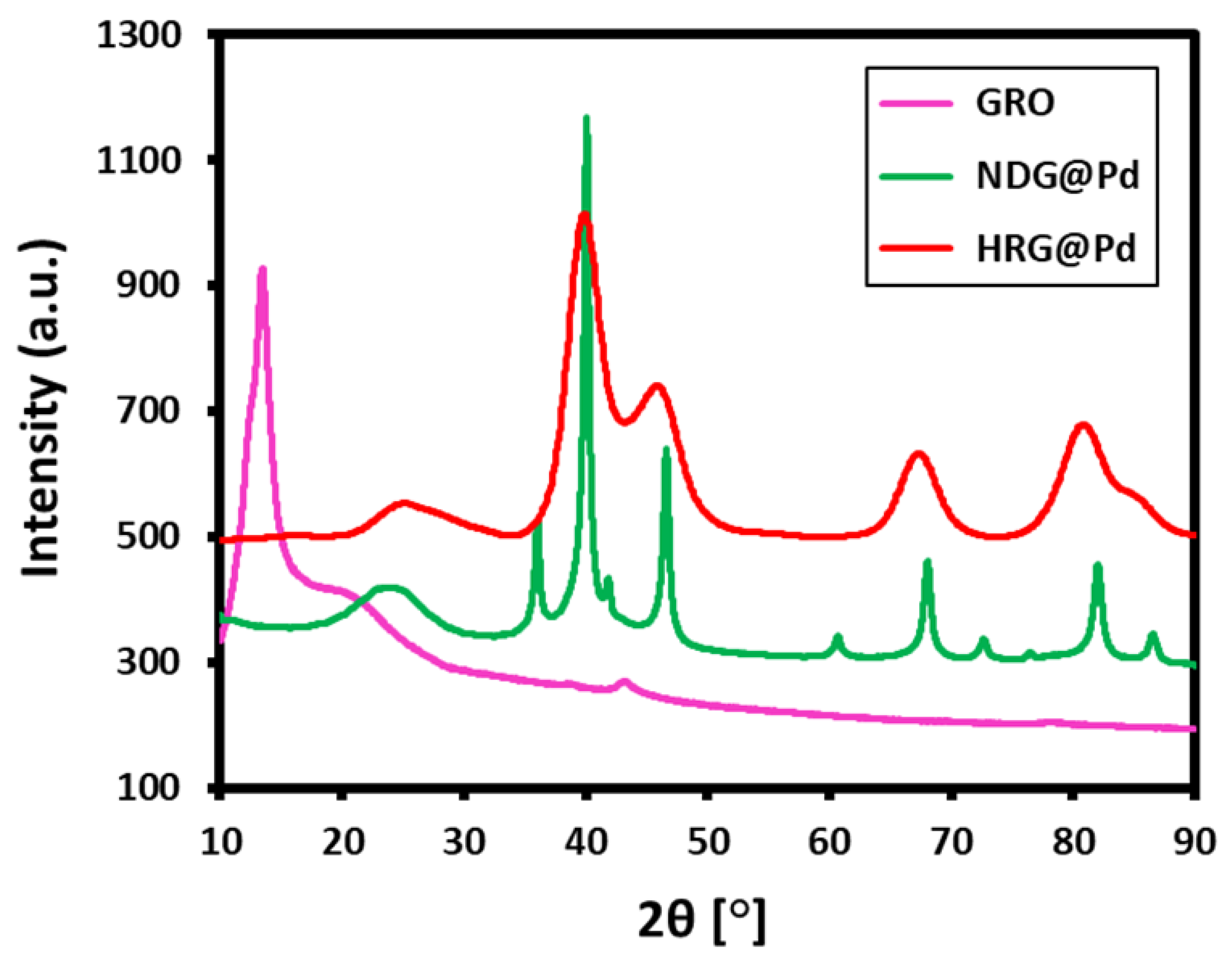
2.3. XRD Analysis
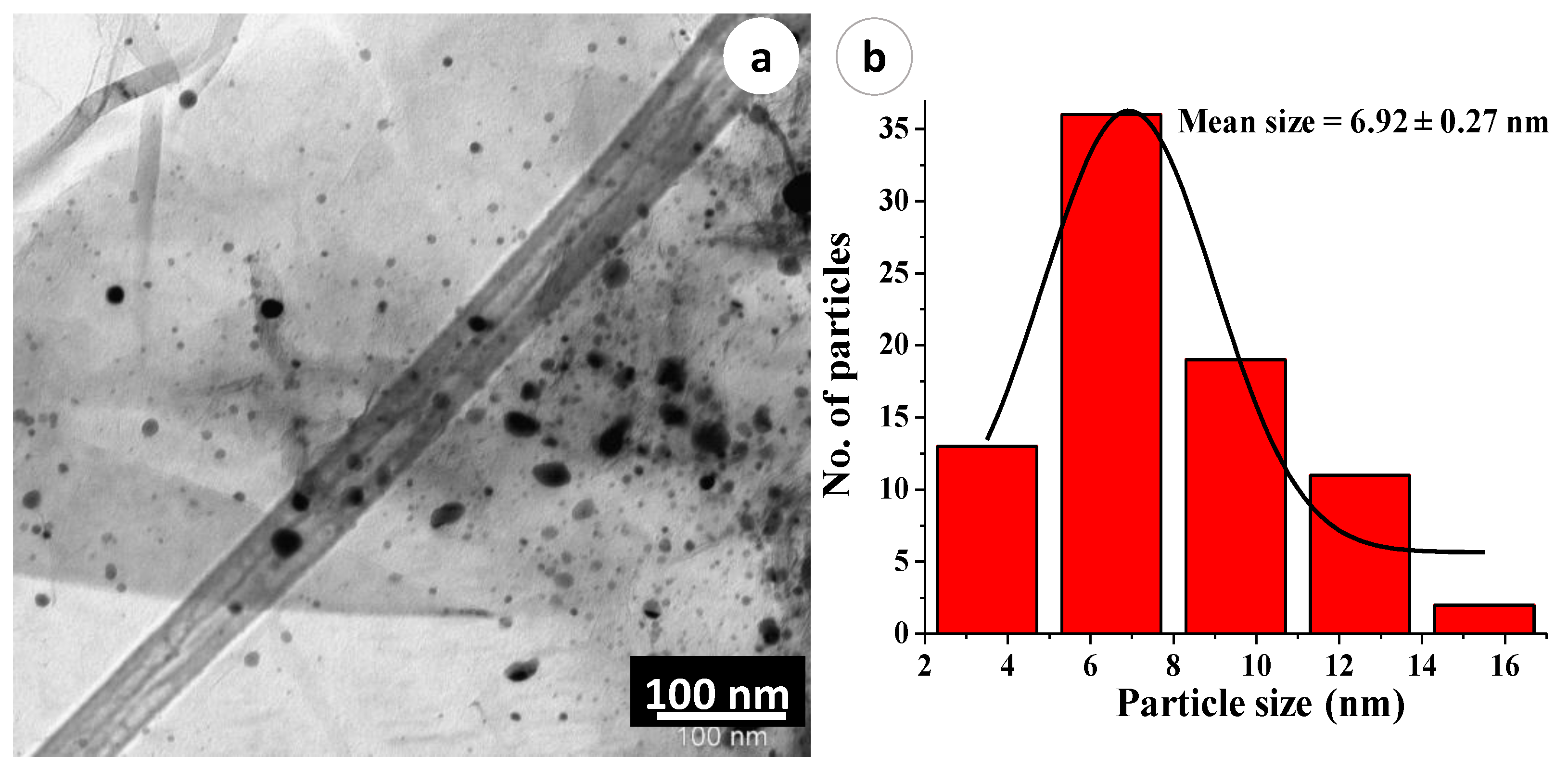
2.4. TEM Analysis of the NDG@Pd Nanocatalyst
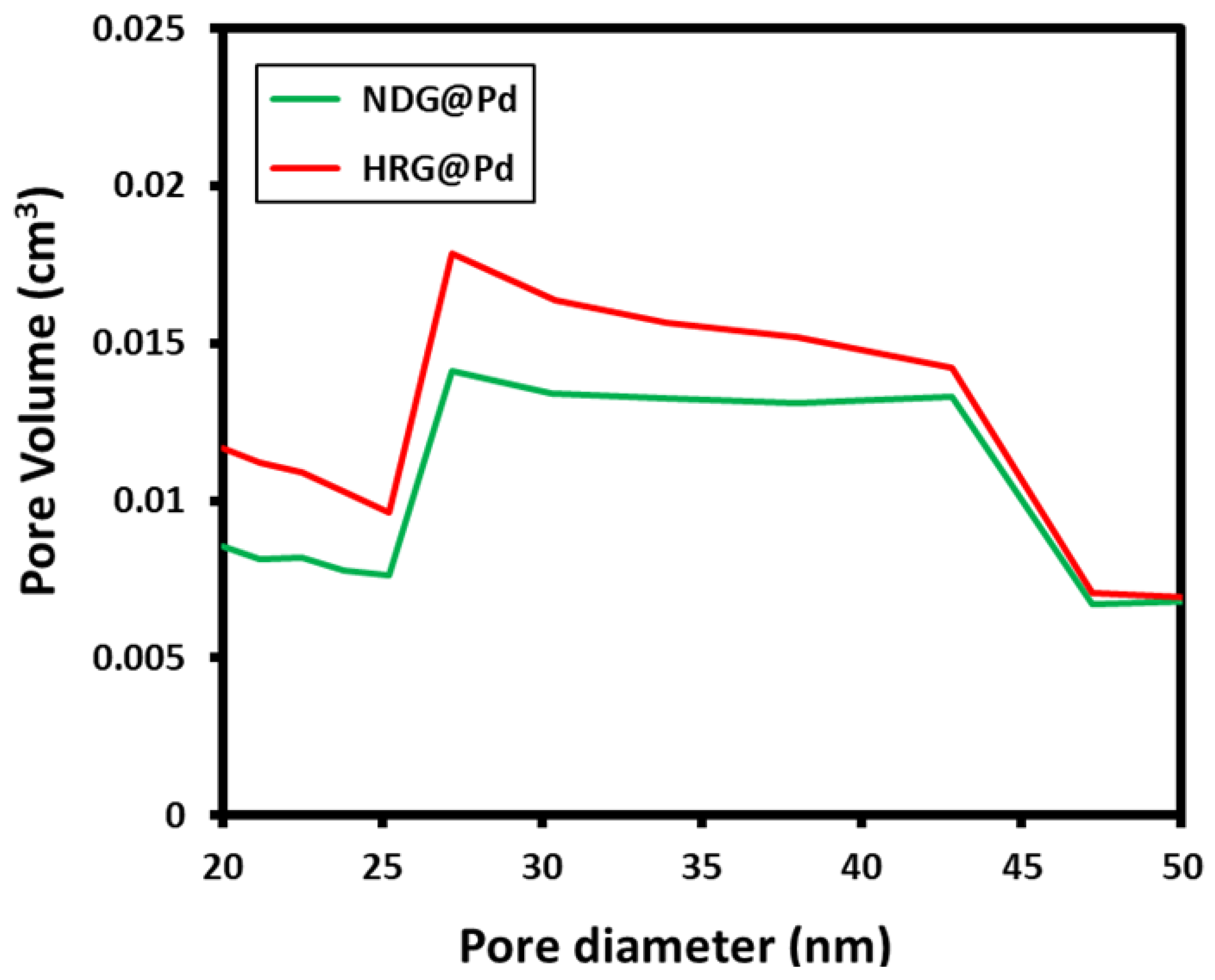
2.5. BET Analysis
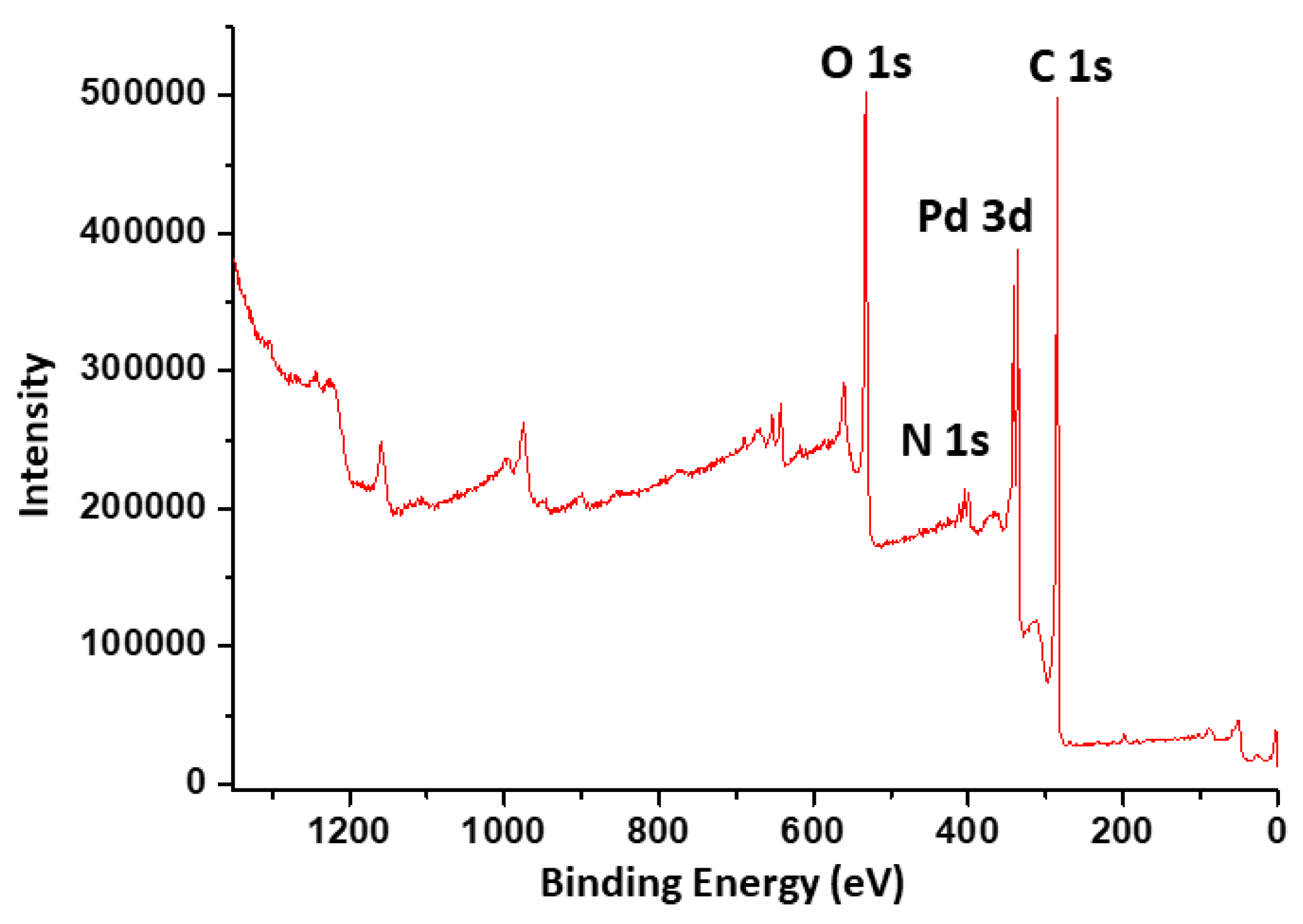
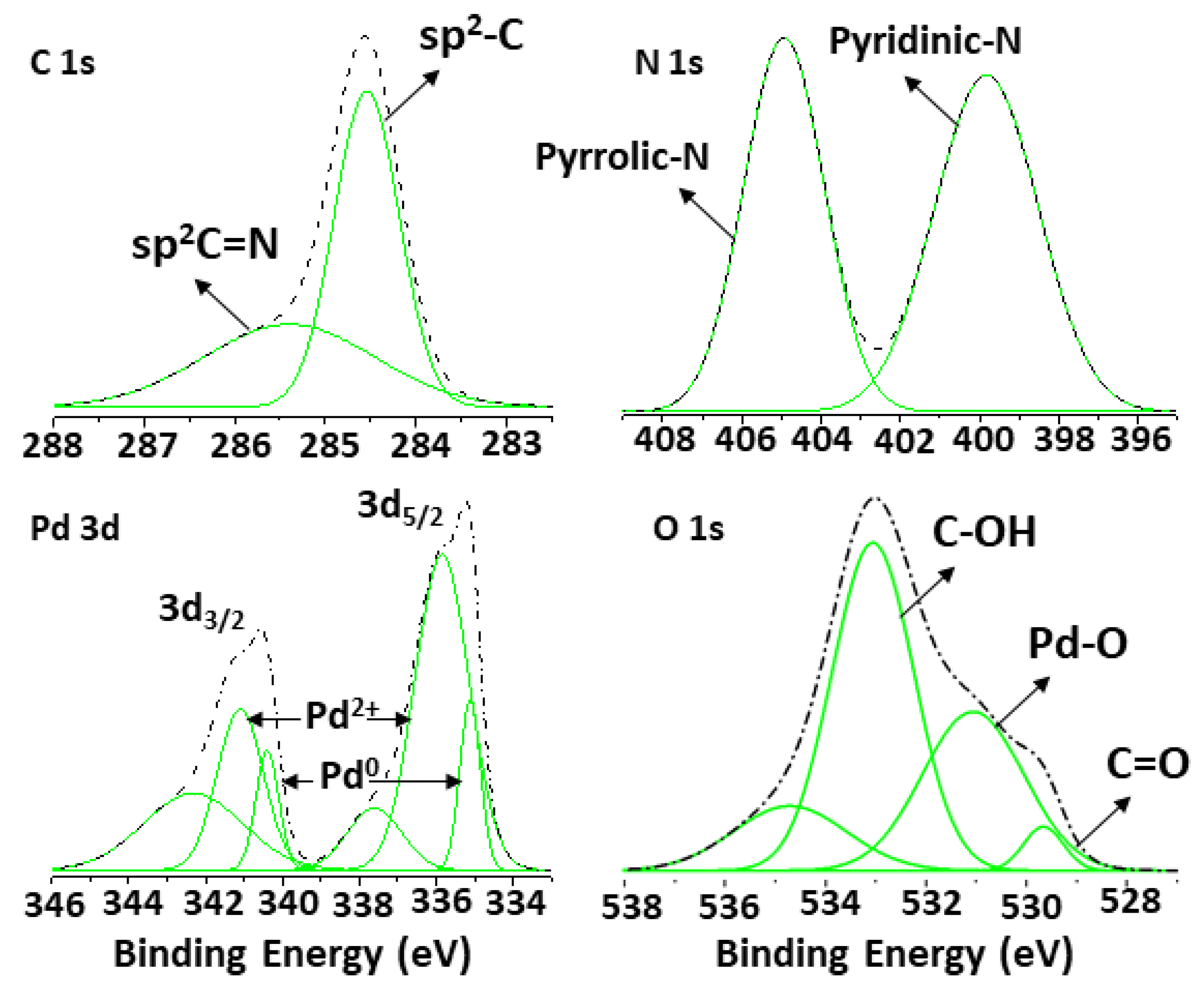
2.6. XPS Analysis of the NDG@Pd Nanocatalyst
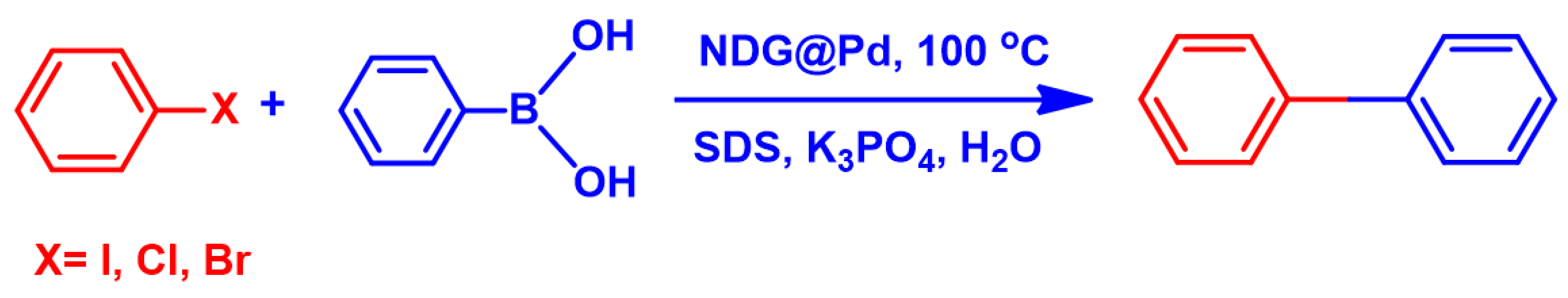
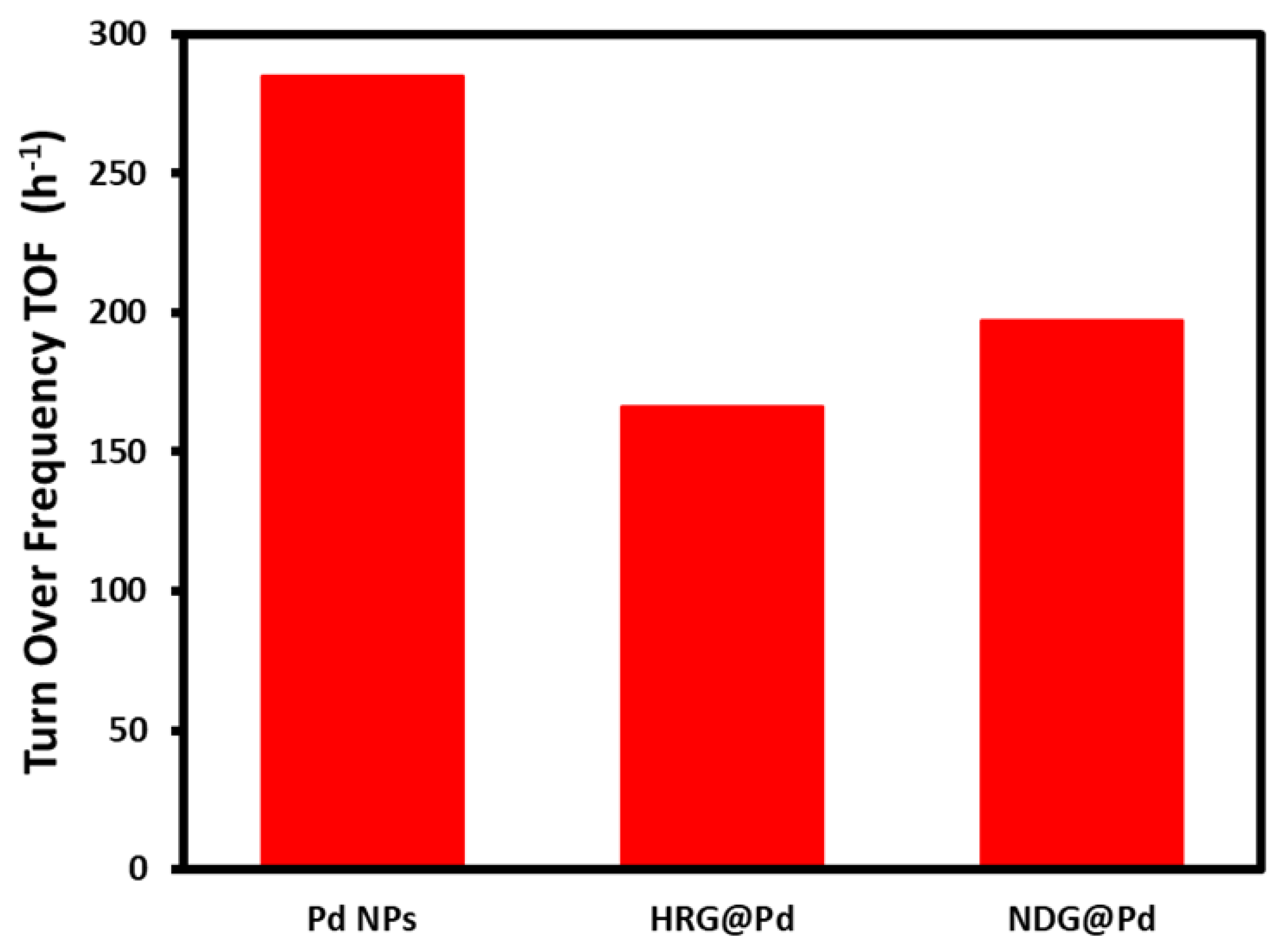
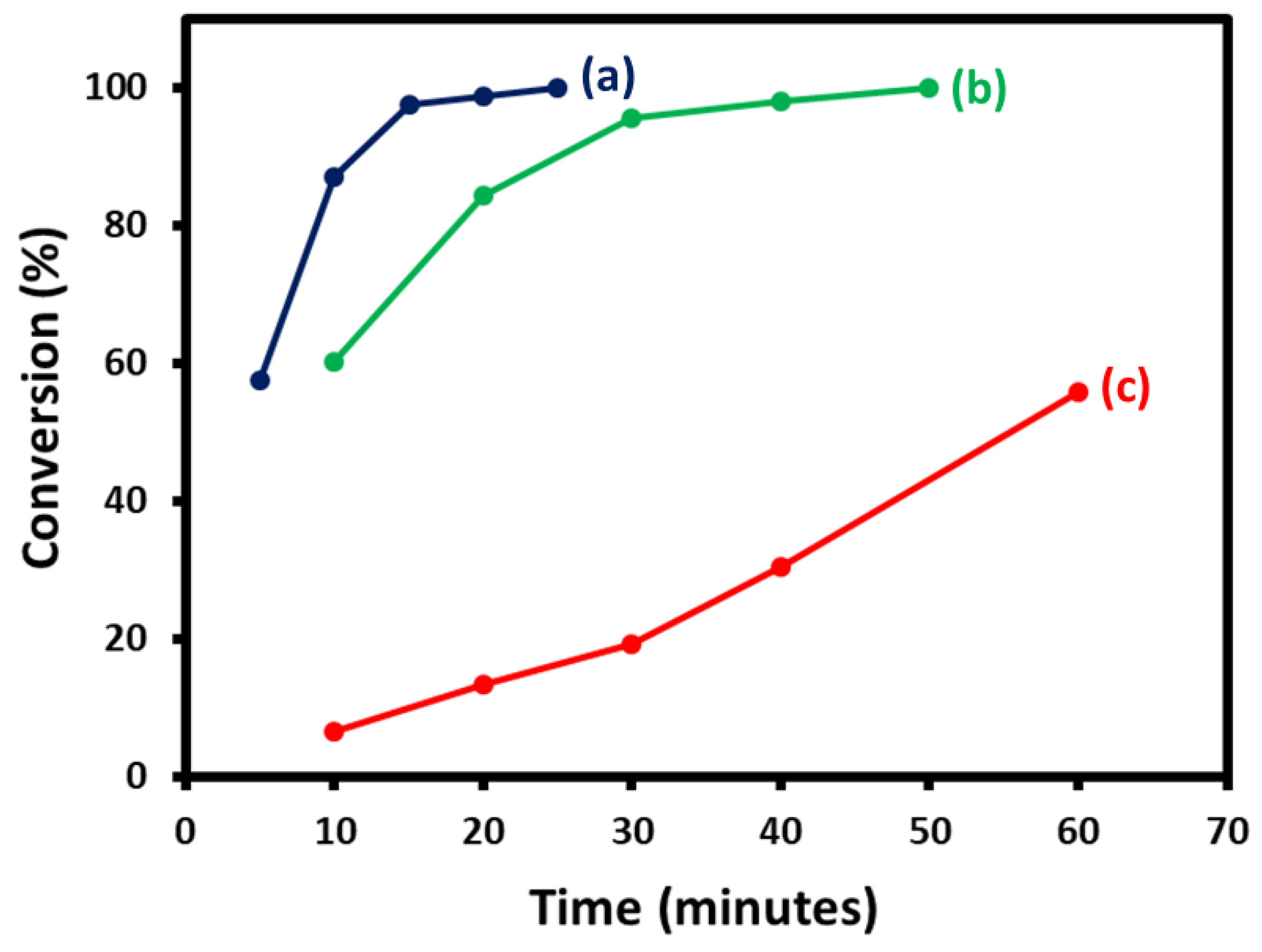
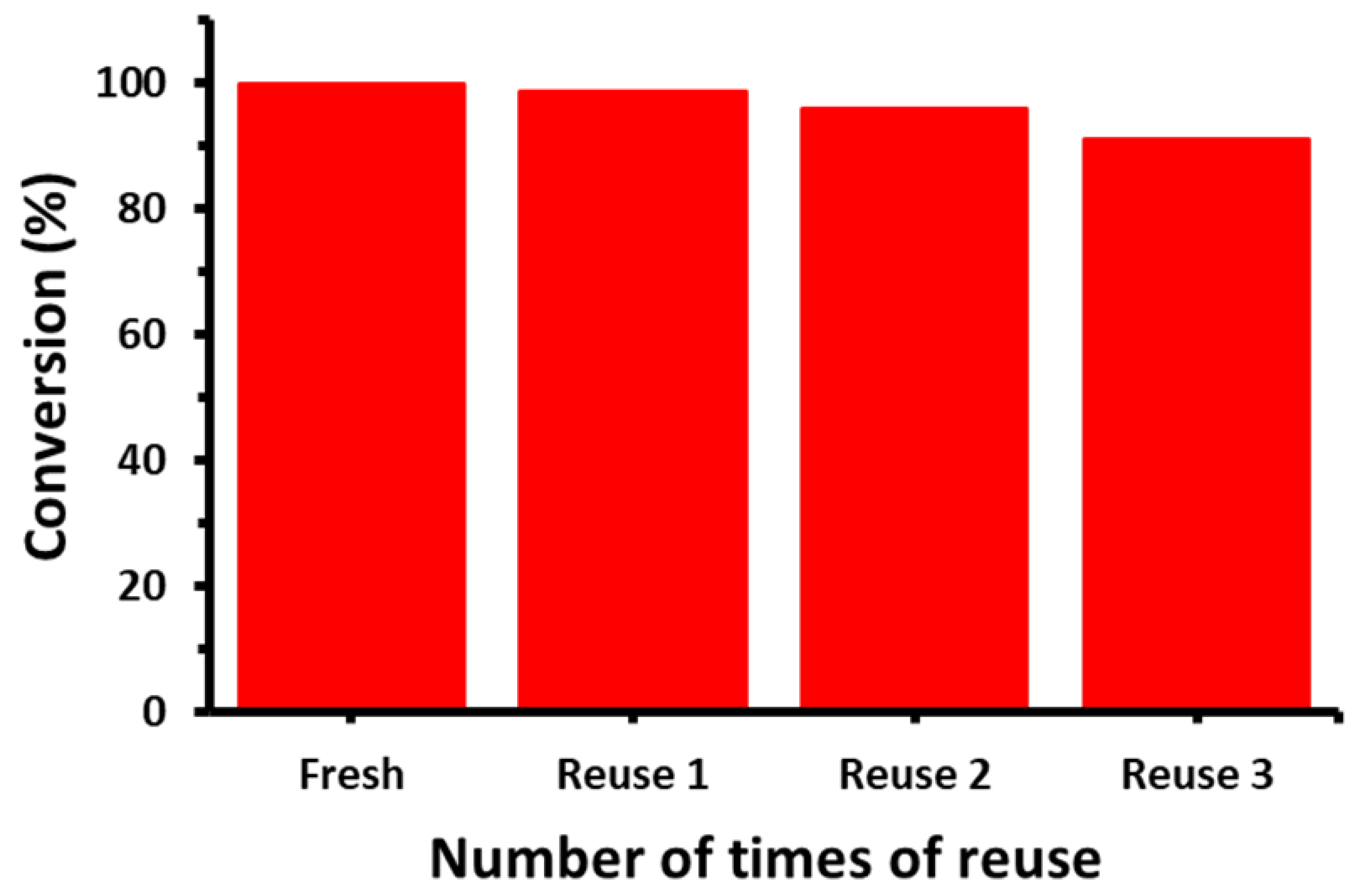
2.7. Suzuki Reaction Catalyzed by NDG@Pd Nanocatalyst
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Graphene Oxide (GRO)
3.2.2. Preparation of N-Doped Graphene-Palladium nanocatalyst (NDG@Pd)
3.2.3. Catalytic Activity
3.2.4. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rao, C.; Sood, A.; Subrahmanyam, K.; Govindaraj, A. Graphene: the new two-dimensional nanomaterial. Angew. Chem. Int. Ed. 2009, 48, 7752–7777. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. In Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific: Singapore, 2010; pp. 11–19. [Google Scholar]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-based materials: synthesis, characterization, properties, and applications. small 2011, 7, 1876–1902. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Huang, Y.; Chen, Y. Focusing on energy and optoelectronic applications: a journey for graphene and graphene oxide at large scale. Acc. Chem. Res. 2012, 45, 598–607. [Google Scholar] [CrossRef]
- Denk, R.; Hohage, M.; Zeppenfeld, P.; Cai, J.; Pignedoli, C.A.; Söde, H.; Fasel, R.; Feng, X.; Müllen, K.; Wang, S. Exciton-dominated optical response of ultra-narrow graphene nanoribbons. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef]
- Assal, M.E.; Shaik, M.R.; Kuniyil, M.; Khan, M.; Al-Warthan, A.; Siddiqui, M.R.H.; Khan, S.M.; Tremel, W.; Tahir, M.N.; Adil, S.F. A highly reduced graphene oxide/ZrOx–MnCO3 or–Mn2O3 nanocomposite as an efficient catalyst for selective aerial oxidation of benzylic alcohols. RSC Adv. 2017, 7, 55336–55349. [Google Scholar] [CrossRef]
- Gao, N.; Fang, X. Synthesis and development of graphene–inorganic semiconductor nanocomposites. Chem. Rev. 2015, 115, 8294–8343. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.T.; Shah, S.; Chhowalla, M.; Lee, K.-B. Design, synthesis, and characterization of graphene–nanoparticle hybrid materials for bioapplications. Chem. Rev. 2015, 115, 2483–2531. [Google Scholar] [CrossRef] [PubMed]
- Chng, L.L.; Erathodiyil, N.; Ying, J.Y. Nanostructured catalysts for organic transformations. Acc. Chem. Res. 2013, 46, 1825–1837. [Google Scholar] [CrossRef]
- Alonso, D.; Baeza, A.; Chinchilla, R.; Gómez, C.; Guillena, G.; Pastor, I.; Ramón, D. Solid-Supported Palladium Catalysts in Sonogashira Reactions: Recent Developments. Catalysts 2018, 8, 202. [Google Scholar] [CrossRef]
- Hervé, G.; Sartori, G.; Enderlin, G.; Mackenzie, G.; Len, C. Palladium-catalyzed Suzuki reaction in aqueous solvents applied to unprotected nucleosides and nucleotides. RSC Adv. 2014, 4, 18558–18594. [Google Scholar] [CrossRef]
- Len, C.; Bruniaux, S.; Delbecq, F.; Parmar, V.S. Palladium-Catalyzed Suzuki–Miyaura Cross-Coupling in Continuous Flow. Catalysts 2017, 7, 146. [Google Scholar] [CrossRef]
- Maluenda, I.; Navarro, O. Recent developments in the Suzuki-Miyaura reaction: 2010–2014. Molecules 2015, 20, 7528–7557. [Google Scholar] [CrossRef]
- Han, F.-S. Transition-metal-catalyzed Suzuki–Miyaura cross-coupling reactions: a remarkable advance from palladium to nickel catalysts. Chem. Soc. Rev. 2013, 42, 5270–5298. [Google Scholar] [CrossRef] [PubMed]
- Erami, R.; Díaz-García, D.; Prashar, S.; Rodríguez-Diéguez, A.; Fajardo, M.; Amirnasr, M.; Gómez-Ruiz, S. Suzuki-Miyaura CC Coupling Reactions Catalyzed by Supported Pd Nanoparticles for the Preparation of Fluorinated Biphenyl Derivatives. Catalysts 2017, 7, 76. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Issaabadi, Z.; Tohidi, M.M.; Mohammad Sajadi, S. Recent progress in application of graphene supported metal nanoparticles in C− C and C− X coupling reactions. The Chemical Record 2018, 18, 165–229. [Google Scholar] [CrossRef]
- Khan, M.; Kuniyil, M.; Shaik, M.R.; Khan, M.; Adil, S.F.; Al-Warthan, A.; Alkhathlan, H.Z.; Tremel, W.; Tahir, M.N.; Siddiqui, M.R.H. Plant Extract Mediated Eco-Friendly Synthesis of Pd@ Graphene Nanocatalyst: An Efficient and Reusable Catalyst for the Suzuki-Miyaura Coupling. Catalysts 2017, 7, 20. [Google Scholar] [CrossRef]
- Wang, J.; Gu, H. Novel metal nanomaterials and their catalytic applications. Molecules 2015, 20, 17070–17092. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical functionalization of graphene and its applications. Prog. Mater Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Vinayan, B.; Sethupathi, K.; Ramaprabhu, S. Facile synthesis of triangular shaped palladium nanoparticles decorated nitrogen doped graphene and their catalytic study for renewable energy applications. Int. J. Hydrogen Energy 2013, 38, 2240–2250. [Google Scholar] [CrossRef]
- Kabir, S.; Artyushkova, K.; Serov, A.; Atanassov, P. Role of Nitrogen Moieties in N-Doped 3D-Graphene Nanosheets for Oxygen Electroreduction in Acidic and Alkaline Media. ACS Appl. Mater. Interfaces 2018, 10, 11623–11632. [Google Scholar] [CrossRef]
- Zhou, Y.; Neyerlin, K.; Olson, T.S.; Pylypenko, S.; Bult, J.; Dinh, H.N.; Gennett, T.; Shao, Z.; O’Hayre, R. Enhancement of Pt and Pt-alloy fuel cell catalyst activity and durability via nitrogen-modified carbon supports. Energy Environ. Sci. 2010, 3, 1437–1446. [Google Scholar] [CrossRef]
- Geng, D.; Chen, Y.; Chen, Y.; Li, Y.; Li, R.; Sun, X.; Ye, S.; Knights, S. High oxygen-reduction activity and durability of nitrogen-doped graphene. Energy Environ. Sci. 2011, 4, 760–764. [Google Scholar] [CrossRef]
- Qiao, X.; Liao, S.; You, C.; Chen, R. Phosphorus and nitrogen dual doped and simultaneously reduced graphene oxide with high surface area as efficient metal-free electrocatalyst for oxygen reduction. Catalysts 2015, 5, 981–991. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Zhu, Y.; Li, L.H.; Han, Y.; Chen, Y.; Du, A.; Jaroniec, M.; Qiao, S.Z. Hydrogen evolution by a metal-free electrocatalyst. Nat. Commun. 2014, 5, 3783. [Google Scholar] [CrossRef] [Green Version]
- Jeon, I.Y.; Zhang, S.; Zhang, L.; Choi, H.J.; Seo, J.M.; Xia, Z.; Dai, L.; Baek, J.B. Edge-selectively sulfurized graphene nanoplatelets as efficient metal-free electrocatalysts for oxygen reduction reaction: the electron spin effect. Adv. Mater. 2013, 25, 6138–6145. [Google Scholar] [CrossRef]
- Sheng, Z.-H.; Shao, L.; Chen, J.-J.; Bao, W.-J.; Wang, F.-B.; Xia, X.-H. Catalyst-free synthesis of nitrogen-doped graphene via thermal annealing graphite oxide with melamine and its excellent electrocatalysis. ACS nano 2011, 5, 4350–4358. [Google Scholar] [CrossRef]
- Jiang, B.; Song, S.; Wang, J.; Xie, Y.; Chu, W.; Li, H.; Xu, H.; Tian, C.; Fu, H. Nitrogen-doped graphene supported Pd@ PdO core-shell clusters for CC coupling reactions. Nano Res. 2014, 7, 1280–1290. [Google Scholar] [CrossRef]
- Khan, M.; Al-Marri, A.H.; Khan, M.; Shaik, M.R.; Mohri, N.; Adil, S.F.; Kuniyil, M.; Alkhathlan, H.Z.; Al-Warthan, A.; Tremel, W. Green approach for the effective reduction of graphene oxide using salvadora persica l. root (miswak) extract. Nanoscale Res. Lett. 2015, 10, 281. [Google Scholar] [CrossRef]
- Vinoth, R.; Babu, S.G.; Bahnemann, D.; Neppolian, B. Nitrogen doped reduced graphene oxide hybrid metal free catalyst for effective reduction of 4-nitrophenol. Sci. Adv. Mater. 2015, 7, 1443–1449. [Google Scholar] [CrossRef]
- Lin, Z.; Waller, G.H.; Liu, Y.; Liu, M.; Wong, C.-p. Simple preparation of nanoporous few-layer nitrogen-doped graphene for use as an efficient electrocatalyst for oxygen reduction and oxygen evolution reactions. Carbon 2013, 53, 130–136. [Google Scholar] [CrossRef]
- Khan, M.; Khan, M.; Kuniyil, M.; Adil, S.F.; Al-Warthan, A.; Alkhathlan, H.Z.; Tremel, W.; Tahir, M.N.; Siddiqui, M.R.H. Biogenic synthesis of palladium nanoparticles using Pulicaria glutinosa extract and their catalytic activity towards the Suzuki coupling reaction. Dalton Trans. 2014, 43, 9026–9031. [Google Scholar] [CrossRef]
- Duan, X.; O’Donnell, K.; Sun, H.; Wang, Y.; Wang, S. Sulfur and nitrogen co-doped graphene for metal-free catalytic oxidation reactions. Small 2015, 11, 3036–3044. [Google Scholar] [CrossRef]
- Cui, L.; Chen, X.; Liu, B.; Chen, K.; Chen, Z.; Qi, Y.; Xie, H.; Zhou, F.; Rümmeli, M.H.; Zhang, Y.; et al. Highly Conductive Nitrogen-Doped Graphene Grown on Glass toward Electrochromic Applications. ACS Appl. Mater. Interfaces 2018, 10, 32622–32630. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, H.; Li, F.; Deng, K.; Wang, X. Palladium nanoparticles supported on graphitic carbon nitride-modified reduced graphene oxide as highly efficient catalysts for formic acid and methanol electrooxidation. J. Mater. Chem.:A 2014, 2, 19084–19094. [Google Scholar] [CrossRef]
- Wang, X.; Hou, Z.; Ikeda, T.; Oshima, M.; Kakimoto, M.-A.; Terakura, K. Theoretical Characterization of X-ray Absorption, Emission, and Photoelectron Spectra of Nitrogen Doped along Graphene Edges. J. Phys. Chem. A 2013, 117, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, A.B.; Imran, M.; Farooq, M.; Kasak, P. Interfacial Phenomenon and Nanostructural Enhancements in Palladium Loaded Lanthanum Hydroxide Nanorods for Heterogeneous Catalytic Applications. Sci. Rep. 2018, 8, 4354. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Chen, B. Macroscopic and Spectroscopic Investigations of the Adsorption of Nitroaromatic Compounds on Graphene Oxide, Reduced Graphene Oxide, and Graphene Nanosheets. Environ. Sci. Technol. 2015, 49, 6181–6189. [Google Scholar] [CrossRef]
- Babaei, A.; Jiang, S.P.; Li, J. Electrocatalytic Promotion of Palladium Nanoparticles on Hydrogen Oxidation on Ni/GDC Anodes of SOFCs via Spillover. J. Electrochem. Soc. 2009, 156, B1022–B1029. [Google Scholar] [CrossRef]
- Mpungose, P.P.; Vundla, Z.P.; Maguire, G.E.M.; Friedrich, H.B. The Current Status of Heterogeneous Palladium Catalysed Heck and Suzuki Cross-Coupling Reactions. Molecules 2018, 23, 1676. [Google Scholar] [CrossRef]
- Hummers Jr, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Cote, L.J.; Kim, F.; Huang, J. Langmuir− Blodgett assembly of graphite oxide single layers. J. Am. Chem. Soc. 2008, 131, 1043–1049. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuniyil, M.; Kumar, J.V.S.; Adil, S.F.; Shaik, M.R.; Khan, M.; Assal, M.E.; Siddiqui, M.R.H.; Al-Warthan, A. One-Pot Synthesized Pd@N-Doped Graphene: An Efficient Catalyst for Suzuki–Miyaura Couplings. Catalysts 2019, 9, 469. https://doi.org/10.3390/catal9050469
Kuniyil M, Kumar JVS, Adil SF, Shaik MR, Khan M, Assal ME, Siddiqui MRH, Al-Warthan A. One-Pot Synthesized Pd@N-Doped Graphene: An Efficient Catalyst for Suzuki–Miyaura Couplings. Catalysts. 2019; 9(5):469. https://doi.org/10.3390/catal9050469
Chicago/Turabian StyleKuniyil, Mufsir, J. V. Shanmukha Kumar, Syed Farooq Adil, Mohammed Rafi Shaik, Mujeeb Khan, Mohamed E. Assal, Mohammed Rafiq H. Siddiqui, and Abdulrahman Al-Warthan. 2019. "One-Pot Synthesized Pd@N-Doped Graphene: An Efficient Catalyst for Suzuki–Miyaura Couplings" Catalysts 9, no. 5: 469. https://doi.org/10.3390/catal9050469







