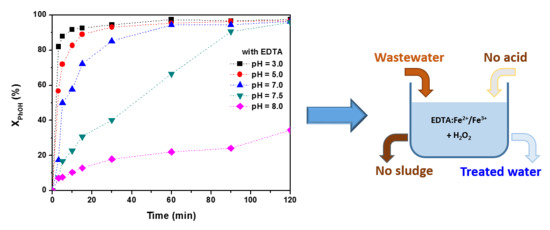
Enhanced Degradation of Phenol by a Fenton-Like System (Fe/EDTA/H2O2) at Circumneutral pH
Abstract
:1. Introduction
2. Results and Discussion
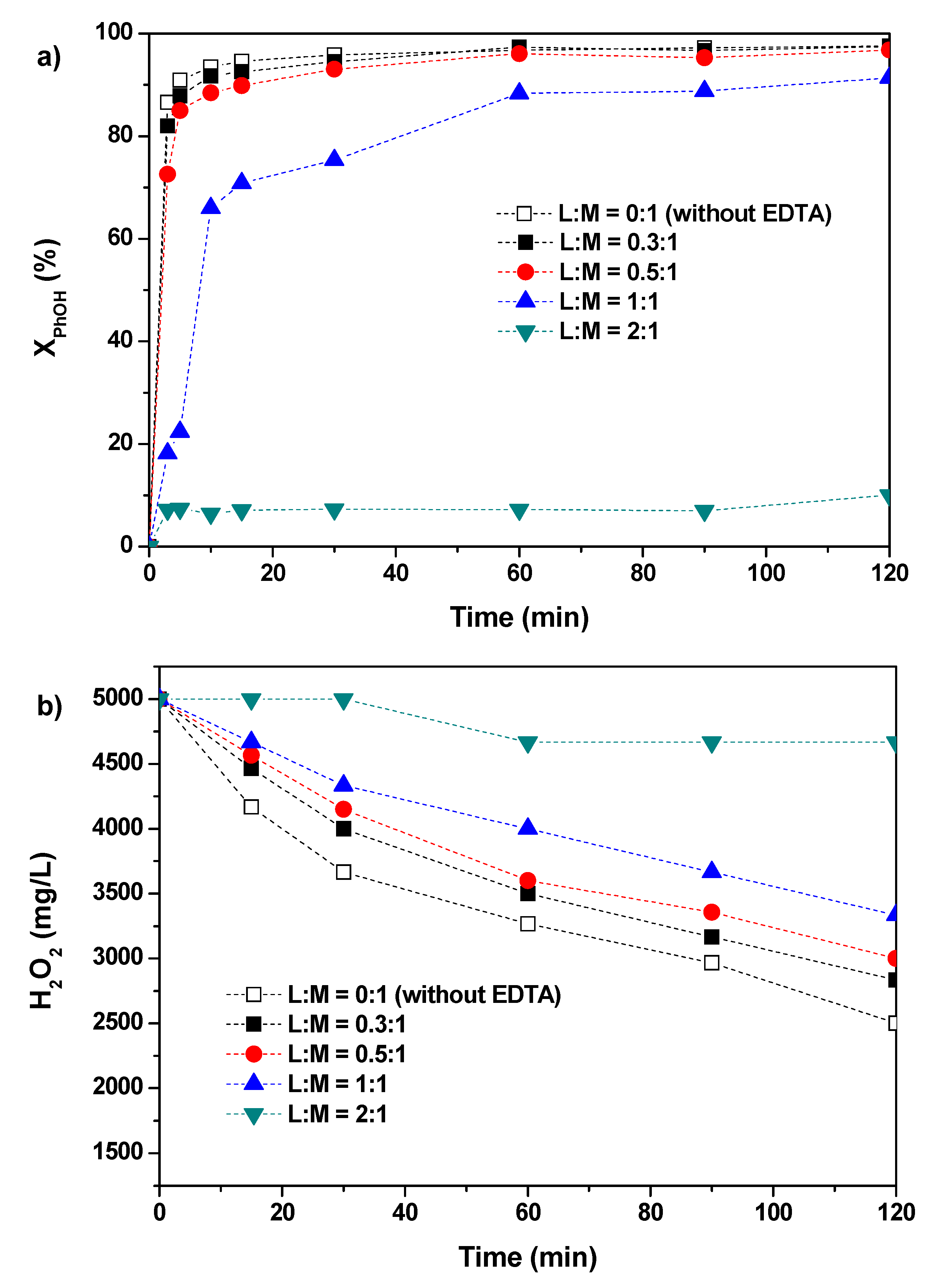
2.1. Effect of Ligand to Metal (L:M) Molar Ratio
2.2. Speciation of Fe2+ and Fe3+ in the Presence of EDTA
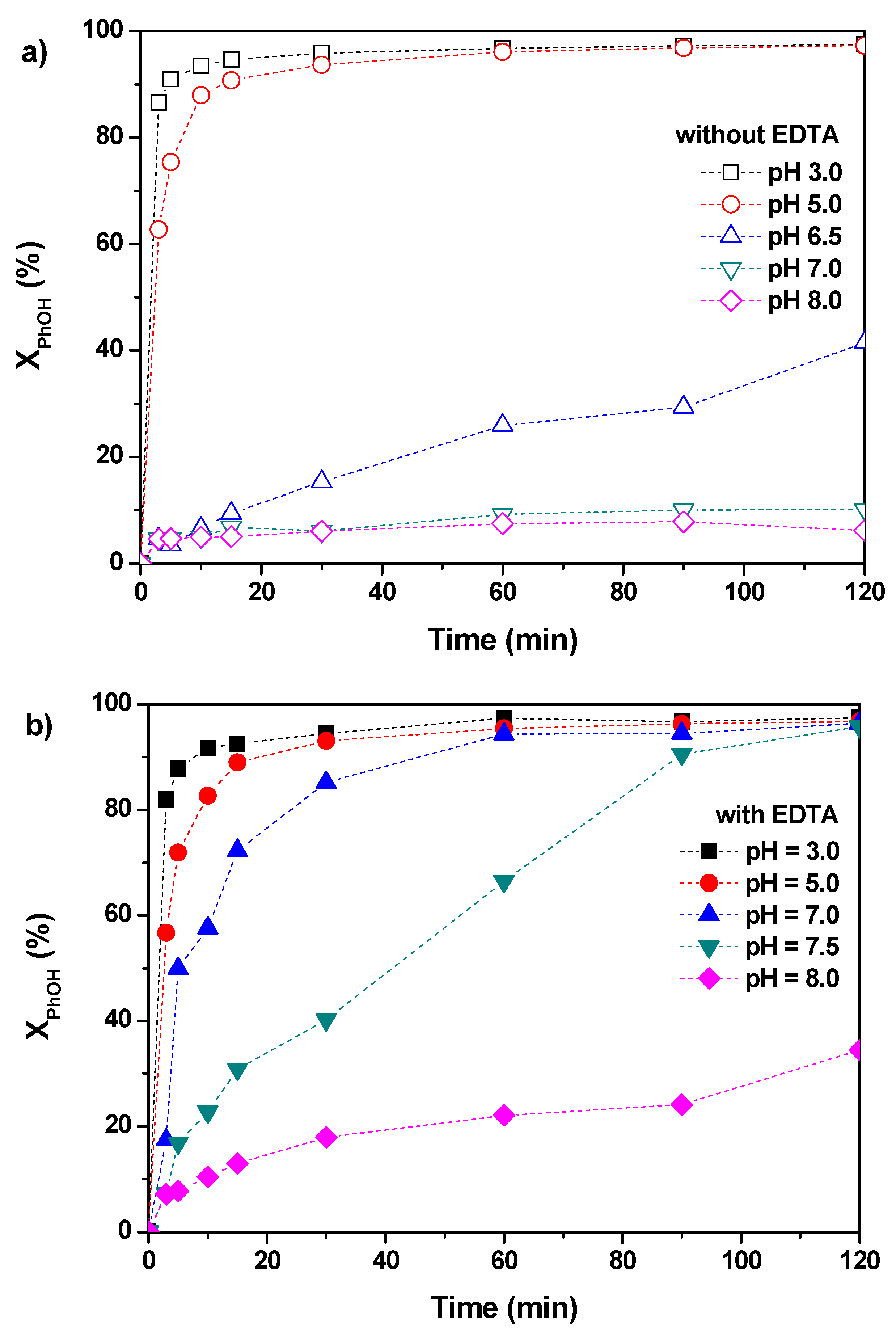
2.3. Effect of Initial pH
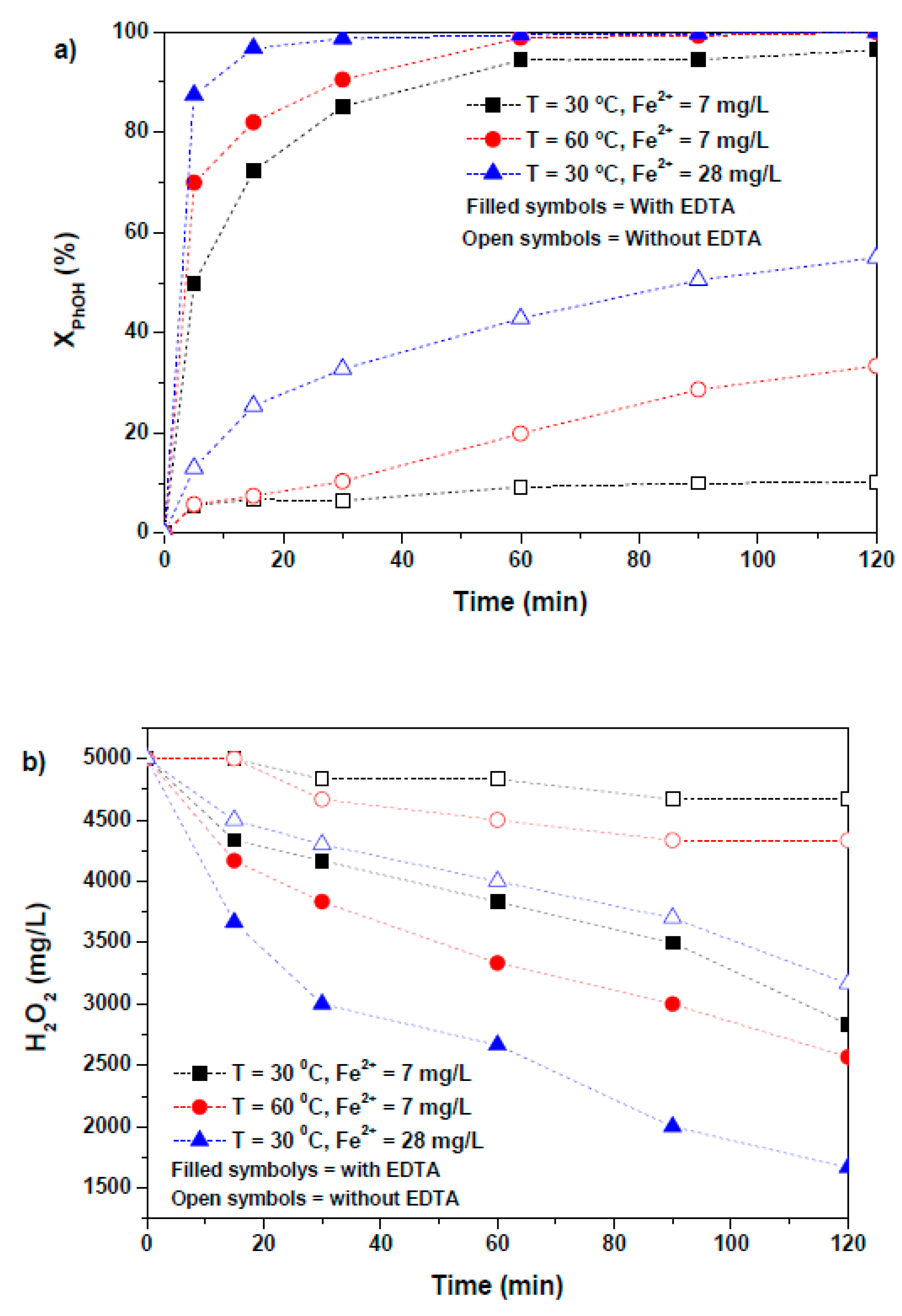
2.4. Effect of Temperature and Iron Concentration
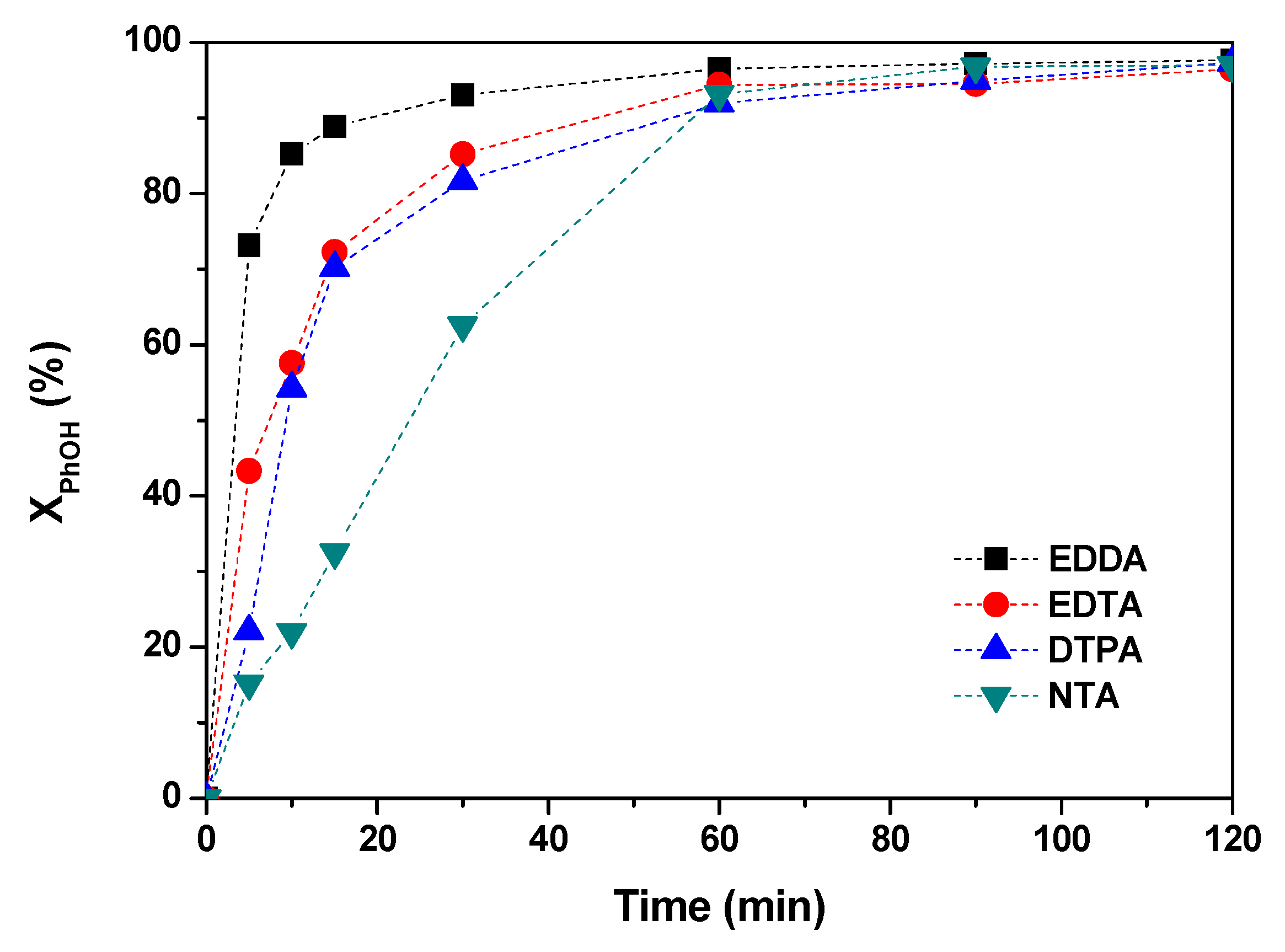
2.5. Effect of the Chelating Agent
3. Materials and Methods
3.1. Chemicals
3.2. Experimental Set-Up and Procedure
3.3. Analytical Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Matatov-Meytal, Y.I.; Sheintuch, M. Catalytic abatement of water pollutants. Ind. Eng. Chem. Res. 1998, 37, 309–326. [Google Scholar] [CrossRef]
- Levec, J.; Pintar, A. Catalytic wet-air oxidation processes. Catal. Today 2007, 124, 172–184. [Google Scholar] [CrossRef]
- Fortuny, A.; Bengoa, C.; Font, J.; Castells, F.; Fabregat, A. Water pollution abatement by catalytic wet air oxidation in a trickle bed reactor. Catal. Today 1999, 53, 107–114. [Google Scholar] [CrossRef]
- Santos, A.; Yustos, P.; Quintanilla, A.; Rodríguez, S.; García-Ochoa, F. Route of the catalytic oxidation of phenol in aqueous phase. Appl. Catal. B 2002, 39, 97–113. [Google Scholar] [CrossRef]
- Pera-Titus, M.; García-Molina, V.; Baños, M.A.; Giménez, J.; Esplugas, S. Degradation of chlorophenols by means of advanced oxidation processes: A general review. Appl. Catal. B 2004, 47, 219–256. [Google Scholar] [CrossRef]
- Jones, C.W. Applications of Hydrogen Peroxide and Derivates; Royal Society of Chemistry: London, UK, 1999. [Google Scholar]
- Azbar, N.; Yonar, T.; Kestioglu, K. Comparison of various advanced oxidation processes and chemical treatment methods for COD and color removal from a polyester and acetate fiber dyeing effluent. Chemosphere 2004, 55, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Esplugas, S.; Giménez, J.; Contreras, S.; Pascual, E.; Rodríguez, M. Comparison of different advanced oxidation processes for phenol degradation. Water Res. 2002, 36, 1034–1042. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.E.; Mackay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Villegas-Guzman, P.; Giannakis, S.; Rtimi, S.; Grandjean, D.; Bensimon, M.; de Alencastro, L.F.; Torres-Palma, R.; Pulgarin, C. A green solar photo-Fenton process for the elimination of bacteria and micropollutants in municipal wastewater treatment using mineral iron and natural organic acids. Appl. Catal. B Environ. 2017, 219, 538–549. [Google Scholar] [CrossRef]
- Villegas-Guzman, P.; Giannakis, S.; Torres-Palma, R.A.; Pulgarin, C. Remarkable enhancement of bacterial inactivation in wastewater through promotion of solar photo-Fenton at near-neutral pH by natural organic acids. Appl. Catal. B Environ. 2017, 205, 219–227. [Google Scholar] [CrossRef]
- Duesterberg, C.K.; Mylon, S.E.; Waite, T.D. pH effects on Iron-catalyzed oxidation using Fenton’s reagent. Environ. Sci. Technol. 2008, 42, 8522–8527. [Google Scholar] [CrossRef]
- Tachiev, G.; Roth, J.A.; Bowers, A.R. Kinetics of hydrogen peroxide decomposition with complexed and “free” iron catalysts. Int. J. Chem. Kinet. 2000, 32, 24–35. [Google Scholar] [CrossRef]
- Usman, M.; Hanna, K.; Haderlein, S. Fenton oxidation to remediate PAHs in contaminated soils: A critical review of major limitations and counter-strategies. Sci. Total Environ. 2016, 569–570, 179–190. [Google Scholar] [CrossRef]
- Barros, W.R.P.; Steter, J.R.; Lanza, M.R.V.; Tavares, A.C. Catalytic activity of Fe3-xCuxO4 (0 ≤ x ≤ 0.25) nanoparticles for the degradation of Amaranth food dye by heterogeneous electro-Fenton process. Appl. Catal. B Environ. 2016, 180, 434–441. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Yu, Y.; Li, S.; Peng, X.; Yang, S.; Zhu, Y.; Chen, L.; Wu, F.; Mailhot, G. Efficient oxidation of bisphenol A with oxysulfur radicals generated by iron-catalyzed autoxidation of sulfite at circumneutral pH under UV irradiation. Environ. Chem. Lett. 2016, 14, 527–532. [Google Scholar] [CrossRef]
- Salazar, L.M.; Grisales, C.M.; Garcia, D.P. How does intensification influence the operational and environmental performance of photo-Fenton processes at acidic and circumneutral pH. Environ. Sci. Pollut. Res. 2019, 26, 4367–4380. [Google Scholar] [CrossRef] [PubMed]
- Bello, M.M.; Raman, A.A.A.; Asghar, A. A review on approaches for addressing the limitations of Fenton oxidation for recalcitrant wastewater treatment. Process Saf. Environ. 2019, 126, 119–140. [Google Scholar] [CrossRef]
- Gupta, S.S.; Stadler, M.; Noser, C.A.; Ghosh, A.; Steinhoff, B.; Lenoir, D.; Horwitz, C.P.; Schramm, K.W.; Collins, T.J. Rapid total destruction of chlorophenols by activated hydrogen peroxide. Science 2002, 296, 326–328. [Google Scholar] [CrossRef]
- Collins, T.J. TAML oxidant activators: A new approach to the activation of hydrogen peroxide for environmentally significant problems. Acc. Chem. Res. 2002, 35, 782–790. [Google Scholar] [CrossRef]
- Wingate, K.G.; Stuthridge, T.R.; Wright, L.J.; Horwitz, C.P.; Collins, T.J. Application of TAML catalysts to remove colour from pulp and paper mill effluents. Water Sci. Technol. 2004, 49, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Chahbane, N.; Popescu, D.; Mitchell, D.A.; Chanda, A.; Lenoir, D.; Ryabov, A.D.; Schramm, K.; Collins, T.J. FeIII–TAML-catalyzed green oxidative degradation of the azo dye Orange II by H2O2 and organic peroxides: Products, toxicity, kinetics, and mechanisms. Green Chem. 2007, 9, 49–57. [Google Scholar] [CrossRef]
- Li, L.; Abea, Y.; Kanagawa, K.; Shoji, T.; Mashino, T.; Mochizuki, M.; Tanaka, M.; Miyata, N. Iron-chelating agents never suppress Fenton reaction but participate in quenching spin-trapped radicals. Anal. Chim. Acta 2007, 599, 315–319. [Google Scholar] [CrossRef]
- Sun, Y.; Pignatello, J.J. Chemical treatment of pesticide wastes: Evaluation of Fe(III) chelates for catalytic hydrogen peroxide oxidation of 2,4-D at circumneutral pH. J. Agric. Food Chem. 1992, 40, 322–327. [Google Scholar] [CrossRef]
- Wink, D.A.; Nims, R.W.; Desrosiers, M.F.; Ford, P.C.; Keefer, L.K. A kinetic investigation of intermediates formed during the Fenton reagent mediated degradation of N-Nitrosodimethylamine: Evidence for an oxidative pathway not involving hydroxyl radical. Chem. Res. Toxicol. 1991, 4, 510–512. [Google Scholar] [CrossRef] [PubMed]
- Bull, C.; McClune, G.J.; Fee, J.A. The mechanism of Fe-EDTA catalyzed superoxide dismutation. J. Am. Chem. Soc. 1983, 105, 5290–5300. [Google Scholar] [CrossRef]
- Wang, Z.; Bush, R.T.; Liu, J. Arsenic(III) and iron(II) co-oxidation by oxygen and hydrogen peroxide: Divergent reactions in the presence of organic ligands. Chemosphere 2013, 93, 1936–1941. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Al-abed, S.R.; Dionysiou, D.D. Effect of inorganic, synthetic and naturally occurring chelating agents on Fe(II) mediated advanced oxidation of chlorophenols. Water Res. 2009, 43, 684–694. [Google Scholar] [CrossRef]
- Sillanpää, M.; Pirkanniemi, K.; Sorokin, A. Oxidation of EDTA with H2O2 catalysed by metallo-phthalocyanines. Environ. Technol. 2009, 30, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Kunz, A.; Peralta-Zamora, P.; Durán, N. Hydrogen peroxide assisted photochemical degradation of ethylenediaminetetraacetic acid. Adv. Environ. Res. 2002, 7, 197–202. [Google Scholar] [CrossRef]
- Engelmann, M.D.; Bobier, R.T.; Hiatt, T.; Cheng, I.F. Variability of the Fenton reaction characteristics of the EDTA, DTPA, and citrate complexes of iron. BioMetals 2003, 16, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Millero, F.J.; Homonnay, Z. The kinetics of the complex formation between iron(III)–ethylenediaminetetraacetate and hydrogen peroxide in aqueous solution. Inorg. Chim. Acta 2004, 357, 3583–3587. [Google Scholar] [CrossRef]
- Rämö, J.; Sillanpää, M. Degradation of EDTA by hydrogen peroxide in alkaline conditions. J. Clean. Prod. 2001, 9, 191–195. [Google Scholar] [CrossRef]
- Chiriţă, P. Hydrogen Peroxide Decomposition by Pyrite in the Presence of Fe(III)-ligands. Chem. Biochem. Eng. Q. 2009, 23, 259–265. [Google Scholar]
- Walling, C.; Kurz, M.; Schugar, H.J. The Iron(III)-Ethylenediaminetetraacetic Acid-Peroxide system. Inorg. Chem. 1970, 9, 931–937. [Google Scholar] [CrossRef]
- Nowack, B.; Sigg, L. Dissolution of Fe(III)(hydr)oxides by metal-EDTA complexes. Geochim. Cosmochim. Acta 1997, 61, 951–963. [Google Scholar] [CrossRef]
- Pierce, E.M.; Wellman, D.M.; Lodge, A.M.; Rodriguez, E.A. Experimental determination of the dissolution kinetics of zero-valent iron in the presence of organic complexants. Environ. Chem. 2007, 4, 260–270. [Google Scholar] [CrossRef]
- Allison, J.D.; Brown, D.S.; Novo-Gradac, K.J. MINTEQA2/PRODEFA2-A Geochemical Model for Environmental Systems: Version 3.0 User’s Manual; EPA/600/3–91/021; US Environmental Protection Agency, Environmental Research Laboratory: Washington, DC, USA, 1991.
- Szpyrkowicz, L.; Juzzolino, C.; Kaul, S.N. A comparative study on oxidation of disperse dyes by electrochemical process, ozone, hypochlorite and Fenton reagent. Water Res. 2001, 35, 2129–2136. [Google Scholar] [CrossRef]
- Lin, S.H.; Chen, M.L. Treatment of textile wastewater by chemical methods for reuse. Water Res. 1997, 31, 868–876. [Google Scholar] [CrossRef]
- Walling, C. Fenton’s reagent revisited. Acc. Chem. Res. 1975, 8, 125–131. [Google Scholar] [CrossRef]
- Oakes, J.; Smith, E.G. Nuclear magnetic resonance studies of transition-metal complexes of Ethylenedi-amine-NNN’N’-tetramethylphosphonate in aqueous solution. J. Chem. Soc. Dalton Trans. 1983, 601–605. [Google Scholar] [CrossRef]
- Francis, K.C.; Cummins, D.; Oakes, J. Kinetic and structural investigations of [FeIII(edta)] − [edta = Ethylenediaminetetra-acetate(4-)] catalysed decomposition of hydrogen peroxide. J. Chem. Soc. Dalton Trans. 1985, 493–501. [Google Scholar] [CrossRef]
- Haber, F.; Weiss, J. The catalytic decomposition of hydrogen peroxide by iron salts. Proc. R. Soc. Lond. Ser. A 1934, 147, 332–351. [Google Scholar]
- Barb, W.G.; Baxendale, J.H.; George, P.; Hargrave, K.R. Reactions of ferrous and ferric ions with hydrogen peroxide. Trans. Faraday Soc. 1951, 47, 462–500. [Google Scholar] [CrossRef]
- Rush, J.D.; Maskos, Z.; Koppenol, W.H. Distinction between hydroxyl radical and ferryl species. Methods Enzymol. 1990, 186, 148–156. [Google Scholar]
- Rahhal, S.; Richter, H.W. Reduction of hydrogen peroxide by the ferrous iron chelate of diethylenetriamine-N,N,N’,N”,N”-pentaacetate. J. Am. Chem. Soc. 1988, 110, 3126–3133. [Google Scholar] [CrossRef]
- Sanchez, I.; Stüber, F.; Font, J.; Fortuny, A.; Fabregat, A.; Bengoa, C. Elimination of phenol and aromatic compounds by zero valent iron and EDTA at low temperature and atmospheric pressure. Chemosphere 2007, 68, 338–344. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association. Method 5310 B. In Standard Methods for the Examination of Water and Wastewater, 17th ed.; Clesceri, L.S., Greenberg, A.E., Trusel, R.R., Franson, M.A., Eds.; American Public Health Association: Washington, DC, USA, 1989. [Google Scholar]


| Compound | Structure | Molecular Formula | Molecular Weight (g/mol) |
|---|---|---|---|
| Ethylenediaminetetraacetic acid (EDTA) | 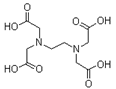 | C10H16N2O8 | 292.24 |
| Ethylenediamine-N,N’-diacetic acid (EDDA) | 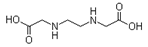 | C6H12N2O4 | 176.17 |
| Diethylenetriaminepenta-acetic acid (DTPA) |  | C14H23N3O10 | 393.35 |
| Nitrilotriacetic acid (NTA) | 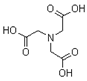 | C6H9NO6 | 191.14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messele, S.A.; Bengoa, C.; Stüber, F.E.; Giralt, J.; Fortuny, A.; Fabregat, A.; Font, J. Enhanced Degradation of Phenol by a Fenton-Like System (Fe/EDTA/H2O2) at Circumneutral pH. Catalysts 2019, 9, 474. https://doi.org/10.3390/catal9050474
Messele SA, Bengoa C, Stüber FE, Giralt J, Fortuny A, Fabregat A, Font J. Enhanced Degradation of Phenol by a Fenton-Like System (Fe/EDTA/H2O2) at Circumneutral pH. Catalysts. 2019; 9(5):474. https://doi.org/10.3390/catal9050474
Chicago/Turabian StyleMessele, Selamawit Ashagre, Christophe Bengoa, Frank Erich Stüber, Jaume Giralt, Agustí Fortuny, Azael Fabregat, and Josep Font. 2019. "Enhanced Degradation of Phenol by a Fenton-Like System (Fe/EDTA/H2O2) at Circumneutral pH" Catalysts 9, no. 5: 474. https://doi.org/10.3390/catal9050474