Thermal Deactivation of Rh/α-Al2O3 in the Catalytic Partial Oxidation of Iso-Octane: Effect of Flow Rate
Abstract
:1. Introduction
2. Results and Discussion
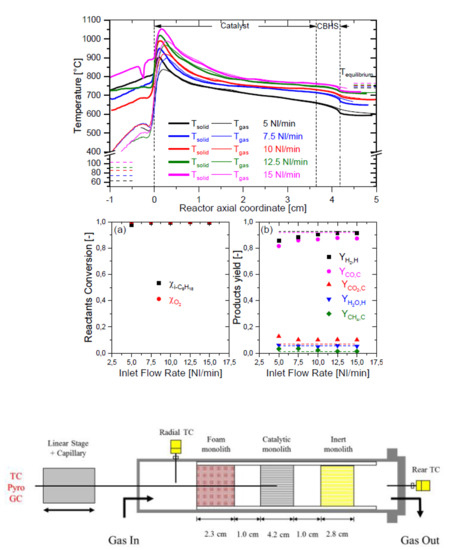
2.1. Conversion and Selectivity Performances
2.2. Modeling Analysis
2.3. Catalyst Stability
3. Materials and Methods
3.1. Catalyst Synthesis, Structural and Morphological Characterizations
3.2. Experimental Setup
3.3. Reactor Modelling
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- U.S. Energy Information Administration. International Energy Outlook 2017; U.S. Energy Information Administration: Washington, DC, USA, 2017.
- Carrera, A.; Beretta, A.; Groppi, G. Catalytic Partial Oxidation of Iso-Octane over Rh/α-Al2O3 in an Adiabatic Reactor: An Experimental and Modeling Study. Ind. Eng. Chem. Res. 2017, 56, 4911–4919. [Google Scholar] [CrossRef]
- Yvonne, R.; Simon, L.; Johannes Pfister, C.D. Fuel Cells and Hydrogen for Green Energy in European Cities and Regions. A Study for the Fuel Cells and Hydrogen Joint Undertaking; Sederanger: Frankfurt, Germany, 2018. [Google Scholar]
- Farrauto, R.J.; Liu, Y.; Ruettinger, W.; Ilinich, O.; Shore, L.; Giroux, T. Precious Metal Catalysts Supported on Ceramic and Metal Monolithic Structures for the Hydrogen Economy. Catal. Rev. 2007, 49, 141–196. [Google Scholar] [CrossRef]
- Heck, R.M.; Gulati, S.; Farrauto, R.J. The Application of Monoliths for Gas Phase Catalytic Reactions. Chem. Eng. J. 2001, 82, 149–156. [Google Scholar] [CrossRef]
- Farrauto, R.; Hwang, S.; Shore, L.; Ruettinger, W.; Lampert, J.; Giroux, T.; Liu, Y.; Ilinich, O. New Material Needs for Hydrocarbon Fuel Processing: Generating Hydrogen for the PEM Fuel Cell. Annu. Rev. Mater. Res. 2003, 33, 1–27. [Google Scholar] [CrossRef]
- Groppi, G.; Tronconi, E. Honeycomb Supports with High Thermal Conductivity for Gas/Solid Chemical Processes. Catal. Today 2005, 105, 297–304. [Google Scholar] [CrossRef]
- Specchia, S. Fuel Processing Activities at European Level: A Panoramic Overview. Int. J. Hydrog. Energy 2014, 39, 17953–17968. [Google Scholar] [CrossRef]
- Kraaij, G.J.; Specchia, S.; Bollito, G.; Mutri, L.; Wails, D. Biodiesel Fuel Processor for APU Applications. Int. J. Hydrog. Energy 2009, 34, 4495–4499. [Google Scholar] [CrossRef]
- Specchia, S.; Tillemans, F.W.A.; van den Oosterkamp, P.F.; Saracco, G. Conceptual Design and Selection of a Biodiesel Fuel Processor for a Vehicle Fuel Cell Auxiliary Power Unit. J. Power Sources 2005, 145, 683–690. [Google Scholar] [CrossRef]
- Kolb, G.; Baier, T.; Schürer, J.; Tiemann, D.; Ziogas, A.; Specchia, S.; Galletti, C.; Germani, G.; Schuurman, Y. A Micro-Structured 5 kW Complete Fuel Processor for Iso-Octane as Hydrogen Supply System for Mobile Auxiliary Power Units Part II—Development of Water–Gas Shift and Preferential Oxidation Catalysts Reactors and Assembly of the Fuel Processor. Chem. Eng. J. 2008, 138, 474–489. [Google Scholar] [CrossRef]
- Hou, Z.; Chen, P.; Fang, H.; Zheng, X.; Yashima, T. Production of Synthesis Gas via Methane Reforming with CO2 on Noble Metals and Small Amount of Noble-(Rh-) Promoted Ni Catalysts. Int. J. Hydrog. Energy 2006, 31, 555–561. [Google Scholar] [CrossRef]
- le Saché, E.; Santos, J.; Smith, T.J.; Centeno, M.A.; Arellano-Garcia, H.; Odriozola, J.A.; Reina, T.R. Multicomponent Ni-CeO2 Nanocatalysts for Syngas Production from CO2/CH4 Mixtures. J. CO2 Util. 2018, 25, 68–78. [Google Scholar]
- Tavazzi, I.; Beretta, A.; Groppi, G.; Maestri, M.; Tronconi, E.; Forzatti, P. Experimental and Modeling Analysis of the Effect of Catalyst Aging on the Performance of a Short Contact Time Adiabatic CH4-CPO Reactor. Catal. Today 2007, 129, 372–379. [Google Scholar] [CrossRef]
- Fichtner, M.; Mayer, J.; Wolf, D.; Schubert, K. Microstructured Rhodium Catalysts for the Partial Oxidation of Methane to Syngas under Pressure. Ind. Eng. Chem. Res. 2001, 40, 3475–3483. [Google Scholar] [CrossRef]
- Maestri, M.; Beretta, A.; Groppi, G.; Tronconi, E.; Forzatti, P. Comparison among Structured and Packed-Bed Reactors for the Catalytic Partial Oxidation of CH4 at Short Contact Times. Catal. Today 2005, 105, 709–717. [Google Scholar] [CrossRef]
- Qi, A.; Wang, S.; Ni, C.; Wu, D. Autothermal Reforming of Gasoline on Rh-Based Monolithic Catalysts. Int. J. Hydrog. Energy 2007, 32, 981–991. [Google Scholar] [CrossRef]
- Costa, D.S.; Gomes, R.S.; Rodella, C.B.; da Silva, R.B.; Fréty, R.; Teixeira Neto, É.; Brandão, S.T. Study of Nickel, Lanthanum and Niobium-Based Catalysts Applied in the Partial Oxidation of Methane. Catal. Today 2018. [Google Scholar] [CrossRef]
- Carrera, A.; Pelucchi, M.; Stagni, A.; Beretta, A.; Groppi, G. Catalytic Partial Oxidation of n-Octane and Iso-Octane: Experimental and Modeling Results. Int. J. Hydrog. Energy 2017, 42, 24675–24688. [Google Scholar] [CrossRef]
- Nogare, D.D.; Degenstein, N.J.; Horn, R.; Canu, P.; Schmidt, L.D. Modeling Spatially Resolved Data of Methane Catalytic Partial Oxidation on Rh Foam Catalyst at Different Inlet Compositions and Flowrates. J. Catal. 2011, 277, 134–148. [Google Scholar] [CrossRef]
- Panuccio, G.J.; Williams, K.A.; Schmidt, L.D. Contributions of Heterogeneous and Homogeneous Chemistry in the Catalytic Partial Oxidation of Octane Isomers and Mixtures on Rhodium Coated Foams. Chem. Eng. Sci. 2006, 61, 4207–4219. [Google Scholar] [CrossRef]
- Wanat, E.C.; Venkataraman, K.; Schmidt, L.D. Steam Reforming and Water–Gas Shift of Ethanol on Rh and Rh–Ce Catalysts in a Catalytic Wall Reactor. Appl. Catal. A Gen. 2004, 276, 155–162. [Google Scholar] [CrossRef]
- Degenstein, N.; Subramanian, R.; Schmidt, L. Partial Oxidation of n-Hexadecane at Short Contact Times: Catalyst and Washcoat Loading and Catalyst Morphology. Appl. Catal. A Gen. 2006, 305, 146–159. [Google Scholar] [CrossRef]
- Donazzi, A.; Maestri, M.; Michael, B.C.; Beretta, A.; Forzatti, P.; Groppi, G.; Tronconi, E.; Schmidt, L.D.; Vlachos, D.G. Microkinetic Modeling of Spatially Resolved Autothermal CH4 Catalytic Partial Oxidation Experiments over Rh-Coated Foams. J. Catal. 2010, 275, 270–279. [Google Scholar] [CrossRef]
- Maestri, M.; Beretta, A.; Faravelli, T.; Groppi, G.; Tronconi, E.; Vlachos, D.G. Two-Dimensional Detailed Modeling of Fuel-Rich H2 Combustion over Rh/Al2O3 Catalyst. Chem. Eng. Sci. 2008, 63, 2657–2669. [Google Scholar] [CrossRef]
- Batista da Silva, R.; Brandão, S.T.; Lucotti, A.; Tommasini, M.S.; Castiglioni, C.; Groppi, G.; Beretta, A. Chemical Pathways in the Partial Oxidation and Steam Reforming of Acetic Acid over a Rh-Al2O3 catalyst. Catal. Today 2017, 289, 162–172. [Google Scholar] [CrossRef]
- Donazzi, A.; Livio, D.; Beretta, A.; Groppi, G.; Forzatti, P. Surface Temperature Profiles in CH4 CPO over Honeycomb Supported Rh Catalyst Probed with in Situ Optical Pyrometer. Appl. Catal. A Gen. 2011, 402, 41–49. [Google Scholar] [CrossRef]
- Beretta, A.; Donazzi, A.; Groppi, G.; Maestri, M.; Tronconi, E.; Forzatti, P. Gaining Insight into the Kinetics of Partial Oxidation of Light Hydrocarbons on Rh, through a Multiscale Methodology Based on Advanced Experimental and Modeling Techniques. Catalysis 2013, 25, 1–49. [Google Scholar]
- Iaquaniello, G.; Antonetti, E.; Cucchiella, B.; Palo, E.; Salladini, A.; Guarinoni, A.; Lainati, A.; Basini, L. Natural Gas Catalytic Partial Oxidation: A Way to Syngas and Bulk Chemicals Production. In Natural Gas—Extraction to End Use; InTech: London, UK, 2012. [Google Scholar] [Green Version]
- Pagani, D.; Batista, R.; Silva, D.; Moioli, E.; Donazzi, A.; Lucotti, A.; Tommasini, M.; Castiglioni, C.; Brandao, S.T.; Beretta, A.; et al. Annular Reactor Testing and Raman Surface Characterization of the CPO of i-Octane and n-Octane on Rh Based Catalyst. Chem. Eng. J. 2016, 294, 9–21. [Google Scholar] [CrossRef]
- Beretta, A.; Groppi, G.; Carrera, A.; Donazzi, A. Analysis of the Impact of Gas-Phase Chemistry in Adiabatic CPO Reactors by Axially Resolved Measurements; Academic Press: Cambridge, MA, USA, 2017; pp. 161–201. [Google Scholar]
- Beretta, A.; Groppi, G.; Lualdi, M.; Tavazzi, I.; Forzatti, P. Experimental and Modeling Analysis of Methane Partial Oxidation: Transient and Steady-State Behavior of Rh-Coated Honeycomb Monoliths. Ind. Eng. Chem. Res. 2009, 48, 3825–3836. [Google Scholar] [CrossRef]
- Cimino, S.; Lisi, L.; Russo, G. Effect of Sulphur during the Catalytic Partial Oxidation of Ethane over Rh and Pt Honeycomb Catalysts. Int. J. Hydrog. Energy 2012, 37, 10680–10689. [Google Scholar] [CrossRef]
- Livio, D.; Donazzi, A.; Beretta, A.; Groppi, G.; Forzatti, P. Experimental and Modeling Analysis of the Thermal Behavior of an Autothermal C3H8 Catalytic Partial Oxidation Reformer. Ind. Eng. Chem. Res. 2012, 51, 7573–7583. [Google Scholar] [CrossRef]
- Donazzi, A.; Beretta, A.; Groppi, G.; Forzatti, P. Catalytic Partial Oxidation of Methane over a 4% Rh/α-Al2O3 catalyst. Part I: Kinetic Study in Annular Reactor. J. Catal. 2008, 255, 241–258. [Google Scholar] [CrossRef]
- Pagani, D.; Livio, D.; Donazzi, A.; Beretta, A.; Groppi, G.; Maestri, M.; Tronconi, E. A Kinetic Analysis of the Partial Oxidation of C3H8 over a 2% Rh/Al2O3 Catalyst in Annular Microreactor. Catal. Today 2012, 197, 265–280. [Google Scholar] [CrossRef]







| Table | χCH4 | χO2 | σH2 | σCO | σCO2 | σH2O |
|---|---|---|---|---|---|---|
| [-] | [-] | [-] | [-] | [-] | [-] | |
| equilibrium | 0.86 | 1.00 | 0.92 | 0.86 | 0.14 | 0.08 |
| fresh catalyst | 0.84 | 1.00 | 0.90 | 0.84 | 0.16 | 0.10 |
| after 5 NL/min | 0.84 | 1.00 | 0.90 | 0.85 | 0.15 | 0.10 |
| after 7.5 NL/min | 0.84 | 1.00 | 0.90 | 0.85 | 0.15 | 0.10 |
| after 10 NL/min | 0.84 | 1.00 | 0.90 | 0.85 | 0.15 | 0.10 |
| after 12.5 NL/min | 0.84 | 1.00 | 0.90 | 0.85 | 0.15 | 0.10 |
| after 15 NL/min | 0.84 | 1.00 | 0.90 | 0.84 | 0.16 | 0.10 |
| Catalyst | Rh Load | Rh Dispersion (%) | ||
|---|---|---|---|---|
| % w/w | Fresh | Aged (4% CH4) | Aged (27% CH4) | |
| Rh/α-Al2O3 | 1.71 | 69 | 23 | 7 |
| Catalyst | Lcat | LCBHS | mcat | tcat | Void Fraction |
|---|---|---|---|---|---|
| [cm] | [cm] | [g] | [μm] | [-] | |
| Rh/α-Al2O3 | 3.65 | 0.53 | 0.63 | 9.43 | 0.714 |
| Gas Phase | |
| Mass Balance | |
| Enthalpy Balance | |
| Solid Phase | |
| Mass Balance | |
| Enthalpy Balance | |
| Boundary Conditions | |
| Reactor Inlet | |
| Reactor Outlet | |
| Initial Conditions |
| Reaction Name and Chemical Equation | Rate Equation [mol/atm/gcat/s] | ki@873K [mol/atm/gcat/s] | Eactivation [kJ/mol] | Ref. | |||
|---|---|---|---|---|---|---|---|
| CH4 oxidation CH4 + 2 O2 → CO2 + 2 H2O | 1.030 × 10−1 | 91.96 | [33] | ||||
| CH4 steam reforming CH4 + H2O → CO + 3 H2 | 1.027 × 10−1 | 91.80 | [33] | ||||
| CO methanation CO +3 H2 → CH4 + H2O | 1.500 × 10−3 | 30.00 | [26] | ||||
| Water Gas Shift CO + H2O → CO2 + H2 | 6.831 × 10−3 | 74.83 | [33] | ||||
| Reverse Water Gas Shift CO2 + H2 → CO + H2O | 1.277 × 10−2 | 62.37 | [26] | ||||
| H2 oxidation H2 + 1/2 O2 → H2O | 2.666 × 103 | 61.65 | [33] | ||||
| CO oxidation CO + 1/2 O2 → CO2 | 1.937 × 101 | 76.07 | [33] | ||||
| iso-C8H18 total oxidation iso-C8H18 + 25/2 O2 → 8 CO2 + 9 H2O | 4.600 × 10−1 | 80.00 | [34] | ||||
| iso-C8H18 steam reforming iso-C8H18 + 8 H2O → 8 CO + 17 H2 | 7.500 × 10−2 | 69.00 | [34] | ||||
| Adsorption | Ki0,ads @873K [1/atm] | ΔHadsorption [kJ/mol] | Ref. | ||||
| O2 | 5.461 × 100 | −72.83 | [33] | ||||
| CO | 2.114 × 102 | −37.15 | [33] | ||||
| H2O | 8.974 × 100 | −57.48 | [33] | ||||
| Poisoning term | Ki0, poisoning @873K [-] | ΔHadsorption [kJ/mol] | Ref. | ||||
| i-C8H18 | 6.000 × 100 | −26.00 | [34] | ||||
| n-C8H18 | 6.000 × 100 | −26.00 | [34] | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista, R.; Carrera, A.; Beretta, A.; Groppi, G. Thermal Deactivation of Rh/α-Al2O3 in the Catalytic Partial Oxidation of Iso-Octane: Effect of Flow Rate. Catalysts 2019, 9, 532. https://doi.org/10.3390/catal9060532
Batista R, Carrera A, Beretta A, Groppi G. Thermal Deactivation of Rh/α-Al2O3 in the Catalytic Partial Oxidation of Iso-Octane: Effect of Flow Rate. Catalysts. 2019; 9(6):532. https://doi.org/10.3390/catal9060532
Chicago/Turabian StyleBatista, Roberto, Andrea Carrera, Alessandra Beretta, and Gianpiero Groppi. 2019. "Thermal Deactivation of Rh/α-Al2O3 in the Catalytic Partial Oxidation of Iso-Octane: Effect of Flow Rate" Catalysts 9, no. 6: 532. https://doi.org/10.3390/catal9060532