Novel Bi3O5I2 Hollow Microsphere and Its Enhanced Photocatalytic Activity
Abstract
:1. Introduction
2. Results
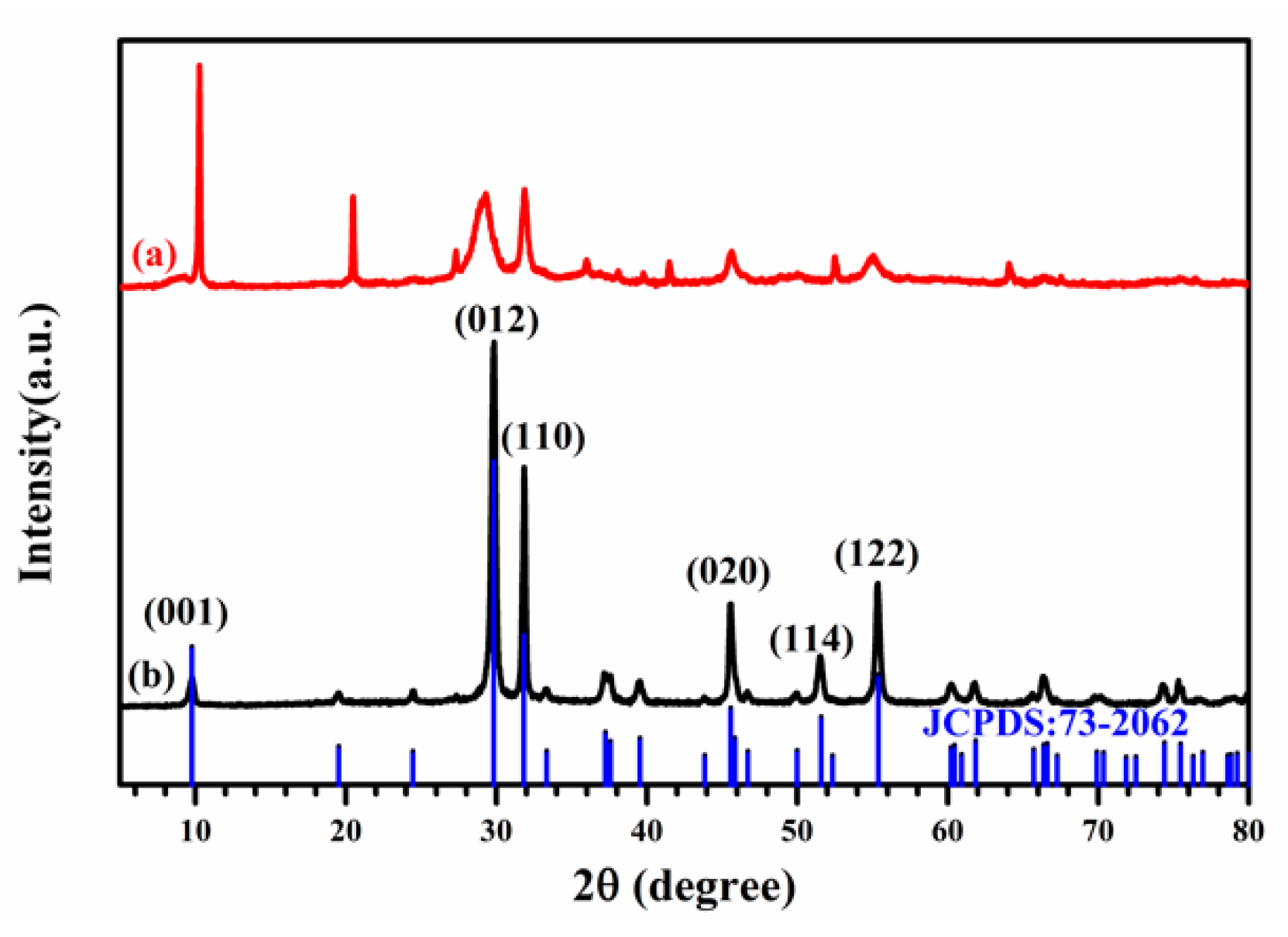
2.1. Molecular Formula Characterization and Phase Transformation
2.2. Structure Investigation and Morphology Observation
2.3. Optical Property and Photoelectric Property of the Photocatalysts
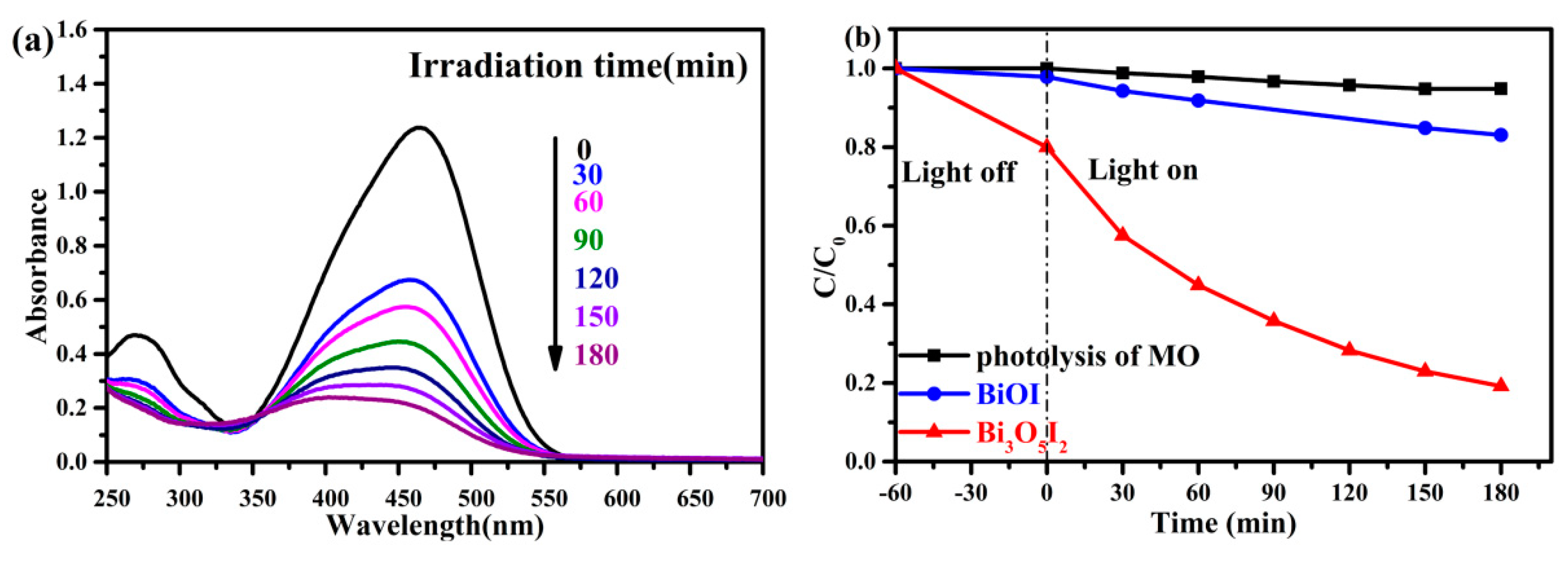
2.4. Photocatalytic Performance of the Photocatalysts
2.5. The Lifetime eValuation of the Photocatalysts
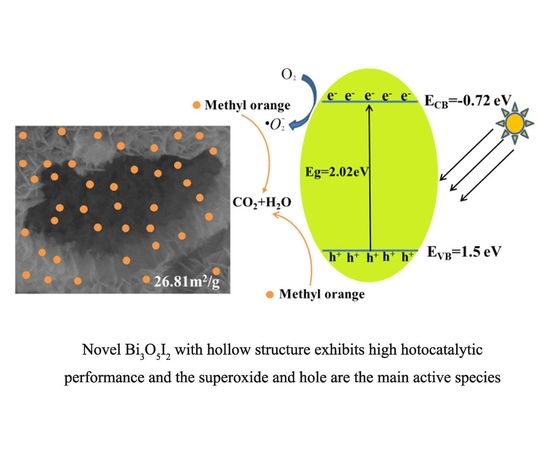
2.6. The Possible Photacatalytic Mechanism
3. Materials and Method
3.1. Materials
3.2. Synthesis of the Bi3O5I2 and BiOI
3.3. Photocatalyst Characterization
3.4. Test of Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Peng, Y.; Yu, P.-P.; Zhou, H.-Y.; Xu, A.-W. Synthesis of BiOI/Bi4O5I2/Bi2O2CO3 p–n–p heterojunctions with superior photocatalytic activities. New J. Chem. 2015, 39, 8321–8328. [Google Scholar] [CrossRef]
- Dong, F.; Xiong, T.; Sun, Y.; Zhang, Y.X.; Zhou, Y. Controlling interfacial contact and exposed facets for enhancing photocatalysis via 2D–2D heterostructures. Chem. Commun. 2015, 51, 8249–8252. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, X. Fast Degradation of Monochloroacetic Acid by BiOI-Enhanced UV/S(IV) Process: Efficiency and Mechanism. Catalysts 2019, 9, 460. [Google Scholar] [CrossRef]
- Liu, H.; Cao, W.-R.; Su, Y.; Chen, Z.; Wang, Y. Bismuth oxyiodide–graphene nanocomposites with high visible light photocatalytic activity. J. Colloid Interface Sci. 2013, 398, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Huang, B.; Dai, Y. Engineering BiOX (X = Cl, Br, I) nanostructures for highly efficient photocatalytic applications. Nanoscale 2014, 6, 2009. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Hojamberdiev, M.; Zhang, S.; Din, S.T.U.; Yang, W. Enhancing visible-light-induced photocatalytic activity of BiOI microspheres for NO removal by synchronous coupling with Bi metal and graphene. Appl. Surf. Sci. 2019, 467, 968–978. [Google Scholar] [CrossRef]
- Ye, L.; Jin, X.; Ji, X.; Liu, C.; Su, Y.; Xie, H.; Liu, C. Facet-dependent photocatalytic reduction of CO2 on BiOI nanosheets. Chem. Eng. J. 2016, 291, 39–46. [Google Scholar] [CrossRef]
- Long, Y.; Wang, Y.; Zhang, D.; Ju, P.; Sun, Y. Facile synthesis of BiOI in hierarchical nanostructure preparation and its photocatalytic application to organic dye removal and biocidal effect of bacteria. J. Colloid Interface Sci. 2016, 481, 47–56. [Google Scholar] [CrossRef]
- Lee, G.-J.; Zheng, Y.-C.; Wu, J.J. Fabrication of hierarchical bismuth oxyhalides (BiOX, X = Cl, Br, I) materials and application of photocatalytic hydrogen production from water splitting. Catal. Today 2018, 307, 197–204. [Google Scholar] [CrossRef]
- Dai, W.W.; Zhao, Z.Y. Electronic structure and optical properties of BiOI as a photocatalyst driven by visible light. Catalysts 2016, 6, 133. [Google Scholar] [CrossRef]
- Huang, H.; Xiao, K.; Zhang, T.; Dong, F.; Zhang, Y. Rational design on 3D hierarchical bismuth oxyiodides via in situ self-template phase transformation and phase-junction construction for optimizing photocatalysis against diverse contaminants. App. Catal. B Environ. 2017, 203, 879–888. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, T.; Wang, P.; Wang, L.; Ye, L. Bismuth-rich Bi4O5X2 (X = Br, and I) nanosheets with dominant {101} facets exposure for photocatalytic H2 eVolution. Chem. Eng. J. 2016, 304, 454–460. [Google Scholar] [CrossRef]
- Hu, J.; Weng, S.; Zheng, Z.; Pei, Z.; Huang, M.; Liu, P. Solvents mediated-synthesis of BiOI photocatalysts with tunable morphologies and their visible-light driven photocatalytic performances in removing of arsenic from water. J. Hazard. Mater. 2014, 264, 293–302. [Google Scholar] [CrossRef]
- Cao, J.; Zhou, C.; Lin, H.; Xu, B.; Chen, S. Direct hydrolysis preparation of plate-like BiOI and their visible light photocatalytic activity for contaminant removal. Mater. Lett. 2013, 109, 74–77. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Ge, Y.; Xu, L.; Xu, H.; He, M.; Zhang, Q.; Li, H. Reactable ionic liquid-assisted rapid synthesis of BiOI hollow microspheres at room temperature with enhanced photocatalytic activity. J. Mater. Chem. A 2014, 2, 15864–15874. [Google Scholar] [CrossRef]
- Zhang, G.; Su, A.; Qu, J.; Xu, Y. Synthesis of BiOI flowerlike hierarchical structures toward photocatalytic reduction of CO2 to CH4. Mater. Res. Bull. 2014, 55, 43–47. [Google Scholar] [CrossRef]
- Han, J.; Zhu, G.; Hojamberdiev, M.; Peng, J.; Zhang, X.; Liu, Y.; Ge, B. Rapid adsorption and photocatalytic activity for Rhodamine B and Cr(vi) by ultrathin BiOI nanosheets with highly exposed {001} facets. New J. Chem. 2015, 39, 1874–1882. [Google Scholar] [CrossRef]
- Shan, L.-W.; He, L.-Q.; Suriyaprakash, J.; Yang, L.-X. Photoelectrochemical (PEC) water splitting of BiOI{001} nanosheets synthesized by a simple chemical transformation. J. Alloys Compd. 2016, 665, 158–164. [Google Scholar] [CrossRef]
- Huang, H.; Xiao, K.; He, Y.; Zhang, T.; Dong, F.; Du, X.; Zhang, Y. In situ assembly of BiOI@Bi12O17Cl2 p—n junction: Charge induced unique front-lateral surfaces coupling heterostructure with high exposure of BiOI {001} active facets for robust and nonselective photocatalysis. Appl. Catal. B Environ. 2016, 199, 75–86. [Google Scholar] [CrossRef]
- Zeng, L.; Zhe, F.; Wang, Y.; Zhang, Q.; Zhao, X.; Hu, X.; Wu, Y.; He, Y. Preparation of interstitial carbon doped BiOI for enhanced performance in photocatalytic nitrogen fixation and methyl orange degradation. J. Colloid Interf. Sci. 2019, 539, 563–574. [Google Scholar] [CrossRef]
- Dai, B.; Zhang, A.; Liu, Z.; Wang, T.; Li, C.; Zhang, C.; Li, H.; Liu, Z.; Zhang, X. Facile synthesis of metallic Bi deposited BiOI composites with the aid of EDTA-2Na for highly efficient Hg-0 removal. Catal. Commun. 2019, 121, 53–56. [Google Scholar] [CrossRef]
- Liu, H.; Cao, W.; Su, Y.; Wang, Y.; Wang, X. Synthesis, characterization and photocatalytic performance of novel visible-light-induced Ag/BiOI. Appl. Catal. B Environ. 2012, 111, 271–279. [Google Scholar] [CrossRef]
- Huang, H.; Liu, K.; Zhang, Y.; Chen, K.; Zhang, Y.; Tian, N. Tunable 3D hierarchical graphene–BiOI nanoarchitectures: Their in situ preparation, and highly improved photocatalytic performance and photoelectrochemical properties under visible light irradiation. RSC Adv. 2014, 4, 49386–49394. [Google Scholar] [CrossRef]
- Shi, X.; Wang, P.; Wang, L.; Bai, Y.; Xie, H.; Zhou, Y.; Ye, L. Change in photocatalytic NO removal mechanisms of ultrathin BiOBr/BiOI via NO3-adsorption. Appl. Catal. B Environ. 2019, 243, 322–329. [Google Scholar] [CrossRef]
- Al-Keisy, A.; Ren, L.; Xu, X.; Hao, W.; Dou, S.X.; Du, Y. Selective ferroelectric BiOI/Bi4Ti3O12 heterostructures for visible light-driven photocatalysis. J. Phys. Chem. C 2019, 123, 517–525. [Google Scholar] [CrossRef]
- Yan, P.; Jiang, D.; Li, H.; Cheng, M.; Xu, L.; Qian, J.; Bao, J.; Xia, J.; Li, H. Exploitation of a photoelectrochemical sensing platform for catechol quantitative determination using BiPO4 nanocrystals/BiOI heterojunction. Anal. Chim. Acta 2018, 1042, 11–19. [Google Scholar] [CrossRef]
- Gao, P.; Yan, T.; Liu, H.; Sun, M.; Wei, Q.; Xu, W.; Wang, X.; Du, B. Facile synthesized highly active BiOI/Zn2GeO4 composites for the elimination of endocrine disrupter BPA under visible light irradiation. New J. Chem. 2015, 39, 3964–3972. [Google Scholar]
- Jin, X.; Lv, C.; Zhou, X.; Zhang, C.; Meng, Q.; Liu, Y.; Chen, G. Molecular adsorption promotes carrier migration: Key step for molecular oxygen activation of defective Bi4O5I2. Appl. Catal. B Environ. 2018, 226, 53–60. [Google Scholar] [CrossRef]
- Cao, J.; Li, X.; Lin, H.; Xu, B.; Luo, B.; Chen, S. Low temperature synthesis of novel rodlike Bi5O7I with visible light photocatalytic performance. Mater. Lett. 2012, 76, 181–183. [Google Scholar] [CrossRef]
- Dai, B.J.; Zhang, A.C.; Zhang, D.; Liu, Z.C.; Li, H.X.; Wang, R.R.; Zhang, X.M. Effect of preparation method on the structure and photocatalytic performance of BiOI and Bi5O7I for Hg-0 removal. Atmos. Pollut. Res. 2019, 10, 355–362. [Google Scholar] [CrossRef]
- Sun, S.M.; Wang, W.Z.; Zhang, L.; Zhou, L.; Yin, W.Z.; Shang, M. Visible light-induced efficient contaminant removal by Bi5O7I. Environ. Sci. Technol. 2009, 43, 2005–2010. [Google Scholar] [CrossRef]
- Chuang, C.-W.; Siao, C.-W.; Lee, W.W.; Lu, C.-S.; Chen, Y.-J.; Fu, J.-Y. Synthesis of bismuth oxyiodides and their composites: Characterization, photocatalytic activity, and degradation mechanisms. RSC Adv. 2015, 5, 23450–23463. [Google Scholar]
- Xiao, X.; Zhang, W.D. Hierarchical Bi7O9I3 micro/nano-architecture: Facile synthesis, growth mechanism, and high visible light photocatalytic performance. Rsc. Adv. 2011, 1, 1099–1105. [Google Scholar] [CrossRef]
- Xiao, X.; Xing, C.; He, G.; Zuo, X.; Nan, J.; Wang, L. Solvothermal synthesis of novel hierarchical Bi4O5I2 nanoflakes with highly visible light photocatalytic performance for the degradation of 4-tert-butylphe. Appl. Catal. B Environ. 2014, 148, 154–163. [Google Scholar] [CrossRef]
- Zhang, L.; Gonçalves, A.A.S.; Jiang, B.; Jaroniec, M. Capture of Iodide by Bismuth Vanadate and Bismuth Oxide: An Insight into the Process and its Aftermath. ChemSusChem 2018, 11, 1486–1493. [Google Scholar] [CrossRef]
- Liu, Q.-C.; Ma, D.-K.; Hu, Y.-Y.; Zeng, Y.-W.; Huang, S.-M. Various Bismuth Oxyiodide Hierarchical Architectures: Alcohothermal-Controlled Synthesis, Photocatalytic Activities, and Adsorption Capabilities for Phosphate in Water. ACS Appl. Mater. Interfaces 2013, 5, 11927–11934. [Google Scholar] [CrossRef]
- Xia, J.; Yin, S.; Li, H.; Xu, H.; Yan, Y.; Zhang, Q. Self-Assembly and Enhanced Photocatalytic Properties of BiOI Hollow Microspheres via a Reactable Ionic Liquid. Langmuir 2011, 27, 1200–1206. [Google Scholar] [CrossRef]
- Ma, D.-K.; Zhou, S.-M.; Hu, X.; Jiang, Q.-R.; Huang, S.-M. Hierarchical BiOI and hollow Bi2WO6 microspheres: Topochemical conversion and photocatalytic activities. Mater. Chem. Phys. 2013, 140, 11–15. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, D.; Liu, J.; Ren, K.; Luo, H.; Peng, Y.; Li, G.; Yu, X. A novel nanoreactor framework of iodine-incorporated BiOCl core–shell structure: Enhanced light-harvesting system for photocatalysis. CrystEngComm 2012, 14, 700–707. [Google Scholar] [CrossRef]
- Ji, M.; Xia, J.; Di, J.; Wang, B.; Yin, S.; Xu, L.; Zhao, J.; Li, H. Ionic liquid-assisted bidirectional regulation strategy for carbon quantum dots (CQDs)/Bi4O5I2 nanomaterials and enhanced photocatalytic properties. J. Colloid Interface Sci. 2016, 478, 324–333. [Google Scholar] [CrossRef]
- Tu, S.; Zheng, C.; Zhong, H.; Lu, M.; Xiao, X.; Zuo, X.; Nan, J. Flower-like Bi4O5I2/Bi5O7 I nanocomposite: Facile hydrothermal synthesis and efficient photocatalytic degradation of propylparaben under visible-light irradiation. RSC Adv. 2016, 6, 44552–44560. [Google Scholar] [CrossRef]
- Ren, K.; Zhang, K.; Liu, J.; Luo, H.; Huang, Y.; Yu, X. Controllable synthesis of hollow/flower-like BiOI microspheres and highly efficient adsorption and photocatalytic activity. CrystEngComm 2012, 14, 4384. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.-J. Room temperature synthesis of Bi 4 O 5 I 2 and Bi 5 O 7 I ultrathin nanosheets with a high visible light photocatalytic performance. Dalton Trans. 2016, 45, 7720–7727. [Google Scholar] [CrossRef]
- Wang, W.; Chen, X.-Q.; Liu, G.; Shen, Z.; Xia, D.; Wong, P.K.; Yu, J.C. Monoclinic dibismuth tetraoxide: A new visible-light-driven photocatalyst for environmental remediation. Appl. Catal. B Environ. 2015, 176, 444–453. [Google Scholar] [CrossRef]
- Long, Y.; Li, L.; Wang, S.; Chen, Y.; Wang, L.; Zhang, S.; Luo, L.; Jiang, F. Photocatalytic Removal of 17α-Ethinyl Estradiol Using the Bi2O3/Bi2O4 Photocatalyst. Catal. Lett. 2018, 148, 3608–3617. [Google Scholar] [CrossRef]
- Xia, J.; Ji, M.; Di, J.; Wang, B.; Yin, S.; He, M.; Zhang, Q.; Li, H. Improved photocatalytic activity of few-layer Bi4O5I2 nanosheets induced by efficient charge separation and lower valence position. J. Alloys Compd. 2017, 695, 922–930. [Google Scholar] [CrossRef]
- Cui, S.; Shan, G.; Zhu, L. Solvothermal synthesis of I-deficient BiOI thin film with distinct photocatalytic activity and durability under simulated sunlight. Appl. Catal. B Environ. 2017, 219, 249–258. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, B.; Dai, Y.; Qin, X.; Zhang, X. One-Step Synthesis of the Nanostructured AgI/BiOI Composites with Highly Enhanced Visible-Light Photocatalytic Performances. Langmuir 2010, 26, 6618–6624. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, K.; Zhang, L. Visible Light Photocatalysis of BiOI and Its Photocatalytic Activity Enhancement by in Situ Ionic Liquid Modification. J. Phys. Chem. C 2011, 115, 14300–14308. [Google Scholar] [CrossRef]
- Ning, S.B.; Lin, H.X.; Tong, Y.C.; Zhang, X.Y.; Lin, Q.Y.; Zhang, Y.Q.; Long, J.L.; Wang, X.X. Dual couples Bi metal depositing and Ag@AgI islanding on BiOI 3D architectures for synergistic bactericidal mechanism of E-coli under visible light. Appl. Catal. B Environ. 2017, 204, 53–60. [Google Scholar] [CrossRef]
- Chang, C.; Zhu, L.; Fu, Y.; Chu, X. Highly active Bi/BiOI composite synthesized by one-step reaction and its capacity to degrade bisphenol A under simulated solar light irradiation. Chem. Eng. J. 2013, 233, 305–314. [Google Scholar] [CrossRef]
- Bao, C.; Wang, C.; Fan, D.; Ma, H.; Hu, L.; Fan, Y.; Wei, Q. A novel sandwich-type photoelectrochemical sensor for SCCA detection based on Ag2S-sensitized BiOI matrix and AucorePdshell nanoflower label for signal amplification. New J. Chem. 2018, 42, 15762–15769. [Google Scholar] [CrossRef]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, B.; Cui, H.; Li, Z.; Dong, H.; Li, X.; Zhao, L.; Wang, J. Novel Bi3O5I2 Hollow Microsphere and Its Enhanced Photocatalytic Activity. Catalysts 2019, 9, 709. https://doi.org/10.3390/catal9090709
Cui B, Cui H, Li Z, Dong H, Li X, Zhao L, Wang J. Novel Bi3O5I2 Hollow Microsphere and Its Enhanced Photocatalytic Activity. Catalysts. 2019; 9(9):709. https://doi.org/10.3390/catal9090709
Chicago/Turabian StyleCui, Baoyin, Haitao Cui, Zhenrong Li, Hongyu Dong, Xin Li, Liangfu Zhao, and Junwei Wang. 2019. "Novel Bi3O5I2 Hollow Microsphere and Its Enhanced Photocatalytic Activity" Catalysts 9, no. 9: 709. https://doi.org/10.3390/catal9090709