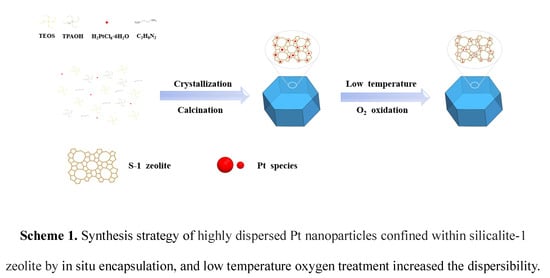
In Situ Encapsulated Pt Nanoparticles Dispersed in Low Temperature Oxygen for Partial Oxidation of Methane to Syngas
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
2.1.1. Phase Composition
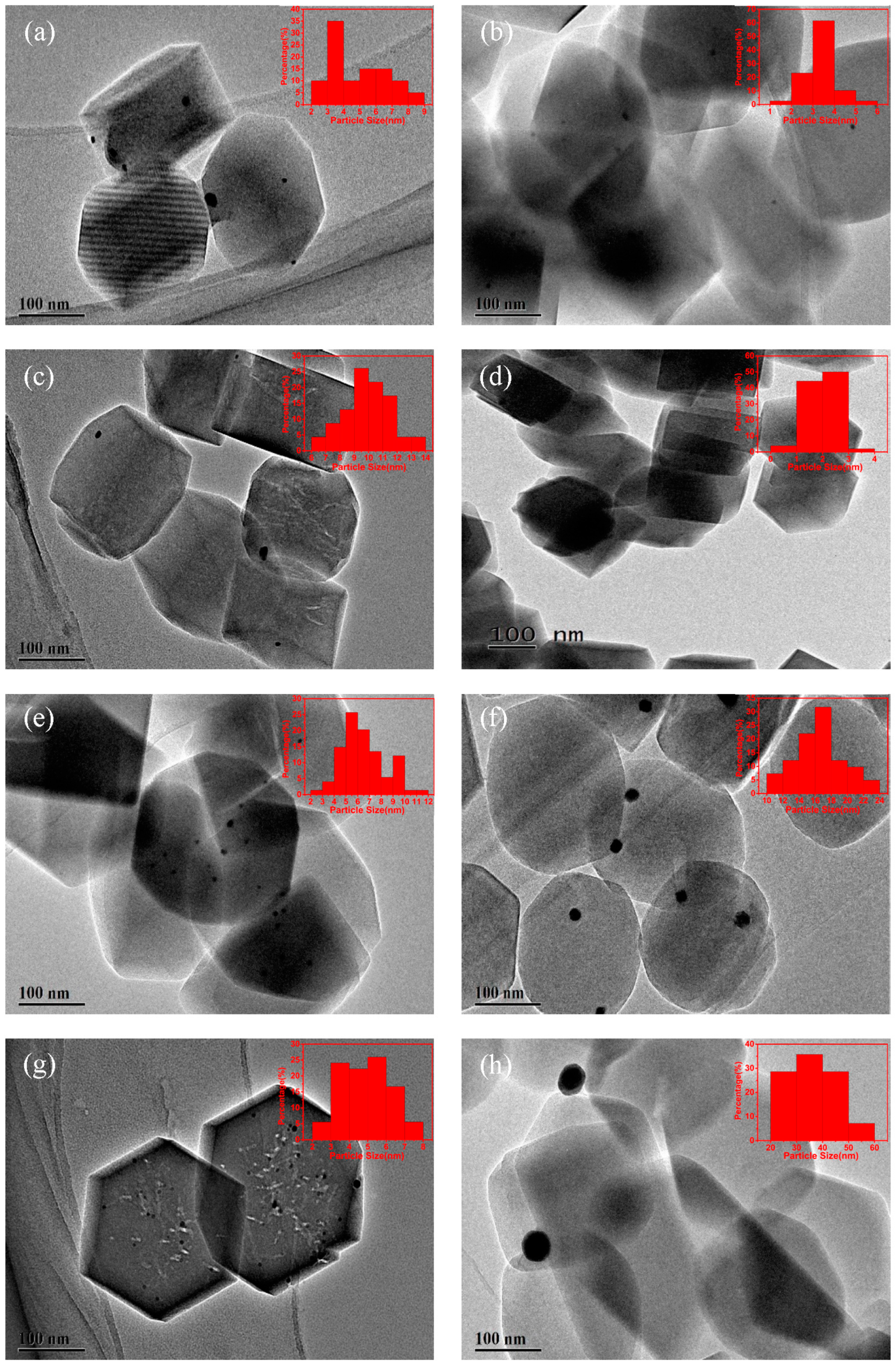
2.1.2. TEM
2.1.3. CO Adsorption
2.1.4. N2 Adsorption-Desorption
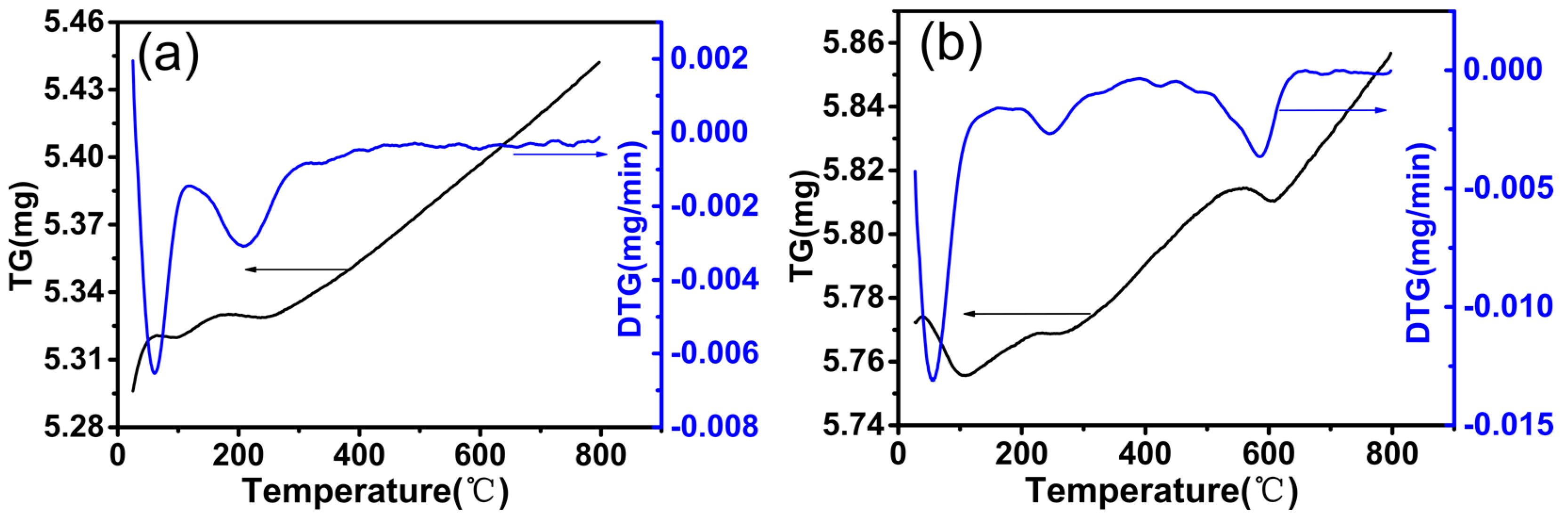
2.1.5. TGA
2.2. POM Catalytic Activity and Stability
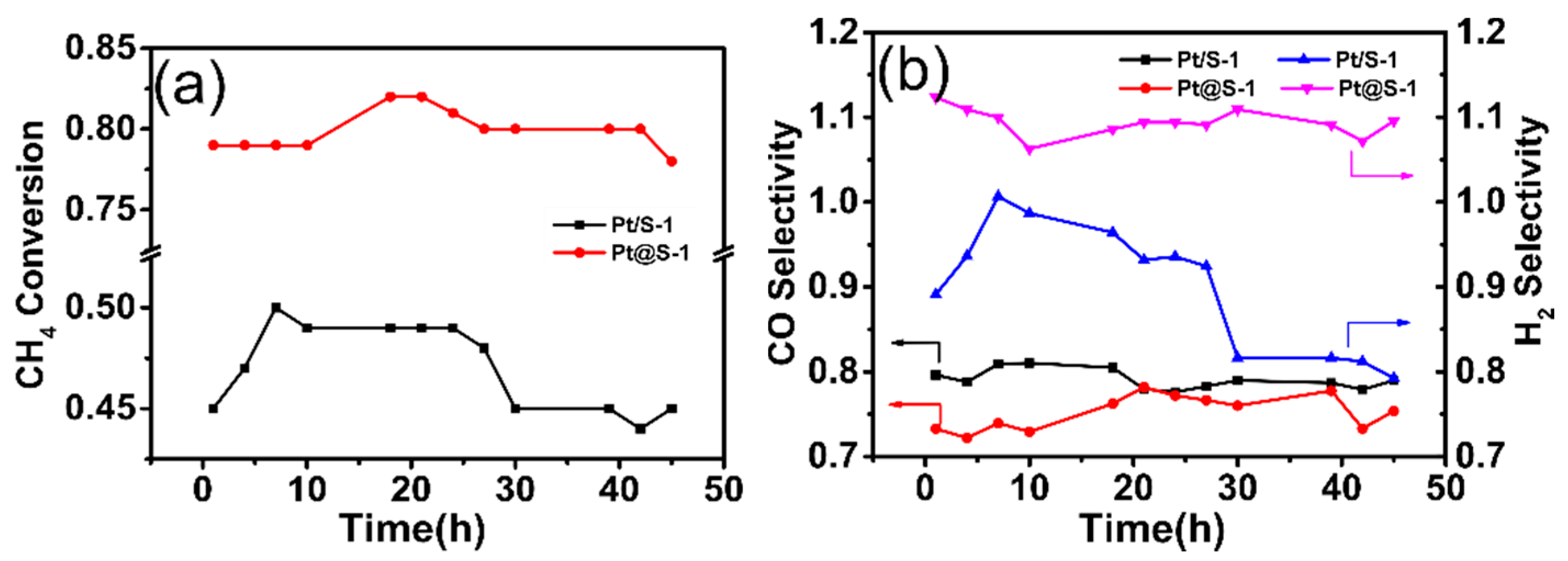
2.2.1. The Catalytic Activity and Stability Comparison of Pt/S-1 and Pt@S-1
2.2.2. Effect of Different Oxygen Temperatures on Catalyst Performance
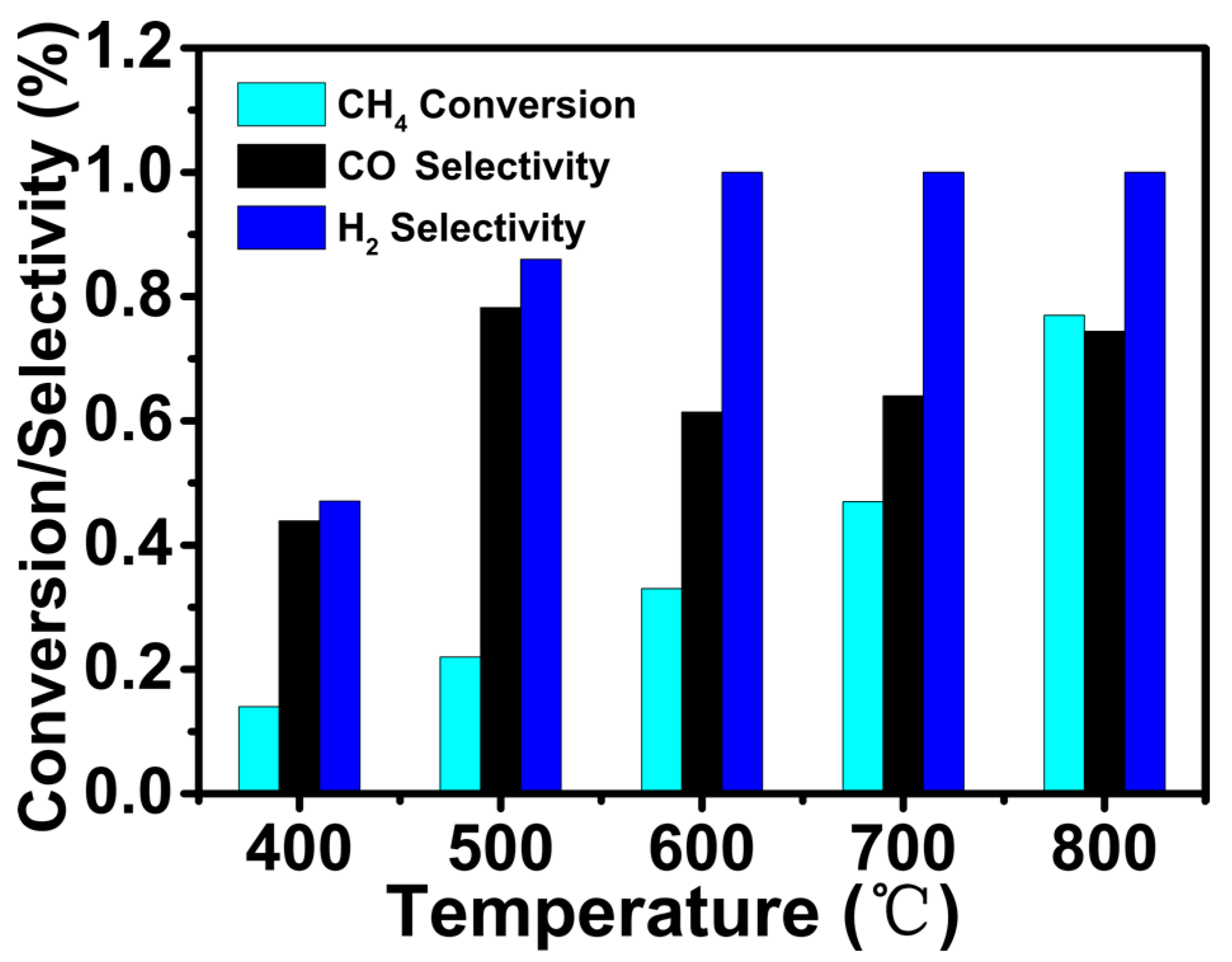
2.2.3. Effect of Different Reaction Temperatures on Catalyst Performance
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.2.1. Synthesis of S-1 Zeolite
3.2.2. Synthesis of Pt/S-1 Zeolite
3.2.3. Synthesis of Pt@S-1 Zeolite
3.3. Catalyst Characterization
3.4. Catalyst Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vora, B.; Chen, J.Q.; Bozzano, A.; Glover, B.; Barger, P. Various routes to methane utilization-SAPO-34 catalysis offers the best option. Catal. Today 2009, 141, 77–83. [Google Scholar] [CrossRef]
- Pei, S.; Kleefisch, M.S.; Kobylinski, T.P.; Faber, J.; Udovich, C.A.; Zhang-McCoy, V.; Dabrowski, B.; Balachandran, U.; Mieville, R.L.; Poeppel, R.B. Failure mechanisms of ceramic membrane reactors in partial oxidation of methane to synthesis gas. Catal. Lett. 1994, 30, 201–212. [Google Scholar] [CrossRef]
- Freni, S.; Calogero, G.; Cavallaro, S. Hydrogen production from methane through catalytic partial oxidation reactions. J. Power Sources 2000, 87, 28–38. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, T. Manufacture of hydrogen. Catal. Today 2005, 106, 293–296. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Al-Fatesh, A.S.; Abasaeed, A.E. Modification of alumina support with TiO2-P25 in CO2 reforming of CH4. J. Ind. Eng. Chem. 2012, 18, 212–217. [Google Scholar] [CrossRef]
- Cheng, Z.X.; Zhao, X.G.; Li, J.L.; Zhu, Q.M. Role of support in CO2 reforming of CH4 over a Ni/γ-Al2O3 catalyst. Appl. Catal. A Gen. 2001, 205, 31–36. [Google Scholar] [CrossRef]
- Hohn, K.L.; Schmidt, L.D. Partial oxidation of methane to syngas at high space velocities over Rh-coated spheres. Appl. Catal. A Gen. 2001, 211, 53–68. [Google Scholar] [CrossRef]
- Li, K.; Wang, H.; Wei, Y.; Yan, D. Syngas production from methane and air via a redox process using Ce–Fe mixed oxides as oxygen carriers. Appl. Catal. B 2010, 97, 361–372. [Google Scholar] [CrossRef]
- Eriksson, S.; Rojas, S.; Boutonnet, M.; Fierro, J. Effect of Ce-doping on Rh/ZrO2 catalysts for partial oxidation of methane. Appl. Catal. A Gen. 2007, 326, 8–16. [Google Scholar] [CrossRef]
- Enger, B.C.; Lødeng, R.; Holmen, A. A review of catalytic partial oxidation of methane to synthesis gas with emphasis on reaction mechanisms over transition metal catalysts. Appl. Catal. A Gen. 2008, 346, 1–27. [Google Scholar] [CrossRef]
- Nematollahi, B.; Rezaei, M.; Khajenoori, M. Combined dry reforming and partial oxidation of methane to synthesis gas on noble metal catalysts. Int. J. Hydrogen Energy 2011, 36, 2969–2978. [Google Scholar] [CrossRef]
- Khajenoori, M.; Rezaei, M.; Nematollahi, B. Preparation of noble metal nanocatalysts and their applications in catalytic partial oxidation of methane. J. Ind. Eng. Chem. 2013, 19, 981–986. [Google Scholar] [CrossRef]
- Sun, W.Z.; Jin, G.Q.; Guo, X.Y. Partial oxidation of methane to syngas over Ni/SiC catalysts. Catal. Commun. 2005, 6, 135–139. [Google Scholar] [CrossRef]
- Hunt, S.T.; Milina, M.; Alba-Rubio, A.C.; Hendon, C.H.; Dumesic, J.A.; Román-Leshkov, Y. Self-assembly of noble metal monolayers on transition metal carbide nanoparticle catalysts. Science 2016, 352, 974–978. [Google Scholar] [CrossRef] [Green Version]
- Yue, Q.; Zhang, Y.; Wang, C.; Wang, X.; Sun, Z.; Hou, X.; Zhao, D.; Deng, Y. Magnetic yolk-shell mesoporous silica microspheres with supported Au nanoparticles as recyclable high-performance nanocatalysts. J. Mater. Chem. A 2015, 3, 4586–4594. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Li, J.; Xu, Q. Immobilizing metal nanoparticles to metal-organic frameworks with size and location control for optimizing catalytic performance. J. Am. Chem. Soc. 2013, 135, 10210–10213. [Google Scholar] [CrossRef]
- Li, G.; Kobayashi, H.; Taylor, J.M.; Ikeda, R.; Kubota, Y.; Kato, K.; Takata, M.; Yamamoto, T.; Toh, S.; Matsumura, S.; et al. Hydrogen storage in Pd nanocrystals covered with a metal-organic framework. Nat. Mater. 2014, 13, 802–806. [Google Scholar] [CrossRef]
- Arnal, P.M.; Weidenthaler, C.; Schüth, F. Highly monodisperse zirconia-coated silica spheres and zirconia/silica hollow spheres with remarkable textural properties. Chem. Mater. 2006, 18, 2733–2739. [Google Scholar] [CrossRef]
- Farrusseng, D.; Tuel, A. Perspectives on zeolite-encapsulated metal nanoparticles and their applications in catalysis. New J. Chem. 2016, 40, 3933–3949. [Google Scholar] [CrossRef]
- Choi, M.; Wu, Z.; Iglesia, E. Mercaptosilane-assisted synthesis of metal clusters within zeolites and catalytic consequences of encapsulation. J. Am. Chem. Soc. 2010, 132, 9129–9137. [Google Scholar] [CrossRef]
- Yang, H.; Chen, H.; Chen, J.; Omotoso, O.; Ring, Z. Shape selective and hydrogen spillover approach in the design of sulfur-tolerant hydrogenation catalysts. J. Catal. 2006, 243, 36–42. [Google Scholar] [CrossRef]
- Goel, S.; Wu, Z.; Zones, S.I.; Iglesia, E. Synthesis and catalytic properties of metal clusters encapsulated within small-pore (SOD, GIS, ANA) zeolites. J. Am. Chem. Soc. 2012, 134, 17688–17695. [Google Scholar] [CrossRef]
- Liu, L.C.; Díaz, U.; Arenal, R.; Agostini, G.; Concepción, P.; Corma, A. Generation of subnanometric platinum with high stability during transformation of a 2D zeolite into 3D. Nat. Mater. 2017, 16, 132–138. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Zhang, J.; Wang, H.; Lewis, J.P.; Xiao, F.S. Product selectivity controlled by zeolite crystals in biomass hydrogenation over a palladium catalyst. J. Am. Chem. Soc. 2016, 138, 7880–7883. [Google Scholar] [CrossRef]
- Niu, X.; Gao, J.; Miao, Q.; Dong, M.; Wang, G.; Fan, W.; Qin, Z.; Wang, J. Influence of preparation method on the performance of Zn-containing HZSM-5 catalysts in methanol-to-aromatics. Microporous Mesoporous Mater. 2014, 197, 252–261. [Google Scholar] [CrossRef]
- Di, Z.; Yang, C.; Jiao, X.; Li, J.; Wu, J.; Zhang, D. A ZSM-5/MCM-48 based catalyst for methanol to gasoline conversion. Fuel 2013, 104, 878–881. [Google Scholar] [CrossRef]
- Bera, P.; Priolkar, K.R.; Gayen, A.; Sarode, P.R.; Hegde, M.S.; Emura, S.; Kumashiro, R.; Jayaram, V.; Subbanna, G.N. Ionic dispersion of Pt over CeO2 by the combustion method: Structural investigation by XRD, TEM, XPS, and EXAFS. Chem. Mater. 2003, 15, 2049–2060. [Google Scholar] [CrossRef]
- Liu, X.; Chen, N.; Han, B.; Xiao, X.; Chen, G.; Djerdj, I.; Wang, Y. Nanoparticle cluster gas sensor: Pt activated SnO2 nanoparticles for NH3 detection with ultrahigh sensitivity. Nanoscale 2015, 7, 14872–14880. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, M.; Jiang, Z.; Shangguan, W. Low-temperature selective catalytic reduction of NO with propylene in excess oxygen over the Pt/ZSM-5 catalyst. J. Hazard. Mater. 2011, 193, 330–334. [Google Scholar] [CrossRef]
- Li, N.; Hu, Z.; Zheng, M.; Lu, H.; Zhao, B.; Zhang, S.; Zheng, J.; Ji, G.; Cao, J. Formation of Pt nanoparticles in mesoporous silica channels via direct low-temperature decomposition of H2PtCl6·6H2O. Mater. Lett. 2013, 106, 193–196. [Google Scholar] [CrossRef]
- Luo, M.; Yao, W.; Huang, C.; Wu, Q.; Xu, Q. Shape effects of Pt nanoparticles on hydrogen production via Pt/CdS photocatalysts under visible light. J. Mater. Chem. A 2015, 3, 13884–13891. [Google Scholar] [CrossRef]
- Kim, J.; Jo, C.; Lee, S.; Ryoo, R. Bulk crystal seeding in the generation of mesopores by organosilane surfactants in zeolite synthesis. J. Mater. Chem. A 2014, 2, 11905–11912. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kolvenbach, R.; Al-Khattaf, S.S.; Jentys, A.; Lercher, J.A. Methanol usage in toluene methylation with medium and large pore zeolites. ACS Catal. 2013, 3, 817–825. [Google Scholar] [CrossRef]
- Liu, L.; Zakharov, D.N.; Arenal, R.; Concepcion, P.; Stach, E.A.; Corma, A. Evolution and stabilization of subnanometric metal species in confined space by in situ TEM. Nat. Commun. 2018, 9, 574. [Google Scholar] [CrossRef]
- Thommes, M. Physical adsorption characterization of nanoporous materials. Chem. Ing. Tech. 2010, 82, 1059–1073. [Google Scholar] [CrossRef]
- Lim, D.H.; Lee, W.D.; Choi, D.H.; Park, D.R.; Lee, H.I. Preparation of platinum nanoparticles on carbon black with mixed binary surfactants: Characterization and evaluation as anode catalyst for low-temperature fuel cell. J. Power Sources 2008, 185, 159–165. [Google Scholar] [CrossRef]
- Rabe, S.; Nachtegaal, M.; Vogel, F. Catalytic partial oxidation of methane to synthesis gas over a ruthenium catalyst: The role of the oxidation state. Phys. Chem. Chem. Phys. 2007, 9, 1461–1468. [Google Scholar] [CrossRef]
- Duan, Q.; Wang, J.; Ding, C.; Ding, H.; Guo, S.; Jia, Y.; Liu, P.; Zhang, K. Partial oxidation of methane over Ni based catalyst derived from order mesoporous LaNiO3 perovskite prepared by modified nanocasting method. Fuel 2017, 193, 112–118. [Google Scholar] [CrossRef]
- Liu, D.; Cheo, W.N.E.; Lim, Y.W.Y.; Borgna, A.; Lau, R.; Yang, Y. A comparative study on catalyst deactivation of nickel and cobalt incorporated MCM-41 catalysts modified by platinum in methane reforming with carbon dioxide. Catal. Today 2010, 154, 229–236. [Google Scholar] [CrossRef]
- Guo, S.; Wang, J.; Ding, C.; Duan, Q.; Ma, Q.; Zhang, K.; Liu, P. Confining Ni nanoparticles in honeycomb-like silica for coking and sintering resistant partial oxidation of methane. Int. J. Hydrogen Energy 2018, 43, 6603–6613. [Google Scholar] [CrossRef]
- Dedov, A.G.; Loktev, A.S.; Komissarenko, D.A.; Mazo, G.N.; Shlyakhtin, O.A.; Parkhomenko, K.V.; Kiennemann, A.A.; Roger, A.C.; Ishmurzin, A.V.; Moiseev, I.I. Partial oxidation of methane to produce syngas over a neodymium–calcium cobaltate-based catalyst. Appl. Catal. A Gen. 2015, 489, 140–146. [Google Scholar] [CrossRef]
- Basile, A.; Paturzo, L.; Lganà, F. The partial oxidation of methane to syngas in a palladium membrane reactor: Simulation and experimental studies. Catal. Today 2001, 67, 65–75. [Google Scholar] [CrossRef]
- Özdemir, H.; Öksüzömer, M.A.F.; Gürkaynak, M.A. Preparation and characterization of Ni based catalysts for the catalytic partial oxidation of methane: Effect of support basicity on H2/CO ratio and carbon deposition. Int. J. Hydrogen Energy 2010, 35, 12147–12160. [Google Scholar] [CrossRef]
- López-Fonseca, R.; Jiménez-González, C.; de Rivas, B.; Gutiérrez-Ortiz, J.I. Partial oxidation of methane to syngas on bulk NiAl2O4 catalyst. Comparison with alumina supported nickel, platinum and rhodium catalysts. Appl. Catal. A Gen. 2012, 437, 53–62. [Google Scholar] [CrossRef]
- Aitbekova, A.; Wu, L.; Wrasman, C.J.; Boubnov, A.; Hoffman, A.S.; Goodman, E.D.; Bare, S.R.; Cargnello, M. Low-Temperature Restructuring of CeO2-Supported Ru Nanoparticles Determines Selectivity in CO2 Catalytic Reduction. J. Am. Chem. Soc. 2018, 140, 13736–13745. [Google Scholar] [CrossRef]
- Kim, D.; Becknell, N.; Yu, Y.; Yang, P. Room-temperature dynamics of vanishing copper nanoparticles supported on silica. Nano Lett. 2017, 17, 2732–2737. [Google Scholar] [CrossRef]
- Morgan, K.; Goguet, A.; Hardacre, C. Metal redispersion strategies for recycling of supported metal catalysts: A perspective. ACS Catal. 2015, 5, 3430–3445. [Google Scholar] [CrossRef]
- Moliner, M.; Gabay, J.E.; Kliewer, C.E.; Carr, R.T.; Guzman, J.; Casty, G.L.; Serna, P.; Corma, A. Reversible transformation of Pt nanoparticles into single atoms inside high-silica chabazite zeolite. J. Am. Chem. Soc. 2016, 138, 15743–15750. [Google Scholar] [CrossRef]
- Dai, C.; Li, X.; Zhang, A.; Liu, C.; Song, C.; Guo, X. Pd and Pd-CuO nanoparticles in hollow silicalite-1 single crystals for enhancing selectivity and activity for the Suzuki-Miyaura reaction. RSC Adv. 2015, 5, 40297–40302. [Google Scholar] [CrossRef]
- Chary, K.V.; Naresh, D.; Vishwanathan, V.; Sadakane, M.; Ueda, W. Vapour phase hydrogenation of phenol over Pd/C catalysts: A relationship between dispersion, metal area and hydrogenation activity. Catal. Commun. 2007, 8, 471–477. [Google Scholar] [CrossRef]
- Nie, R.; Liang, D.; Shen, L.; Gao, J.; Chen, P.; Hou, Z. Selective oxidation of glycerol with oxygen in base-free solution over MWCNTs supported PtSb alloy nanoparticles. Appl. Catal. B Environ. 2012, 127, 212–220. [Google Scholar] [CrossRef]


| Catalyst | Pt Loading (wt %) a | Pt Dispersion b | CO Uptake (μmolg−1 cat) b | Particle Size (nm) | ||
|---|---|---|---|---|---|---|
| Pt Area (m2g−1 cat) | Dispersion (%) | CO b | TEM c | |||
| Pt/S-1 | 0.382 | 0.16 | 37.25 | 3.22 | 6.14 | 6.09 |
| Pt@S-1 | 0.312 | 0.24 | 86.49 | 5.08 | 3.57 | 3.67 |
| Pt@S-1-300O2 | 0.312 | 0.56 | 89.41 | 13.70 | 2.41 | 2.32 |
| Pt@S-1-700O2 | 0.312 | 0.12 | 31.72 | 2.74 | 7.57 | 7.54 |
| Sample | SBET (m2g−1) a | Smicro (m2g−1) b | Sext (m2g−1) b | Vtotal (cm3g−1) c | Vmicro (cm3g−1) d |
|---|---|---|---|---|---|
| S-1 | 460 | 283 | 176.48 | 0.37 | 0.11 |
| Pt/S-1 | 435 | 318 | 116.73 | 0.37 | 0.13 |
| Pt@S-1 | 374 | 235 | 139.43 | 0.27 | 0.09 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Ma, L.; Ding, C.; Xue, Y.; Zhang, Y.; Gao, Z. In Situ Encapsulated Pt Nanoparticles Dispersed in Low Temperature Oxygen for Partial Oxidation of Methane to Syngas. Catalysts 2019, 9, 720. https://doi.org/10.3390/catal9090720
Wang J, Ma L, Ding C, Xue Y, Zhang Y, Gao Z. In Situ Encapsulated Pt Nanoparticles Dispersed in Low Temperature Oxygen for Partial Oxidation of Methane to Syngas. Catalysts. 2019; 9(9):720. https://doi.org/10.3390/catal9090720
Chicago/Turabian StyleWang, Junwen, Lichao Ma, Chuanmin Ding, Yanan Xue, Yongkang Zhang, and Zhiting Gao. 2019. "In Situ Encapsulated Pt Nanoparticles Dispersed in Low Temperature Oxygen for Partial Oxidation of Methane to Syngas" Catalysts 9, no. 9: 720. https://doi.org/10.3390/catal9090720