A Five-Coordinate Copper(II) Complex Constructed from Sterically Hindered 4-Chlorobenzoate and Benzimidazole: Synthesis, Crystal Structure, Hirshfeld Surface Analysis, DFT, Docking Studies and Antibacterial Activity
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Physical Measurements
2.2. Crystallographic Analysis
2.3. Hirshfeld Surface Analysis
2.4. DFT Calculation
2.5. Molecular Docking Method
2.6. Antimicrobial Test
2.7. Synthesis
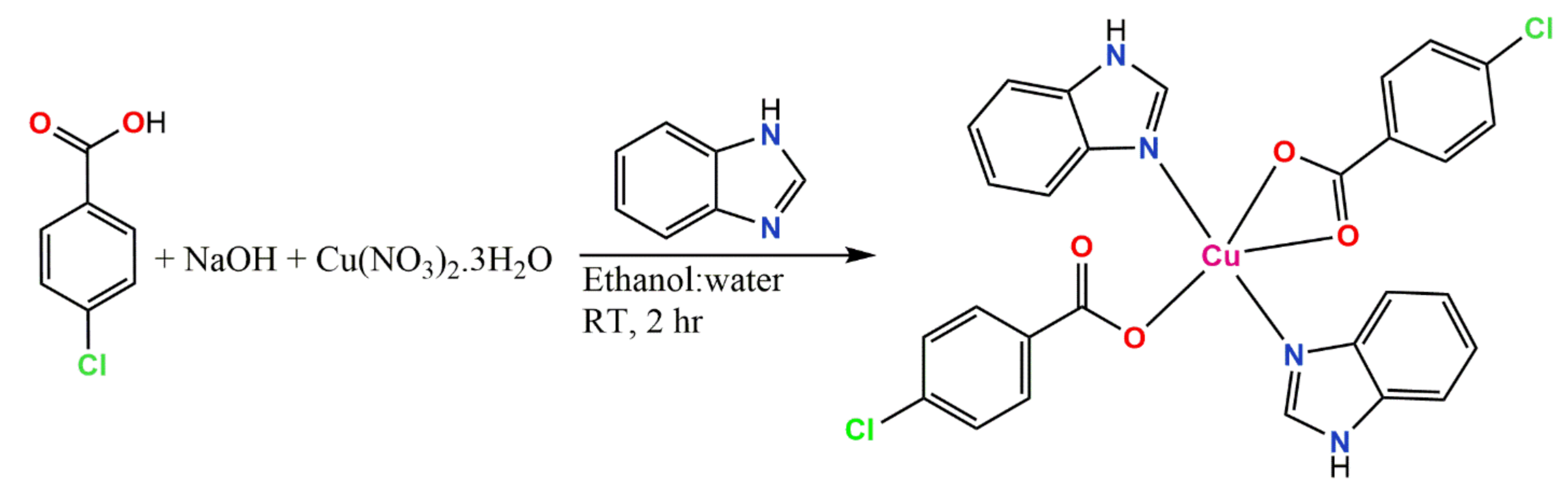
Synthesis of Cu(BZDH)2(4Clbz)2 (1)
3. Result and Discussion
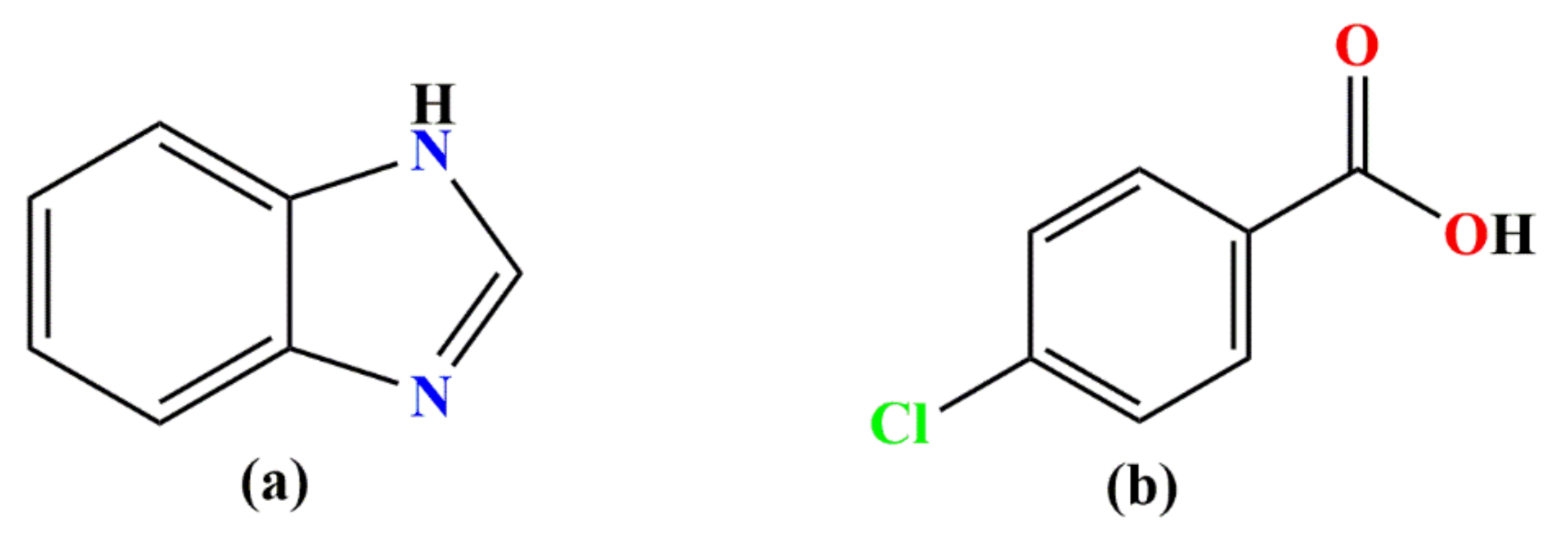
3.1. Synthesis
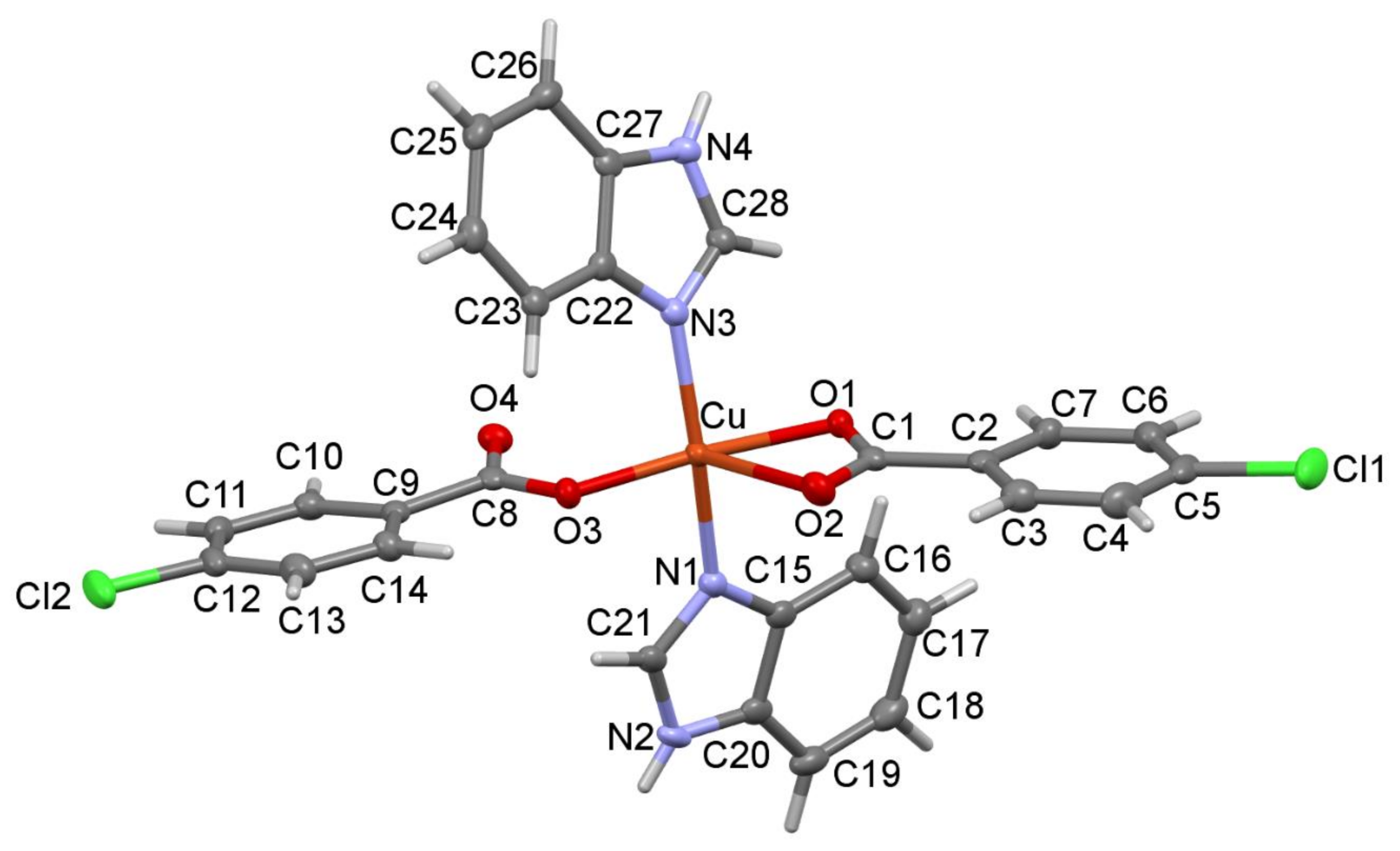
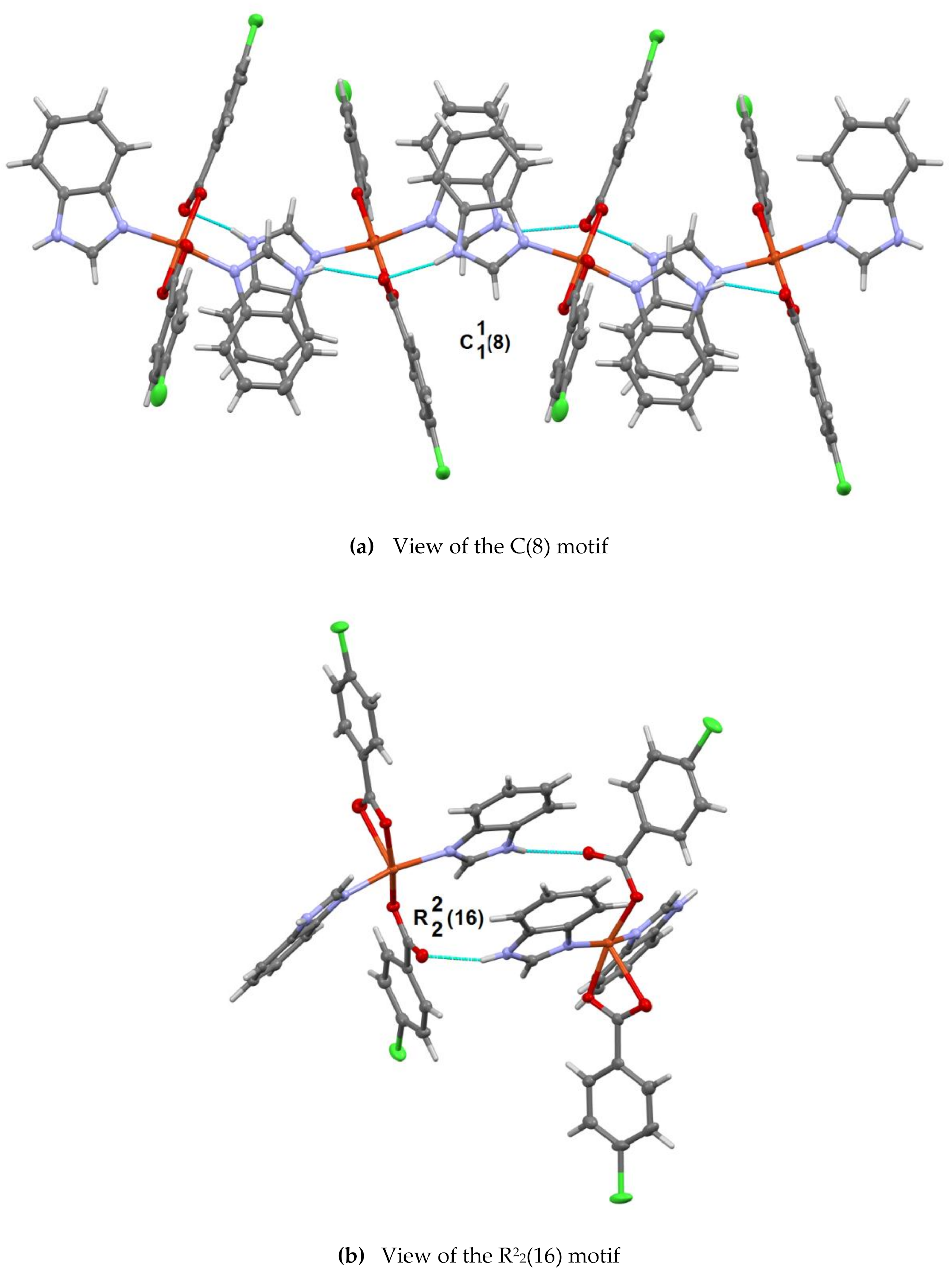
3.2. Structural Description
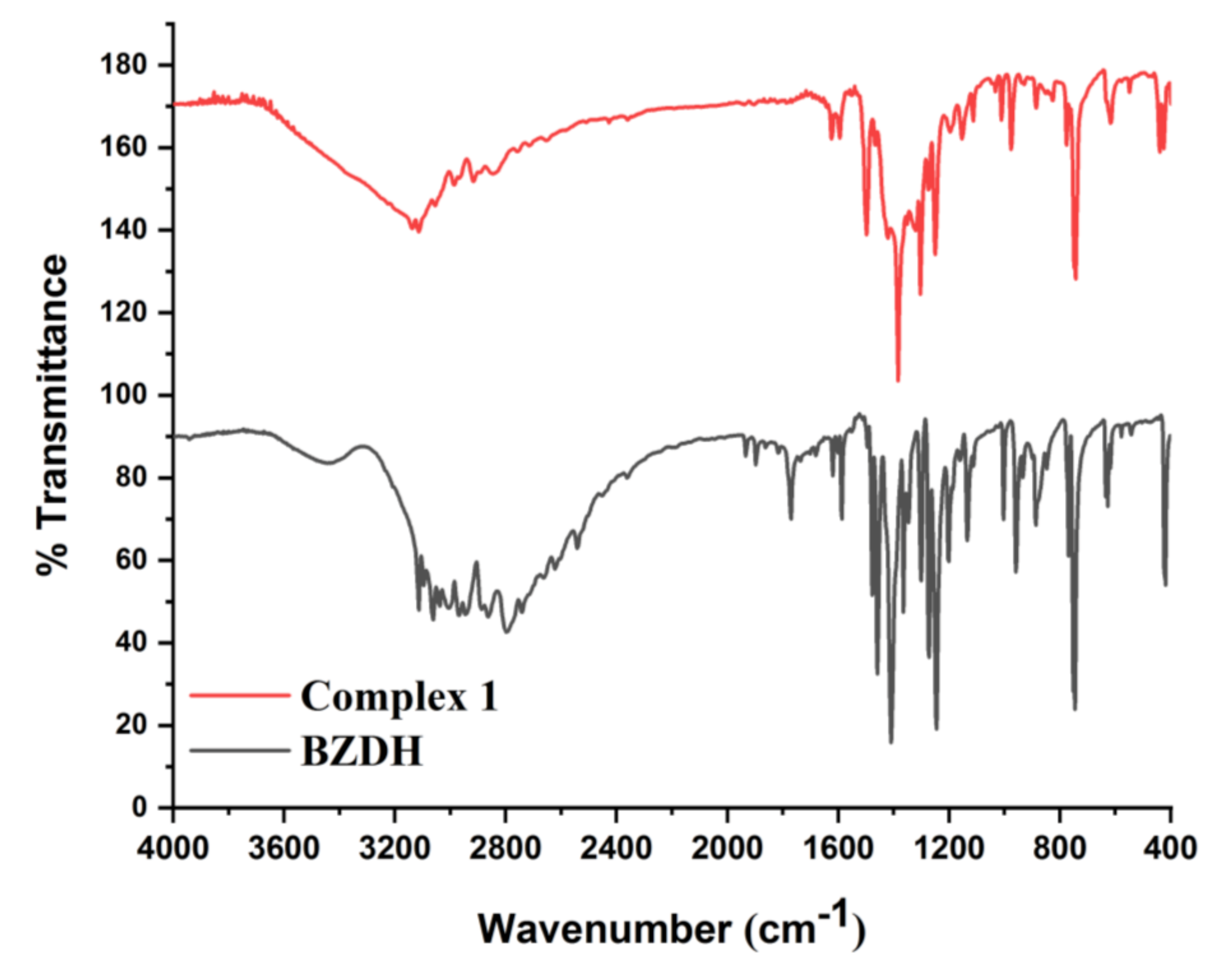
3.3. Infrared Spectra
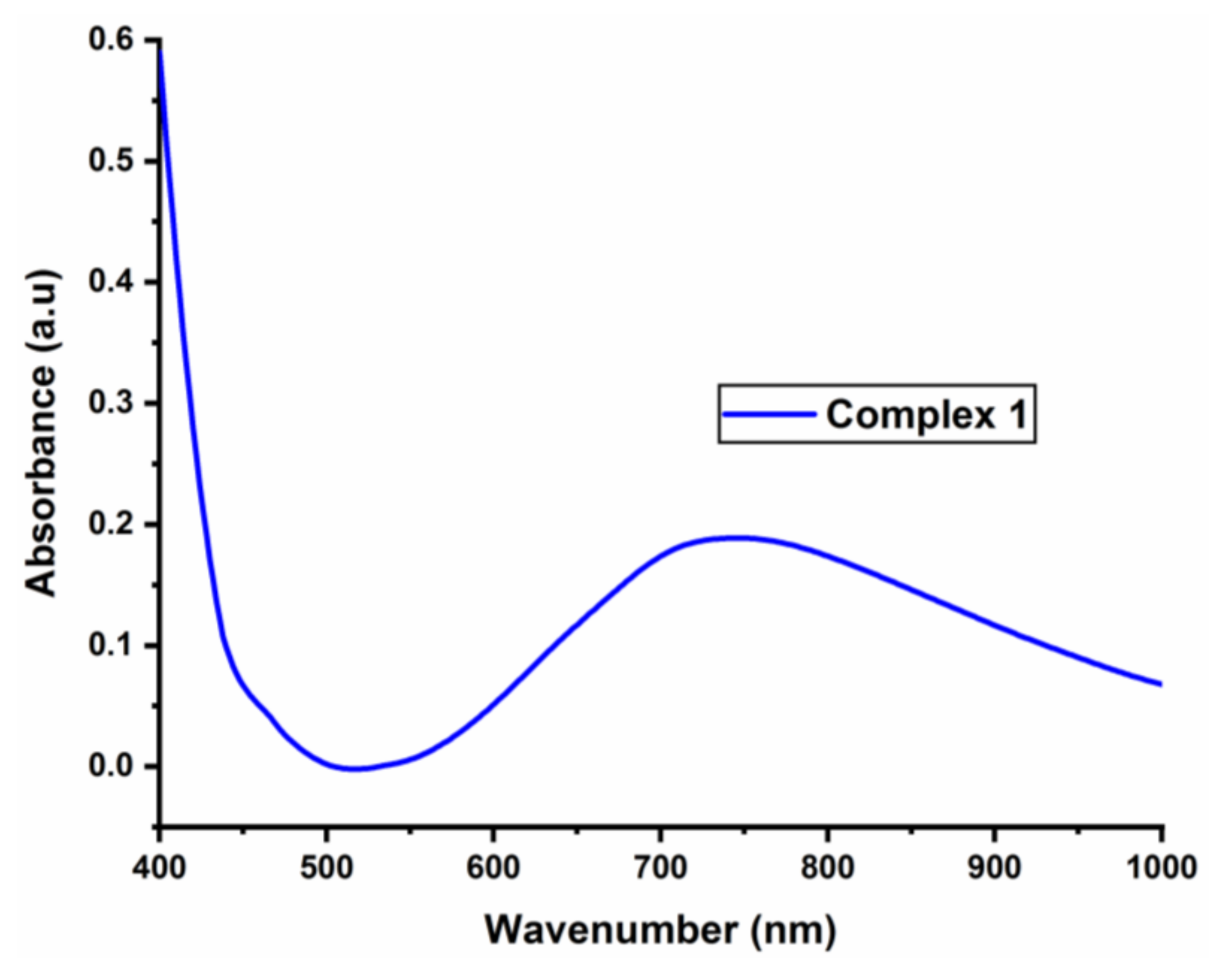
3.4. The UV Spectrum of the Complex
3.5. Hirshfeld Surfaces Analysis
3.6. Structural Analysis
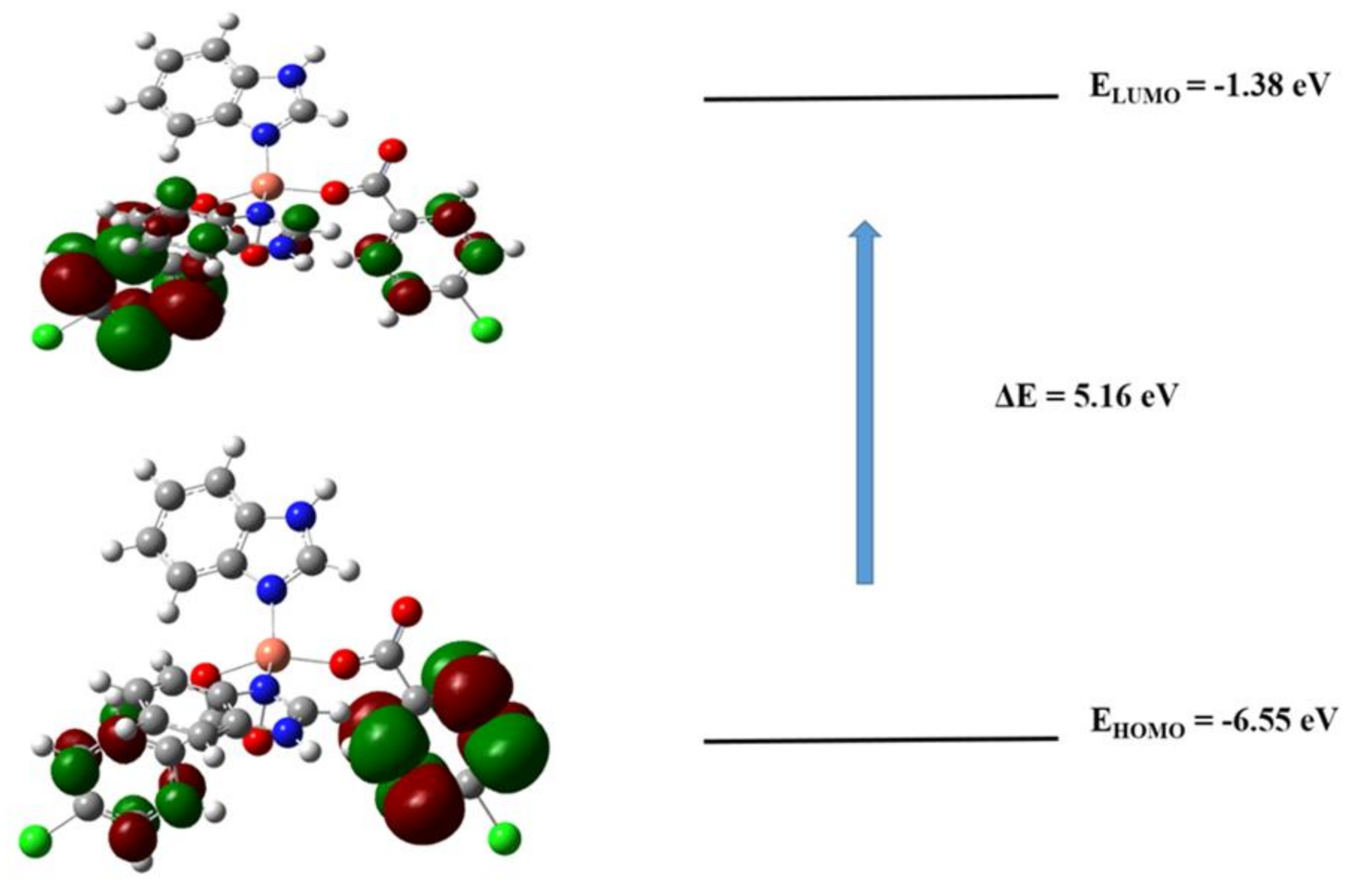
3.6.1. Frontier Molecular Orbitals (FMOs) Analysis
3.6.2. Molecular Electrostatic Potential Analysis
3.6.3. Reactivity Parameters
- Ionization potential (I) = −EHOMO;
- Electron affinity (A) = −ELUMO;
- Chemical hardness (η) = (I − A)/2;
- Chemical potential (μ) = −(I + A)/2;
- Softness (S) = ½η;
- Electronegativity (χ) = (I + A)/2;
- Electrophilicity index (ω) = μ2 /2η.
3.7. Molecular Docking Studies
3.8. Antibacterial Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rafique, S.; Idrees, M.; Nasim, A.; Akbar, A.; Athar, A. Transition metal complexes as potential therapeutic agents. Biotechnol. Mol. Biol. Rev. 2010, 5, 38–45. [Google Scholar] [CrossRef]
- Schatzschneider, U. Photoactivated Biological Activity of Transition-Metal Complexes. Eur. J. Inorg. Chem. 2010, 2010, 1451–1467. [Google Scholar] [CrossRef]
- Frei, A. Metal Complexes, an Untapped Source of Antibiotic Potential? Antibiotics 2020, 9, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iakovidis, I.; Delimaris, I.; Piperakis, S.M. Copper and Its Complexes in Medicine: A Biochemical Approach. Mol. Biol. Int. 2011, 2011, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, C.; White, A.R. Copper complexes as therapeutic agents. Metallomics 2012, 4, 127–138. [Google Scholar] [CrossRef]
- Zhang, C.X.; Lippard, S.J. New metal complexes as potential therapeutics. Curr. Opin. Chem. Biol. 2003, 7, 481–489. [Google Scholar] [CrossRef]
- Obaleye, J.A.; Ajibola, A.A.; Bernardus, V.B.; Hosten, E.C. Synthesis, X-ray crystallography, spectroscopic and in vitro antimicrobial studies of a new Cu(II) complex of trichloroacetic acid and imidazole. J. Mol. Struct. 2020, 1203, 127435. [Google Scholar] [CrossRef]
- Baldini, M.; Belicchi-Ferrari, M.; Bisceglie, F.; Dall’Aglio, P.P.; Pelosi, G.; Pinelli, S.; Tarasconi, P. Copper(II) Complexes with Substituted Thiosemicarbazones of α-Ketoglutaric Acid: Synthesis, X-ray Structures, DNA Binding Studies, and Nuclease and Biological Activity. Inorg. Chem. 2004, 43, 7170–7179. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Katsaros, N.; Karaliota, A.; Psomas, G. Mononuclear copper(II) complexes with quinolones and nitrogen-donor heterocyclic ligands: Synthesis, characterization, biological activity and interaction with DNA. Inorg. Chim. Acta 2007, 360, 4093–4102. [Google Scholar] [CrossRef]
- Renfrew, A.K. Transition metal complexes with bioactive ligands: Mechanisms for selective ligand release and applications for drug delivery. Metallomics 2014, 6, 1324–1335. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Yin, K.-L.; Xu, D.-J. catena-Poly[[bis(1H-benzimidazole-κN3)(salicylato-κO)copper(II)]-μ-salicylato-O,O′:O′′]. Acta Cryst. 2005, C61, m19–m21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansal, Y.; Silakari, O. The therapeutic journey of benzimidazoles: A review. Bioorganic Med. Chem. 2012, 20, 6208–6236. [Google Scholar] [CrossRef] [PubMed]
- Gurer-Orhan, H.; Orhan, H.; Suzen, S.; Püsküllü, M.O.; Buyukbingol, E. Synthesis and evaluation of in vitro antioxidant capacities of some benzimidazole derivatives. J. Enzym. Inhib. Med. Chem. 2006, 21, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Spasov, A.A.; Yozhitsa, I.N.; Bugaeva, L.I.; Anisimova, V.A. Benzimidazole derivatives: Spectrum of pharmacological activity and toxicological properties (a review). Pharm. Chem. J. 1999, 33, 232–243. [Google Scholar] [CrossRef]
- Matejová, S.; Puchoňová, M.; Mazúr, M.; Valigura, D.; Rohlíček, J.; Jorík, V.; Moncoľ, J. Preparation, spectral properties and structure of bis(salicylato)bis(benzimidazole)-copper(II) complexes with two different benzimidazole spatial orientation. Polyhedron 2019, 170, 86–94. [Google Scholar] [CrossRef]
- Yoe, F.; Flores-Álamo, M.; Morales, F.; Escudero, R.; Cortes-Hernández, H.; Castro, M.; Barba-Behrens, N. Structural, magnetic and theoretical study of mononuclear nickel(II) and cobalt(II) compounds of a benzimidazole thiobutanoic acid derivative. Inorg. Chim. Acta 2014, 423, 36–45. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, L.-P.; Xu, D.-J. Bis(benzimidazole-κN)bis(3,5-dihydroxybenzoato-κO)copper(II) trihydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2006, 62, 5. [Google Scholar] [CrossRef] [Green Version]
- Devereux, M.; O’Shea, D.; O’Connor, M.; Grehan, H.; Connor, G.; McCann, M.; Rosair, G.M.; Lyng, F.M.; Kellett, A.; Walsh, M.; et al. Synthesis, catalase, superoxide dismutase and antitumour activities of copper(II) carboxylate complexes incorporating benzimidazole, 1,10-phenanthroline and bipyridine ligands: X-ray crystal structures of [Cu(BZA)2(bipy)(H2O)], [Cu(SalH)2(BZDH)2] and [Cu(CH3COO)2(5,6-DMBZDH)2] (SalH2 = salicylic acid; BZAH = benzoic acid; BZDH = benzimidazole and 5,6-DMBZDH = 5,6-dimethylbenzimidazole). Polyhedron 2007, 26, 4073–4084. [Google Scholar] [CrossRef]
- Su, J.-R.; Gu, J.-M.; Xu, D.-J. Bis(1H-benzimidazole-κN3)bis(4-hydroxybenzoato-κ2O,O′)copper(II). Acta Crystallogr. Sect. E Struct. Rep. Online 2005, 61, 244. [Google Scholar] [CrossRef]
- Yıldırım, M.H.; Heren, Z.; Paşaoğlu, H.; Hıra, D.; Buyukgungor, O. Bis-(benzimidazole-κN)bis(2-benzoyl-benzoato-κO)copper(II). Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, m638–m639. [Google Scholar] [CrossRef] [Green Version]
- Puchoňová, M.; Švorec, J.; Švorc, Ľ.; Pavlik, J.; Mazúr, M.; Dlháň, Ľ.; Růžičková, Z.; Moncol, J.; Valigura, D. Synthesis, spectral, magnetic properties, electrochemical evaluation and SOD mimetic activity of four mixed-ligand Cu(II) complexes. Inorg. Chim. Acta 2017, 455, 298–306. [Google Scholar] [CrossRef]
- Song, W.-D.; Huang, X.-H.; Wang, H. Bis(1H-benzimidazole-κN)bis-(4-methyl-benzoato-κO,O′)copper(II). Acta Crystallogr. Sect. E Struct. Rep. Online 2008, 64, m764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, M.; Kellett, A.; McCann, M.; Rosair, G.; McNamara, M.; Howe, O.; Creaven, B.S.; McClean, S.; Kia, A.F.-A.; O’Shea, D.; et al. Copper(II) Complexes of Salicylic Acid Combining Superoxide Dismutase Mimetic Properties with DNA Binding and Cleaving Capabilities Display Promising Chemotherapeutic Potential with Fast Acting in Vitro Cytotoxicity against Cisplatin Sensitive and Resistant Cancer Cell Lines. J. Med. Chem. 2012, 55, 1957–1968. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Q.; Yang, B.; Shi, Q.; Gao, Y.; Zhou, Z. Synthesis, crystal structure and forming mechanism of two novel copper (II) α-methacrylate complexes with benzimidazole. Sci. China Ser. B Chem. 1999, 42, 363–372. [Google Scholar] [CrossRef]
- Obaleye, J.A.; Ajibola, A.A.; Bernardus, V.B.; Hosten, E.C.; Ozarowski, A. Synthesis, spectroscopic, structural and antimicrobial studies of a dimeric complex of copper(II) with trichloroacetic acid and metronidazole. Inorg. Chim. Acta 2020, 503, 119404. [Google Scholar] [CrossRef]
- Chakraborty, I.; Pinto, M.; Stenger-Smith, J.; Martinez-Gonzalez, J.; Mascharak, P.K. Synthesis, structures and antibacterial properties of Cu(II) and Ag(I) complexes derived from 2,6-bis(benzothiazole)-pyridine. Polyhedron 2019, 172, 1–7. [Google Scholar] [CrossRef]
- Festa, R.A.; Thiele, D.J. Copper: An essential metal in biology. Curr. Biol. 2011, 21, R877–R883. [Google Scholar] [CrossRef] [Green Version]
- Yeşilel, O.Z.; Ilker, I.; Soylu, M.S.; Darcan, C.; Süzen, Y. Synthesis, crystal structures and antimicrobial properties of copper(II)-thiophene-2,5-dicarboxylate complexes with N-donor ligands. Polyhedron 2012, 39, 14–24. [Google Scholar] [CrossRef]
- Yenikaya, C.; Poyraz, M.; Sarı, M.; Demirci, F.; Ilkimen, H.; Büyükgüngör, O.; Sarı, M. Synthesis, characterization and biological evaluation of a novel Cu(II) complex with the mixed ligands 2,6-pyridinedicarboxylic acid and 2-aminopyridine. Polyhedron 2009, 28, 3526–3532. [Google Scholar] [CrossRef]
- Carter, K.P.; Zulato, C.H.F.; Cahill, C. Exploring supramolecular assembly and luminescent behavior in a series of RE-p-chlorobenzoic acid-1,10-phenanthroline complexes. CrystEngComm 2014, 16, 10189–10202. [Google Scholar] [CrossRef] [Green Version]
- Carter, K.P.; Pope, S.J.A.; Cahill, C.L. A series of Ln-p-chlorobenzoic acid–terpyridine complexes: Lanthanide contraction effects, supramolecular interactions and luminescent behavior. CrystEngComm 2014, 16, 1873–1884. [Google Scholar] [CrossRef]
- Khosravi, I.; Mirzaei, M.; Bauzá, A.; Frontera, A.; Eftekhar, M. A new oxo centered basic p-chlorobenzoate bridging heterotrinuclear complex, [Cr2MnO(C7H4O2Cl)6(Py)3]C7H5O2Cl: Synthesis, X-ray crystal structure and theoretical DFT study. Polyhedron 2014, 81, 349–355. [Google Scholar] [CrossRef]
- Abdullah, N.; Sharmin, N.; Ozair, L.N.; Nordin, A.R.; Nasir, W.S.N.M.; Mohamadin, M.I. Structures and mesomorphism of complexes of tetrakis(4-chlorobenzoate-μ-O,O′)bis(ethanol)dicopper(II) with different N-donor ligands. J. Co-Ord. Chem. 2015, 68, 1347–1360. [Google Scholar] [CrossRef]
- Sundberg, M.R.; Uggla, R.; Kivekäs, R. Conformational isomerism and effect of complexation on carboxylate group in two crystallographically independent coordination units of trans-di(4-chlorobenzoato-O)bis(1,3-diaminopropane-N,N′)cobalt(III) 4-chlorobenzoate dihydrate. Inorg. Chim. Acta 1995, 232, 1–8. [Google Scholar] [CrossRef]
- Köse, D.A.; Gökçe, G.; Gökçe, S.; Uzun, I. bis(N,N-diethylnicotinamide) p-chlorobenzoate complexes of Ni(II), Zn(II) and Cd(II). J. Therm. Anal. Calorim. 2008, 95, 247–251. [Google Scholar] [CrossRef]
- CrysAlis PRO, version 40; Rigaku Oxford Diffraction Ltd.: Yarnton, UK, 2019.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- CrystalExplorer, version 17.5; University of Western Australia: Perth, Australia, 2017.
- McKinnon, J.J.; Spackman, M.A.; Mitchell, A.S. Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Crystallogr. Sect. B Struct. Sci. 2004, 60, 627–668. [Google Scholar] [CrossRef] [PubMed]
- Gaussian, version 0, revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2010.
- GaussView, version 05; Semichem Inc.: Shawnee, OK, USA, 2009.
- Ahmed, M.N.; Yasin, K.A.; Ayub, K.; Mahmood, T.; Tahir, M.N.; Khan, B.A.; Hafeez, M.; Ahmed, M.; Haq, I.U. Click one pot synthesis, spectral analyses, crystal structures, DFT studies and brine shrimp cytotoxicity assay of two newly synthesized 1,4,5-trisubstituted 1,2,3-triazoles. J. Mol. Struct. 2016, 1106, 430–439. [Google Scholar] [CrossRef]
- Available online: https://www.rcsb.org/structure/1KIJ (accessed on 5 September 2020).
- Perveen, F.; Arshad, N.; Qureshi, R.; Nowsherwan, J.; Sultan, A.; Nosheen, B.; Rafique, H. Electrochemical, spectroscopic and theoretical monitoring of anthracyclines’ interactions with DNA and ascorbic acid by adopting two routes: Cancer cell line studies. PLoS ONE 2018, 13, e0205764. [Google Scholar] [CrossRef] [PubMed]
- Abu Ali, H.; Omar, S.N.; Darawsheh, M.D.; Fares, H. Synthesis, characterization and antimicrobial activity of zinc(II) ibuprofen complexes with nitrogen-based ligands. J. Co-Ord. Chem. 2016, 69, 1110–1122. [Google Scholar] [CrossRef]
- Brown, I.D. Bond-Length—Bond-Valence Relationships in Inorganic Solids. In Structure Correlation; Wiley: New York, NY, USA, 1994; pp. 405–429. [Google Scholar]
- O’Keefe, M.; Brese, N.E. Atom sizes and bond lengths in molecules and crystals. J. Am. Chem. Soc. 1991, 113, 3226–3229. [Google Scholar] [CrossRef]
- Sieroń, L.; Bukowska-Strzyzewska, M. catena-Poly[[bis(benzimidazole-N3)copper(II)]-μ-suberato-O,O′:O″,O‴] andcatena-poly[[[bis(benzimidazole-N3)copper(II)]-μ-sebacato-O,O′:O″,O‴] dihydrate]. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1999, 55, 1230–1234. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; Van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Etter, M.C.; Macdonald, J.C.; Bernstein, J. Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Crystallogr. Sect. B Struct. Sci. 1990, 46, 256–262. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chem. Int. Ed. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Kannan, S.; Venkatachalam, G.; Lee, H.-J.; Min, B.K.; Kim, W.; Koo, E.; Do, Y.R.; Yoon, S. Mononuclear transition metal complexes with sterically hindered carboxylate ligands: Synthesis, structural and spectral properties. Polyhedron 2011, 30, 340–346. [Google Scholar] [CrossRef]
- Yoon, S.; Lippard, S.J. Synthesis and Characterization of Carboxylate-Rich Complexes Having the {Fe2(μ-OH)2(μ-O2CR)}3+ and {Fe2(μ-O)(μ-O2CR)}3+ Cores of O2-Dependent Diiron Enzymes. J. Am. Chem. Soc. 2004, 126, 2666–2667. [Google Scholar] [CrossRef]
- Ross, K.A.; Dyreson, C.E.; Skiadopoulos, S.; Sirangelo, C.; Larsgaard, M.L.; Kifer, D.; Deutsch, A.; Nash, A.; Dyreson, C.; Mitra, P.; et al. ||-Coords. Encycl. Database Syst. 2009, 33, 496. [Google Scholar] [CrossRef]
- Vicente, M.; Bastida, R.; Macıas, A.; Valencia, L.; Geraldes, C.F.G.C.; Brondino, C.D. Copper complexes with new oxaaza-pendant-armed macrocyclic ligands: X-ray crystal structure of a macrocyclic copper(II) complex. Inorg. Chim. Acta 2005, 358, 1141–1150. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.M.; Nolan, S.P. Efficient Cross-Coupling Reactions of Aryl Chlorides and Bromides with Phenyl- or Vinyltrimethoxysilane Mediated by a Palladium/Imidazolium Chloride System. Org. Lett. 2000, 2, 2053–2055. [Google Scholar] [CrossRef] [PubMed]
- Polshettiwar, V.; Molnár, Á. Silica-supported Pd catalysts for Heck coupling reactions. Tetrahedron 2007, 63, 6949–6976. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Kolandaivel, P. Structure, conformation and NMR studies on 1,2-dioxane and halogen substituted 1,2-dioxane molecules. Comput. Biol. Chem. 2003, 27, 173–183. [Google Scholar] [CrossRef]
- Arab, A.; Habibzadeh, M. Comparative hydrogen adsorption on the pure Al and mixed Al–Si nano clusters: A first principle DFT study. Comput. Theor. Chem. 2015, 1068, 52–56. [Google Scholar] [CrossRef]
- Zgou, H.; Boussaidi, S.; Eddiouane, A.; Chaib, H.; Tripathi, R.; Ben Hadda, T.; Bouachrine, M.; Hamidi, M. New low band-gap conjugated organic materials based on fluorene, thiophene and phenylene for photovoltaic applications: Theoretical study. Mater. Today: Proc. 2016, 3, 2578–2586. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpály, L.V.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Chandraleka, S.; Ramya, K.; Chandramohan, G.; Dhanasekaran, D.; Priyadharshini, A.; Panneerselvam, A. Antimicrobial mechanism of copper (II) 1,10-phenanthroline and 2,2′-bipyridyl complex on bacterial and fungal pathogens. J. Saudi Chem. Soc. 2014, 18, 953–962. [Google Scholar] [CrossRef] [Green Version]
- Neelakantan, M.A.; Esakkiammal, M.; Mariappan, S.S.; Dharmaraja, J.; Jeyakumar, T. Synthesis, Characterization and Biocidal Activities of Some Schiff Base Metal Complexes. Indian J. Pharm. Sci. 2010, 72, 216–222. [Google Scholar] [CrossRef] [Green Version]





| Parameters | 1 |
|---|---|
| Empirical formula | C28H20Cl2CuN4O4 |
| Formula weight | 610.93 |
| Temperature (K) | 100(3) |
| Wavelength (Å) | 0.71073 |
| Crystal system | Monoclinic |
| Space group | P21/c |
| a (Å) | 14.5042(3) |
| b (Å) | 13.2293(2) |
| c (Å) | 13.6286(2) |
| α (º) | 90 |
| β (º) | 104.816(2) |
| γ(º) | 90 |
| Volume (Å3) | 2528.12(8) |
| Z | 4 |
| Density (Mg/m3) | 1.605 |
| Absorp. coeff. (mm−1) | 1.120 |
| F(000) | 1244 |
| Crystal size (mm3) | 0.35 × 0.25 × 0.15 |
| Theta range for data collection | 2.4, 25.0 |
| Index ranges | −1 7≤ h ≤ 17; −15 ≤ k ≤ 15; −16 ≤ l ≤ 16 |
| Reflection collected | 71647 |
| Independent reflections | 4469 [R(int) = 0.055] |
| Goodness of fit on F2 | 1.13 |
| Final R indices [I > 2sigma(I)] | 0.0361 |
| R indices (all data) | 0.0427 |
| Largest diff. peak and hole (eÅ−3) | 1.12 and −0.42 |
| Atoms | 1 Bond Length (Å) |
| Cu-O1 | 1.9873(18) |
| Cu-O2 | 2.440(2) |
| Cu-O3 | 1.9406(18) |
| Cu-N1 | 2.015(2) |
| Cu-N3 | 2.004(2) |
| Atoms | 1 Bond Angle (º) |
| O1-Cu-O2 | 58.89(7) |
| O1-Cu-O3 | 167.73(7) |
| O1-Cu-N1 | 91.40(8) |
| O1-Cu-N3 | 89.00(8) |
| O2-Cu-O3 | 108.84(7) |
| O2-Cu-N1 | 96.30(8) |
| O2-Cu-N3 | 96.85(8) |
| O3-Cu-N1 | 90.41(9) |
| O3-Cu-N3 | 92.40(8) |
| N1-Cu-N3 | 164.87(9) |
| Compound | D–H…A | d(D–H) | d(H…A) | d(D…A) | <(DHA) |
|---|---|---|---|---|---|
| 1 | N2-H1…O4#I | 0.79(4) | 2.32(4) | 3.041(3) | 153(3) |
| N4-H2…O4#II | 0.83(4) | 2.03(4) | 2.813(3) | 160(3) | |
| C16-H16-O1 | 0.95 | 2.32 | 2.991(3) | 128 | |
| C21-H21-O2#III | 0.95 | 2.30 | 2.969(4) | 127 |
| Complex | I (eV) | A (eV) | η (eV) | μ (eV) | S (eV) | χ (eV) | ω (eV) |
|---|---|---|---|---|---|---|---|
| 1 | 6.655 | 1.387 | 2.633 | −4.0214 | 0.18985 | 4.02140 | 3.07026 |
| Complexes | Molecular Docking | |
|---|---|---|
| “Kb”/M−1 | ∆G/KJmol−1 | |
| Complex 1 | 4.09 × 104 | −26.31 |
| Benzimidazole | 9.54 × 102 | −17.00 |
| 4-Chlorobenzoic acid | 1.94 × 103 | −18.75 |
| Test Organisms Compounds | Inhibition Zone Diameter (mm) (± Standard Deviation) Data Are Stated as Average Standard Deviation (N = 3) | |||
|---|---|---|---|---|
| S. aureus (G−) | K. pneumoniae (G+) | A. baumannii (G+) | P. aeruginosa (G+) | |
| BZDH | 12.2 ± 1.3 | 0 | 9.5 ± 1.1 | 0 |
| 1 | 20.4 ± 0.8 | 0 | 11.0 ± 1.2 | 17.3 ± 0.3 |
| Ofloxacin | 18.2 ± 0.9 | 0 | 18.0 ± 1.3 | 0 |
| Amoxicillin/Clavulanate | 0 | 0 | 20.6 ± 1.2 | 0 |
| Ceftazidime | 0 | 0 | 14.3 ± 1.2 | 0 |
| Cefuroxime | 0 | 0 | 14.1 ± 0.2 | 0 |
| Gentamicin | 12.0 ± 1.1 | 16.3 ± 1.2 | 18.5 ± 0.6 | 11 ± 0.9 |
| Nitrofurantoin | NA | 25.1 ± 0.2 | 23.2 ± 1.1 | 0 |
| Ciprofloxacin | NA | 18.3 ± 0.7 | 20.7 ± 1.1 | 0 |
| Ampicillin | NA | 0 | 16.5 ± 0.4 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajibola, A.A.; Perveen, F.; Jan, K.; Anibijuwon, I.I.; Shaibu, S.E.; Sieroń, L.; Maniukiewicz, W. A Five-Coordinate Copper(II) Complex Constructed from Sterically Hindered 4-Chlorobenzoate and Benzimidazole: Synthesis, Crystal Structure, Hirshfeld Surface Analysis, DFT, Docking Studies and Antibacterial Activity. Crystals 2020, 10, 991. https://doi.org/10.3390/cryst10110991
Ajibola AA, Perveen F, Jan K, Anibijuwon II, Shaibu SE, Sieroń L, Maniukiewicz W. A Five-Coordinate Copper(II) Complex Constructed from Sterically Hindered 4-Chlorobenzoate and Benzimidazole: Synthesis, Crystal Structure, Hirshfeld Surface Analysis, DFT, Docking Studies and Antibacterial Activity. Crystals. 2020; 10(11):991. https://doi.org/10.3390/cryst10110991
Chicago/Turabian StyleAjibola, Abiodun A., Fouzia Perveen, Kalsoom Jan, Ibikunle I. Anibijuwon, Solomon E. Shaibu, Lesław Sieroń, and Waldemar Maniukiewicz. 2020. "A Five-Coordinate Copper(II) Complex Constructed from Sterically Hindered 4-Chlorobenzoate and Benzimidazole: Synthesis, Crystal Structure, Hirshfeld Surface Analysis, DFT, Docking Studies and Antibacterial Activity" Crystals 10, no. 11: 991. https://doi.org/10.3390/cryst10110991







