Performance Characteristics of Custom Thermocouples for Specialized Applications
Abstract
:1. Introduction
2. Theoretical Background
3. Experimental
3.1. Materials
3.2. Materials Characterization
3.3. Design of Custom Thermocouples
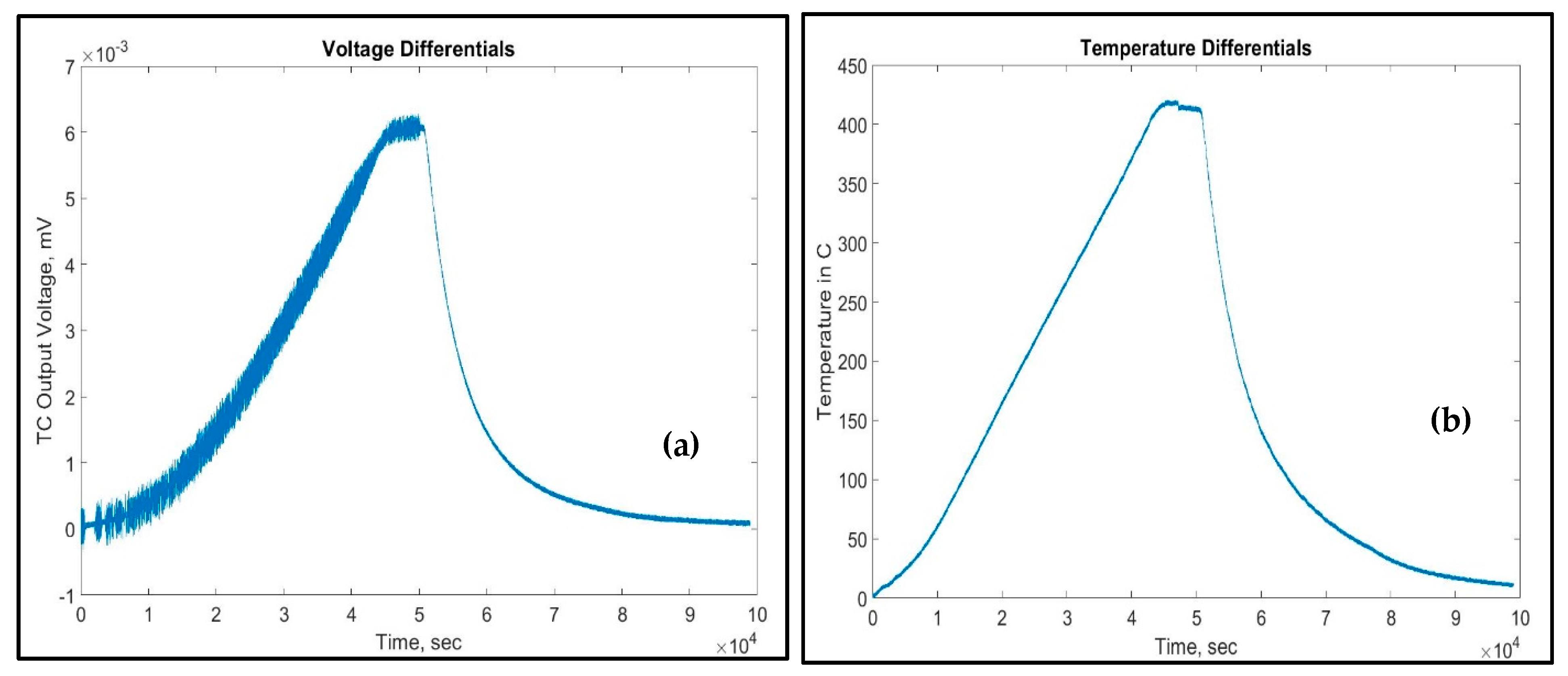
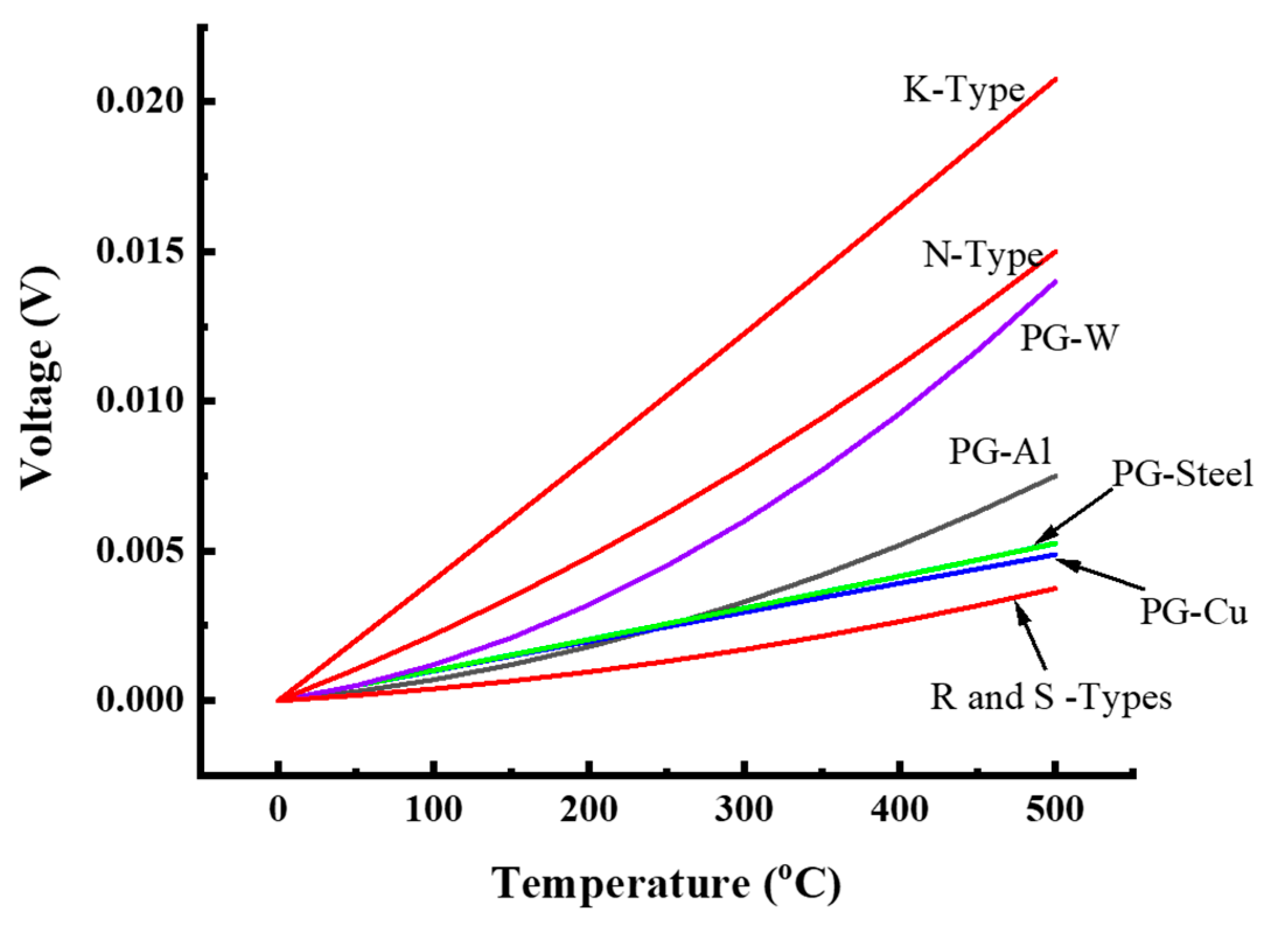
4. Results and Discussion
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kasap, S.O. Principles of Electronic Materials and Devices; McGraw-Hill: Saskatoon, SK, Canada, 2006. [Google Scholar]
- Schön, G. Thermoelectric Effects in Superconductors; Department of Electronic Engineering University of Saskatchewan: Saskatoon, SK, Canada, 2007; pp. 342–362. [Google Scholar]
- Martin, J.; Tritt, T.; Uher, C. High Temperature Seebeck Coefficient Metrology. J. Appl. Phys. 2010, 108, 1–12. [Google Scholar] [CrossRef]
- Yilmaz, N.; Gill, W.; Donaldson, A.B.; Lucero, R.E. Problems Encountered in Fluctuating Flame Temperature Measurements by Thermocouple. Sensors 2008, 8, 7882–7893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abouellail, A.A.; Kostina, M.A.; Bortalevich, S.I.; Loginov, E.L.; Shinyakov, Y.A.; Sukhorukov, M.P. Mathematical Simulation of Thermocouple Characteristics. IOP Conf. Ser. Mater. Sci. Eng. 2018, 327, 022002. [Google Scholar] [CrossRef]
- Shannon, K.S.; Butler, B.W. A Review of Error Associated with Thermocouple Temperature Measurement in Fire Environments. In Proceedings of the 2nd International Wildland Fire Ecology and Fire Management Congress, Orlando, FL, USA, 16–20 November 2003; pp. 7–9. [Google Scholar]
- Webster, E.S.; White, D.R.; Edgar, H. Measurement of Inhomogeneities in MIMS Thermocouples Using a Linear-Gradient Furnace and Dual Heat-Pipe Scanner. Int. J. Thermophys. 2015, 36, 444–466. [Google Scholar] [CrossRef]
- Bhatt, H.D.; Vedula, R.; Desu, S.B.; Fralick, G.C. Thin Film TiC/TaC Thermocouples. Thin Solid Film. 1999, 342, 214–220. [Google Scholar] [CrossRef]
- Sims, C.T.; Gaines, G.B.; Jaffee, R.I. Refractory-Metal Thermocouples Containing Rhenium. Rev. Sci. Instrum. 1959, 30, 112–115. [Google Scholar] [CrossRef]
- Asamoto, R.R.; Novak, P.E. Tungsten-Rhenium Thermocouples for Use at High Temperatures. Rev. Sci. Instrum. 1967, 38, 1047–1052. [Google Scholar] [CrossRef] [Green Version]
- Walker, B.E.; Ewing, C.T.; Miller, R.R. Study of the Instability of Noble Metal Thermocouples in Vacuum. Rev. Sci. Instrum. 1965, 36, 601–606. [Google Scholar] [CrossRef]
- Pavlasek, P.; Elliott, C.J.; Pearce, J.V.; Duris, S.; Palencar, R.; Koval, M.; Machin, G. Hysteresis Effects and Strain-Induced Homogeneity Effects in Base Metal Thermocouples. Int. J. Thermophys. 2015, 36, 467–481. [Google Scholar] [CrossRef]
- Hadi, A.S.; Hill, B.E. Thermopower Determination Using Pyrolytic Graphite and Aluminum Thermocouple. Sens. Actuators A Phys. 2020, 303, 111814. [Google Scholar] [CrossRef]
- Bennett, J.; Kwong, K.S.; Powell, C.; Thomas, H.; Krabbe, R. Improving Thermocouple Service Life in Slagging Gasifiers. In Proceedings of the 22nd Annual International Pittsburgh Coal Conference 2005 (PCC 2005), Pittsburgh, PA, USA, 15–18 September 2005; Volume 3, pp. 2077–2088. [Google Scholar]
- Choi, H.; Datta, A.; Cheng, X.; Li, X. Microfabrication and Characterization of Metal-Embedded Thin-Film Thermomechanical Microsensors for Applications in Hostile Manufacturing Environments. J. Microelectromech. Syst. 2006, 15, 322–329. [Google Scholar] [CrossRef]
- Hadi, A.S.; Hill, B.E. Custom Pyrolytic Graphite–Steel Thermocouple for High-Temperature Measurements. In TMS 2019 148th Annual Meeting & Exhibition Supplemental Proceedings; Springer International Publishing: New York City, NY, USA, 2019; pp. 413–420. [Google Scholar]
- Pollock, D.D. Thermocouples: Theory and Properties; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Hadi, A.S.; Lann, J.; Fricks, T.; Hill, B.E. Pyrolytic Graphite-Copper Thermocouple for Non-Invasive Direct Temperature Measurement. Adv. Ceram. Environ. Funct. Struct. Energy Appl. II 2019, 266, 39–48. [Google Scholar]
- Tougas, I.M.; Gregory, O.J. Thin Film Platinum-Palladium Thermocouples for Gas Turbine Engine Applications. Thin Solid Film. 2013, 539, 345–349. [Google Scholar] [CrossRef]
- Webster, J. Electrocaloric Effect: Theory, Measurements, and Applications; Wiley Encyclopedia of Electrical and Electronics Engineering: Hoboken, NJ, USA, 2015. [Google Scholar]
- Drebushchak, V.A. Universality of the Emf of Thermocouples. Thermochim. Acta 2009, 496, 50–53. [Google Scholar] [CrossRef]
- Slack, G.A. Anisotropic Thermal Conductivity of Pyrolytic Graphite. Phys. Rev. 1962, 127, 694–701. [Google Scholar] [CrossRef]
- Taylor, R. The Thermal Conductivity of Pyrolytic Graphite. Philos. Mag. 1966, 13, 157–166. [Google Scholar] [CrossRef]
- Hoi, Y.M.; Chung, D.D. Flexible Graphite as a Compliant Thermoelectric Material. Carbon 2002, 40, 1134–1136. [Google Scholar] [CrossRef]
- Savvatimskiy, A. Carbon at High Temperatures; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Luo, X.; Chugh, R.; Biller, B.C.; Hoi, Y.M.; Chung, D.D. Electronic Applications of Flexible Graphite. J. Electron. Mater. 2002, 31, 535–544. [Google Scholar] [CrossRef]
- Chung, D.D. Flexible Graphite for Gasketing, Adsorption, Electromagnetic Interference Shielding, Vibration Damping, Electrochemical Applications, and Stress Sensing. J. Mater. Eng. Perform. 2000, 9, 161–163. [Google Scholar] [CrossRef]
- Prabhu, K.N.; Suresha, K.M. Effect of Superheat, Mold, and Casting Materials on the Metal/Mold Interfacial Heat Transfer during Solidification in Graphite-Lined Permanent Molds. J. Mater. Eng. Perform. 2004, 13, 619–626. [Google Scholar] [CrossRef]
- ASM International. Properties and Selection: Nonferrous Alloys and Special-Purpose Materials. In ASM International Handbook; Version 2; ASM International: Phoenix, AZ, USA, 1990; Volume 2, p. 1300. [Google Scholar]
- Issahaq, M.N.; Chandrasekar, S.; Trumble, K.P. Single-Step Shear-Based Deformation Processing of Electrical Conductor Wires. J. Manuf. Sci. Eng. 2021, 143, 1–9. [Google Scholar] [CrossRef]
- Reed-Hill R, A.R. Physical Metallurgy. Phys. Metall. Princ. 1991, 3, 2215–2290. [Google Scholar]
- Davis, J.R. Copper and Copper Alloys. In ASM Specialty Handbook-Copper and Copper Alloys; ASM International: Phoenix, AZ, USA, 2001; pp. 457–494. [Google Scholar]
- Asting, A.V. Copper Wire Tables, Google-Digitized; US Government Printing Office: Washington, DC, USA, 1956. [Google Scholar]
- Snyder, G.J.; Snyder, A.H. Figure of Merit ZT of a Thermoelectric Device Defined from Materials Properties. Energy Environ. Sci. 2017, 10, 2280–2283. [Google Scholar] [CrossRef]
- Washko, R.S.; Aggen, G. Steels. In Properties and Selection: Irons, Steels, and High-Performance Alloys; ASM International: Phoenix, AZ, USA, 2018; Volume 1, pp. 841–907. [Google Scholar]
- Ciulik, J.R.; Shields, J.A.; Kumar, P.; Todd Leonhardt, J.L. Properties and Selection of Powder Metallurgy Refractory Metals. Powder Metall. 2018, 7, 593–598. [Google Scholar]
- Schmidt, F.F. The Engineering Properties of Tungsten and Tungsten Alloys; Defense Documentation Center: Alexandria, VA, USA, 1963. [Google Scholar]
- Thomas, D.B. Studies on the Tungsten-Rhenium Thermocouple to 2000 °C. Eng. Instrum. 1963, 67, 337. [Google Scholar] [CrossRef]
- Maxim Integrated. MAX31855 Cold-Junction Compensated Thermocouple-to-Digital Converter; Datasheet; Maxim Integrated: San Jose, CA, USA, 2015. [Google Scholar]
- Duff, M.; Towey, J. Two Ways to Measure Temperature Using Thermocouples Feature Simplicity, Accuracy, and Flexibility. Analog Dialogue 2010, 44, 1–6. [Google Scholar]
- ASM International. Properties and Selection: Irons, Steels, and High-Performance Alloys. In ASM International Handbook; Version 1; ASM International: Phoenix, AZ, USA, 2018. [Google Scholar]




| Composition | Al | Cu | Steel | W | |
|---|---|---|---|---|---|
| Fe | C | ||||
| Main element (wt%) | 97.89 | 100 | 95.47 | 3.34 | 89.31 |
| Other (wt%) | 2.11 | 0 | 1.19 | 10.63 | |
| Material | Tm (°C) | Thermal Conductivity (W/m·K) | Electrical Resistivity (Ω·m) | Seebeck Coefficient, µV/°C | R2 Value | |
|---|---|---|---|---|---|---|
| Heating | Cooling | |||||
| Al | 660 | 243 | 2.67 × 10−8 | 15.8 | 14 | 0.96 |
| Cu | 1083 | 391 | 1.71 × 10−8 | 18.8 | 17.7 | 0.91 |
| Steel | 1400 | 21.5 | 7.40 × 10−7 | 13.8 | 14 | 0.89 |
| W | 3180 | 155 | 5.30 × 10−8 | 26 | 24.2 | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadi, A.-S.; Hill, B.E.; Issahaq, M.N. Performance Characteristics of Custom Thermocouples for Specialized Applications. Crystals 2021, 11, 377. https://doi.org/10.3390/cryst11040377
Hadi A-S, Hill BE, Issahaq MN. Performance Characteristics of Custom Thermocouples for Specialized Applications. Crystals. 2021; 11(4):377. https://doi.org/10.3390/cryst11040377
Chicago/Turabian StyleHadi, Abdul-Sommed, Bryce E. Hill, and Mohammed Naziru Issahaq. 2021. "Performance Characteristics of Custom Thermocouples for Specialized Applications" Crystals 11, no. 4: 377. https://doi.org/10.3390/cryst11040377






