Green Synthesis of Chitosan Nanoparticles Using of Martynia annua L. Ethanol Leaf Extract and Their Antibacterial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Sample Collection, Identification, and Processing
2.2. Leaf Extract Preparation
2.3. Thin Layer Chromatographic (TLC) Analysis
2.4. Phytochemical (Qualitative) Analysis
2.5. Synthesis of Chitosan Nanoparticles
2.6. Characterization of MA-CNPs
2.7. Antibacterial Activity Analysis
3. Results and Discussion
3.1. Plant Extract Yield and TLC Analyses
3.2. Qualitative Phytochemicals Screening
3.3. Chitosan Nanoparticle Synthesis and Characterization
3.3.1. UV-Visible Spectra of MA-CNPs
3.3.2. FT-IR Analysis
3.3.3. SEM and DLS Analyses
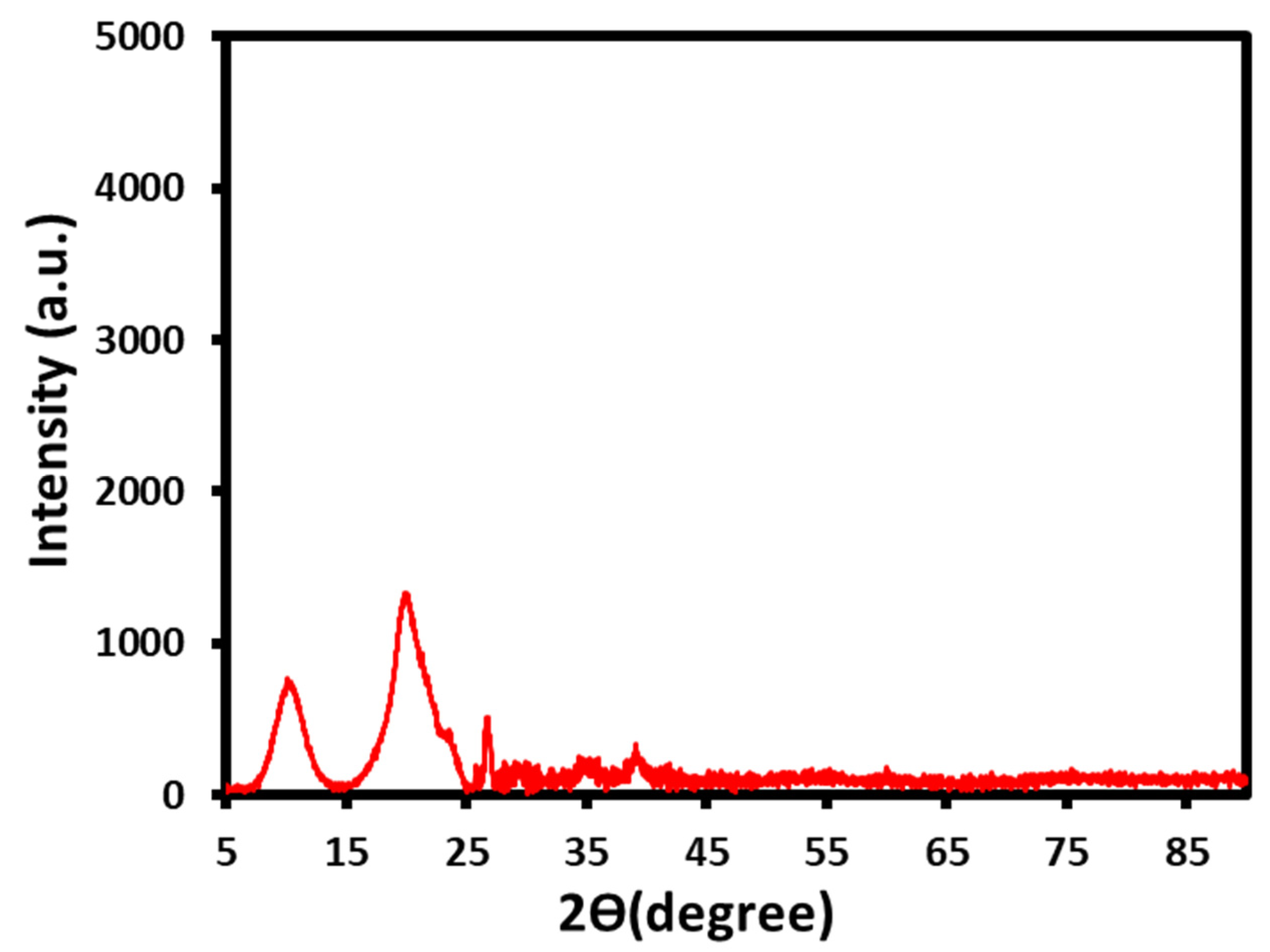
3.3.4. XRD Analysis
3.4. Antibacterial Activity Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Xianyu, Y.; Jiang, X. Surface modification of gold nanoparticles with small molecules for biochemical analysis. Acc. Chem. Res. 2017, 50, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Chau, T.P.; Veeraragavan, G.R.; Narayanan, M.; Chinnathambi, A.; Alharbi, S.A.; Subramani, B.; Brindhadevi, K.; Pimpimon, T.; Pikulkaew, S. Green synthesis of Zirconium nanoparticles using Punica granatum (pomegranate) peel extract and their antimicrobial and antioxidant potency. Environ. Res. 2022, 209, 112771. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, M.; El-Sheekh, M.; Ma, Y.; Pugazhendhi, A.; Natarajan, D.; Kandasamy, G.; Raja, R.; Kumar, R.S.; Kumarasamy, S.; Sathiyan, G. Current status of microbes involved in the degradation of pharmaceutical and personal care products (PPCPs) pollutants in the aquatic ecosystem. Environ. Pollut. 2022, 300, 118922. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Malar, D.S.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Nabavi, S.M.; Daglia, M. Kaempferol and inflammation: From chemistry to medicine. Pharmacol. Res. 2015, 99, 1–10. [Google Scholar] [CrossRef]
- Sheng, Y.; Narayanan, M.; Basha, S.; Elfasakhany, A.; Brindhadevi, K.; Xia, C.; Pugazhendhi, A. In vitro and in vivo efficacy of green synthesized AgNPs against Gram negative and Gram positive bacterial pathogens. Process Biochem. 2022, 112, 241–247. [Google Scholar] [CrossRef]
- Narayanan, M.; Kiran, A.; Natarajan, D.; Kandasamy, S.; Shanmugam, S.; Alshiekheid, M.; Almoallim, H.S.; Pugazhendhi, A. The pharmaceutical potential of crude ethanol leaf extract of Pedalium murex (L.). Process Biochem. 2022, 112, 234–240. [Google Scholar] [CrossRef]
- Kirtane, A.R.; Verma, M.; Karandikar, P.; Furin, J.; Langer, R.; Traverso, G. Nanotechnology approaches for global infectious diseases. Nat. Nanotechnol. 2021, 16, 369–384. [Google Scholar] [CrossRef]
- Manandhar, S.; Luitel, S.; Dahal, R.K. In vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria. J. Trop. Med. 2019, 2019, 1895340. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, M.; Vijay, A.; Kandasamy, S.; Nasif, O.; Alharbi, S.A.; Srinivasan, R.; Kavitha, R. Phytochemical profile and larvicidal activity of aqueous extract of Ocimum americanum against mosquito vectors. Appl. Nanosci. 2021. [Google Scholar] [CrossRef]
- Thanh, N.C.; Rajeswari, V.D.; Narayanan, M.; Kandasamy, S.; Chinnathambi, A.; Alharbi, S.A.; Van Le, Q.; Kathirvel, B. Evaluation of probiotic susceptibility of virulent Aeromonas sp. by a study on gut histology of Cyprinus carpio. Process Biochem. 2021, 111, 154–159. [Google Scholar] [CrossRef]
- Prema, P.; Ranjani, S.S.; Kumar, K.R.; Veeramanikandan, V.; Mathiyazhagan, N.; Nguyen, V.-H.; Balaji, P. Microbial synthesis of silver nanoparticles using Lactobacillus plantarum for antioxidant, antibacterial activities. Inorg. Chem. Commun. 2022, 136, 109139. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Shyichuk, A.; Sojka, Z.; Gryboś, J.; Naushad, M.; Kotsyubynsky, V.; Kowalska, M.; Kwiatkowska-Marks, S.; Danyliuk, N. Green synthesis, structure, cations distribution and bonding characteristics of superparamagnetic cobalt-zinc ferrites nanoparticles for Pb(II) adsorption and magnetic hyperthermia applications. J. Mol. Liq. 2021, 328, 115375. [Google Scholar] [CrossRef]
- Ahmad, T.; Bustam, M.A.; Irfan, M.; Moniruzzaman, M.; Asghar, H.M.A.; Bhattacharjee, S. Mechanistic investigation of phytochemicals involved in green synthesis of gold nanoparticles using aqueous Elaeis guineensis leaves extract: Role of phenolic compounds and flavonoids. Biotechnol. Appl. Biochem. 2019, 66, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Kumarasamy, S.; Narayanan, M.; Ranganathan, M.; Rathinavel, T.; Chinnathambi, A.; Alahmadi, T.A.; Karuppusamy, I.; Pugazhendhi, A.; Whangchai, K. Spectral and structure characterization of Ferula assafoetida fabricated silver nanoparticles and evaluation of its cytotoxic, and photocatalytic competence. Environ. Res. 2022, 204, 111987. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.M.; El-Nahrawy, A.M.; Abou Hammad, A.B. Sol-gel synthesis and characterizations of hybrid chitosan-PEG/calcium silicate nanocomposite modified with ZnO-NPs and (E102) for optical and antibacterial applications. Int. J. Biol. Macromol. 2017, 97, 561–567. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; Saber, W.I.; Zweil, A.M.; Bashir, S.I. An innovative green synthesis approach of chitosan nanoparticles and their inhibitory activity against phytopathogenic Botrytis cinerea on strawberry leaves. Sci. Rep. 2022, 12, 3515. [Google Scholar] [CrossRef]
- Lodhi, S.; Jain, A.; Jain, A.P.; Pawar, R.S.; Singhai, A.K. Effects of flavonoids from Martynia annua and Tephrosia purpurea on cutaneous wound healing. Avicenna J. Phytomedicine 2016, 6, 578. [Google Scholar]
- Lodhi, S.; Singhai, A. Preliminary pharmacological evaluation of Martynia annua Linn leaves for wound healing. Asian Pac. J. Trop. Biomed. 2011, 1, 421–427. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, L.; Salleh, R.M.; Murugaiyah, V. Phytochemical and bioactive compounds identification of Ocimum tenuiflorum leaves of methanol extract and its fraction with an anti-diabetic potential. Int. J. Food Prop. 2018, 21, 2390–2399. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yang, Y.; Shang, Y.; Liang, C.; He, J.; Li, J.; Chang, Y. Optimization of the Purifying Process for Columbianetin-β-D-Glucopyranoside from Angelicae Pubescentis Radix and Evaluation of Its Analgesic Activity Using Hot Plate Test. Evid. Based Complement. Altern. Med. 2021, 2021, 9944270. [Google Scholar] [CrossRef]
- Olech, M.; Komsta, Ł.; Nowak, R.; Cieśla, Ł.; Waksmundzka-Hajnos, M. Investigation of antiradical activity of plant material by thin-layer chromatography with image processing. Food Chem. 2012, 132, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rao, K.; Bhuvaneswari, C.; Giri, A.; Mangamoori, L.N. Phytochemical analysis of Andrographis paniculata extract and its antimicrobial activity. World J. Microbiol. Biotechnol. 2010, 26, 85–91. [Google Scholar] [CrossRef]
- Soni, A.; Sosa, S. Phytochemical analysis and free radical scavenging potential of herbal and medicinal plant extracts. J. Pharmacogn. Phytochem. 2013, 2, 22–29. [Google Scholar]
- Duraisamy, N.; Dhayalan, S.; Shaik, M.R.; Shaik, A.H.; Shaik, J.P.; Shaik, B. Evaluation of Antioxidant, Cytotoxic, Mutagenic and Other Inhibitory Potentials of Green Synthesized Chitosan Nanoparticles. Crystals 2022, 12, 1540. [Google Scholar]
- El-Aziz, A.R.M.A.; Al-Othman, M.R.; Mahmoud, M.A.; Shehata, S.M.; Abdelazim, N.S. Chitosan nanoparticles as a carrier for Mentha longifolia extract: Synthesis, characterization and antifungal activity. Curr. Sci. 2018, 114, 2116–2122. [Google Scholar]
- Othman, M.; San Loh, H.; Wiart, C.; Khoo, T.J.; Lim, K.H.; Ting, K.N. Optimal methods for evaluating antimicrobial activities from plant extracts. J. Microbiol. Methods 2011, 84, 161–166. [Google Scholar] [CrossRef]
- Kaushik, S.; Jain, P.; Satapathy, T.; Purabiya, P.; Roy, A. Evaluation of anti-arthritic and anti-inflammatory activities of Martynia annua L. Ethanolic extract. Clin. Phytoscience 2021, 7, 7. [Google Scholar] [CrossRef]
- Macchioni, V.; Carbone, K.; Cataldo, A.; Fraschini, R.; Bellucci, S. Lactic acid-based deep natural eutectic solvents for the extraction of bioactive metabolites of Humulus lupulus L.: Supramolecular organization, phytochemical profiling and biological activity. Sep. Purif. Technol. 2021, 264, 118039. [Google Scholar] [CrossRef]
- Martinez-Fernandez, J.S.; Seker, A.; Davaritouchaee, M.; Gu, X.; Chen, S. Recovering valuable bioactive compounds from potato peels with sequential hydrothermal extraction. Waste Biomass Valorization 2021, 12, 1465–1481. [Google Scholar] [CrossRef]
- Mandal, S.; Patra, A.; Samanta, A.; Roy, S.; Mandal, A.; Mahapatra, T.D.; Pradhan, S.; Das, K.; Nandi, D.K. Analysis of phytochemical profile of Terminalia arjuna bark extract with antioxidative and antimicrobial properties. Asian Pac. J. Trop. Biomed. 2013, 3, 960–966. [Google Scholar] [CrossRef] [Green Version]
- Kagan, I.A.; Flythe, M.D. Thin-layer chromatographic (TLC) separations and bioassays of plant extracts to identify antimicrobial compounds. JOVE J. Vis. Exp. 2014, e51411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galib, N.A.; Ali, K.S.S.; Munaiem, R.T.; Mohammed, A.S.A. Phytochemical Screening and Thin Layer Chromatography of Acacia Etbaica Ssp. Uncinata Leaves. World J. Pharm. Res. 2017, 6, 1278–1283. [Google Scholar]
- Baldwin, A.; Booth, B.W. Biomedical applications of tannic acid. J. Biomater. Appl. 2022, 36, 1503–1523. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef] [PubMed]
- Vaezifar, S.; Razavi, S.; Golozar, M.A.; Karbasi, S.; Morshed, M.; Kamali, M. Effects of some parameters on particle size distribution of chitosan nanoparticles prepared by ionic gelation method. J. Clust. Sci. 2013, 24, 891–903. [Google Scholar] [CrossRef]
- Oh, J.-W.; Chun, S.C.; Chandrasekaran, M. Preparation and in vitro characterization of chitosan nanoparticles and their broad-spectrum antifungal action compared to antibacterial activities against phytopathogens of tomato. Agronomy 2019, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Rai, S.; Dutta, P.; Mehrotra, G. Lignin incorporated antimicrobial chitosan film for food packaging application. J. Polym. Mater. 2017, 34, 171. [Google Scholar]
- Thamilarasan, V.; Sethuraman, V.; Gopinath, K.; Balalakshmi, C.; Govindarajan, M.; Mothana, R.A.; Siddiqui, N.A.; Khaled, J.M.; Benelli, G. Single Step Fabrication of Chitosan Nanocrystals Using Penaeus semisulcatus: Potential as New Insecticides, Antimicrobials and Plant Growth Promoters. J. Clust. Sci. 2018, 29, 375–384. [Google Scholar] [CrossRef]
- Abdallah, Y.; Liu, M.; Ogunyemi, S.O.; Ahmed, T.; Fouad, H.; Abdelazez, A.; Yan, C.; Yang, Y.; Chen, J.; Li, B. Bioinspired green synthesis of chitosan and zinc oxide nanoparticles with strong antibacterial activity against rice pathogen Xanthomonas oryzae pv. oryzae. Molecules 2020, 25, 4795. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnology 2018, 16, 84. [Google Scholar] [CrossRef]
- Manne, A.A.; Mangamuri, U.; Podha, S. Pterocarpus marsupium Roxb. heartwood extract synthesized chitosan nanoparticles and its biomedical applications. J. Genet. Eng. Biotechnol. 2020, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Shetta, A.; Kegere, J.; Mamdouh, W. Comparative study of encapsulated peppermint and green tea essential oils in chitosan nanoparticles: Encapsulation, thermal stability, in-vitro release, antioxidant and antibacterial activities. Int. J. Biol. Macromol. 2019, 126, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Ilk, S.; Saglam, N.; Özgen, M. Kaempferol loaded lecithin/chitosan nanoparticles: Preparation, characterization, and their potential applications as a sustainable antifungal agent. Artif. Cells Nanomed. Biotechnol. 2017, 45, 907–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garavand, F.; Cacciotti, I.; Vahedikia, N.; Rehman, A.; Tarhan, Ö.; Akbari-Alavijeh, S.; Shaddel, R.; Rashidinejad, A.; Nejatian, M.; Jafarzadeh, S. A comprehensive review on the nanocomposites loaded with chitosan nanoparticles for food packaging. Crit. Rev. Food Sci. Nutr. 2022, 62, 1383–1416. [Google Scholar] [CrossRef]
- Kalaichelvi, K.; Dhivya, S. Phytochemical screening and antibacterial activity of leaf extract of Martynia annua L. and Premna latifolia, Roxb. J. Med. Plants Stud. 2016, 4, 84–87. [Google Scholar]
- Khan, M.F.; Tang, H.; Lyles, J.T.; Pineau, R.; Mashwani, Z.-U.-R.; Quave, C.L. Antibacterial properties of medicinal plants from Pakistan against multidrug-resistant ESKAPE pathogens. Front. Pharmacol. 2018, 9, 815. [Google Scholar] [CrossRef] [Green Version]
- Alamgir, A. Secondary metabolites: Secondary metabolic products consisting of C and H; C, H, and O; N, S, and P elements; and O/N heterocycles. In Therapeutic Use of Medicinal Plants and Their Extracts; Springer: Berlin/Heidelberg, Germany, 2018; Volume 2, pp. 165–309. [Google Scholar]
- Deng, Y.; Zhao, Y.; Padilla-Zakour, O.; Yang, G. Polyphenols, antioxidant and antimicrobial activities of leaf and bark extracts of Solidago canadensis L. Ind. Crops Prod. 2015, 74, 803–809. [Google Scholar] [CrossRef]
- Willems, T.; De Mol, M.L.; De Bruycker, A.; De Maeseneire, S.L.; Soetaert, W.K. Alkaloids from marine fungi: Promising antimicrobials. Antibiotics 2020, 9, 340. [Google Scholar] [CrossRef]
- Sivakumar, S.R.; Sasikala, C.; Sheela, T. Bio-Synthesis of Silver Nanoparticles from Leaf Extracts of Martynia Annua L. and Its Antimicrobial Activity. Int. J. Pharm. Sci. Invent. 2018, 7, 40–47. [Google Scholar]
- Subitha, R.; Senthilkumar, P.; Gobinath, K. In vivo wound healing potential of chitosan gel based silver nanoparticles synthesized from Martynia annua. Res. J. Biotechnol. 2022, 17, 119–133. [Google Scholar] [CrossRef]

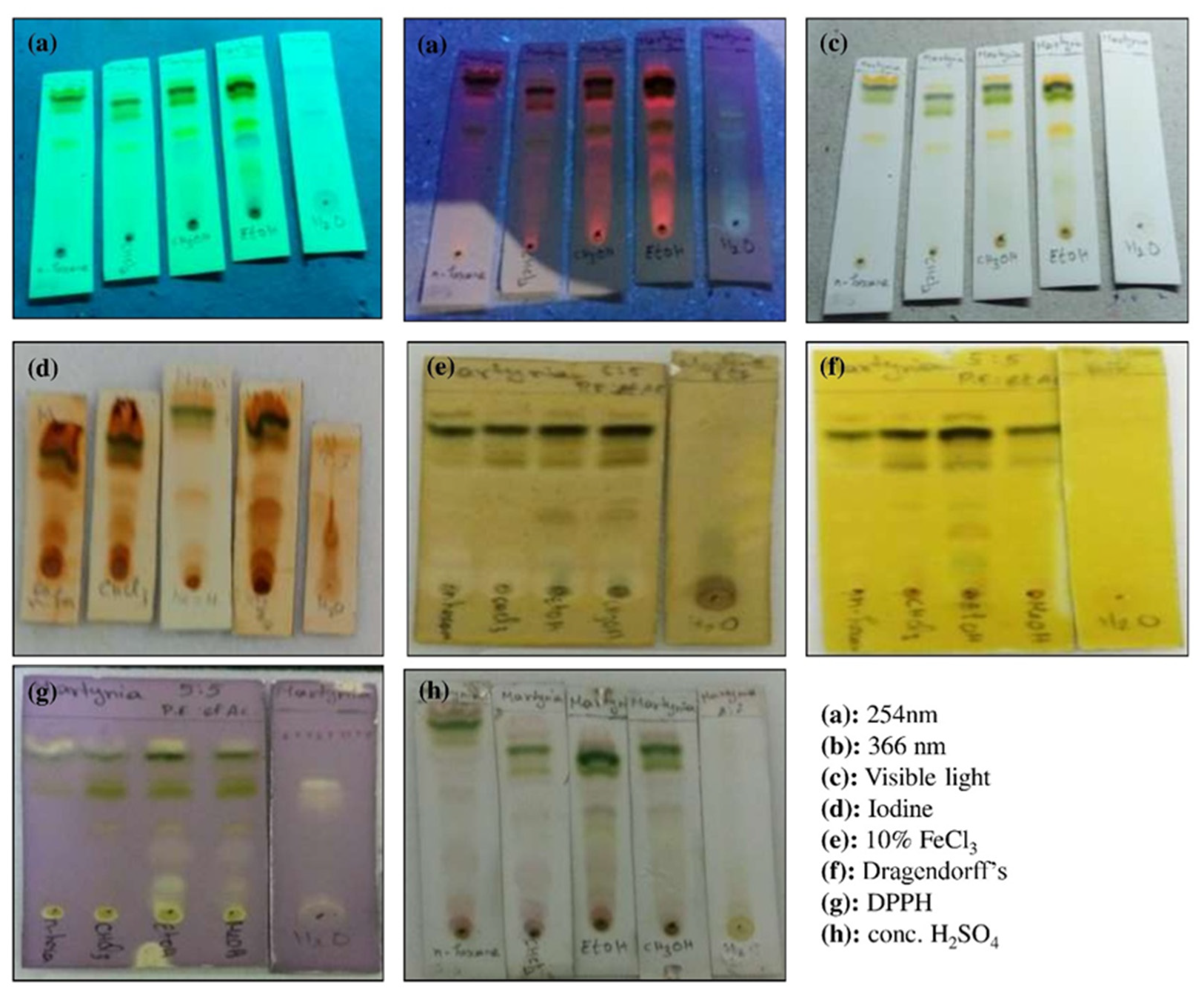
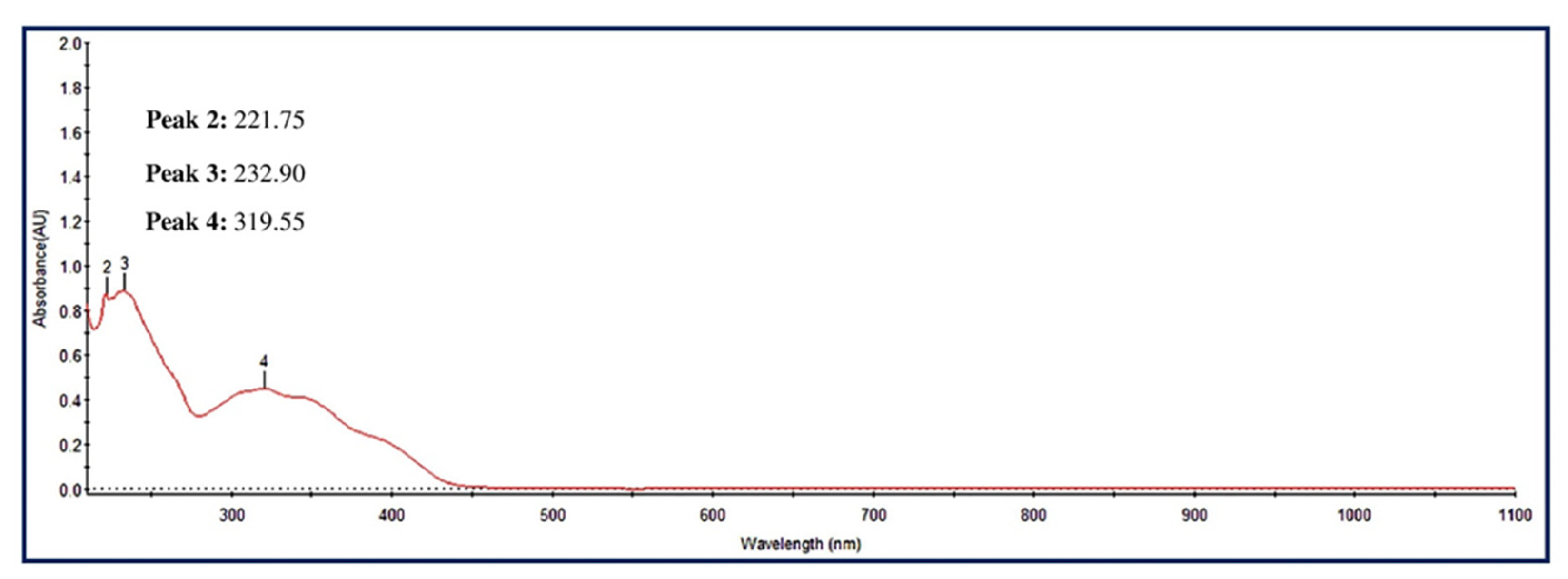
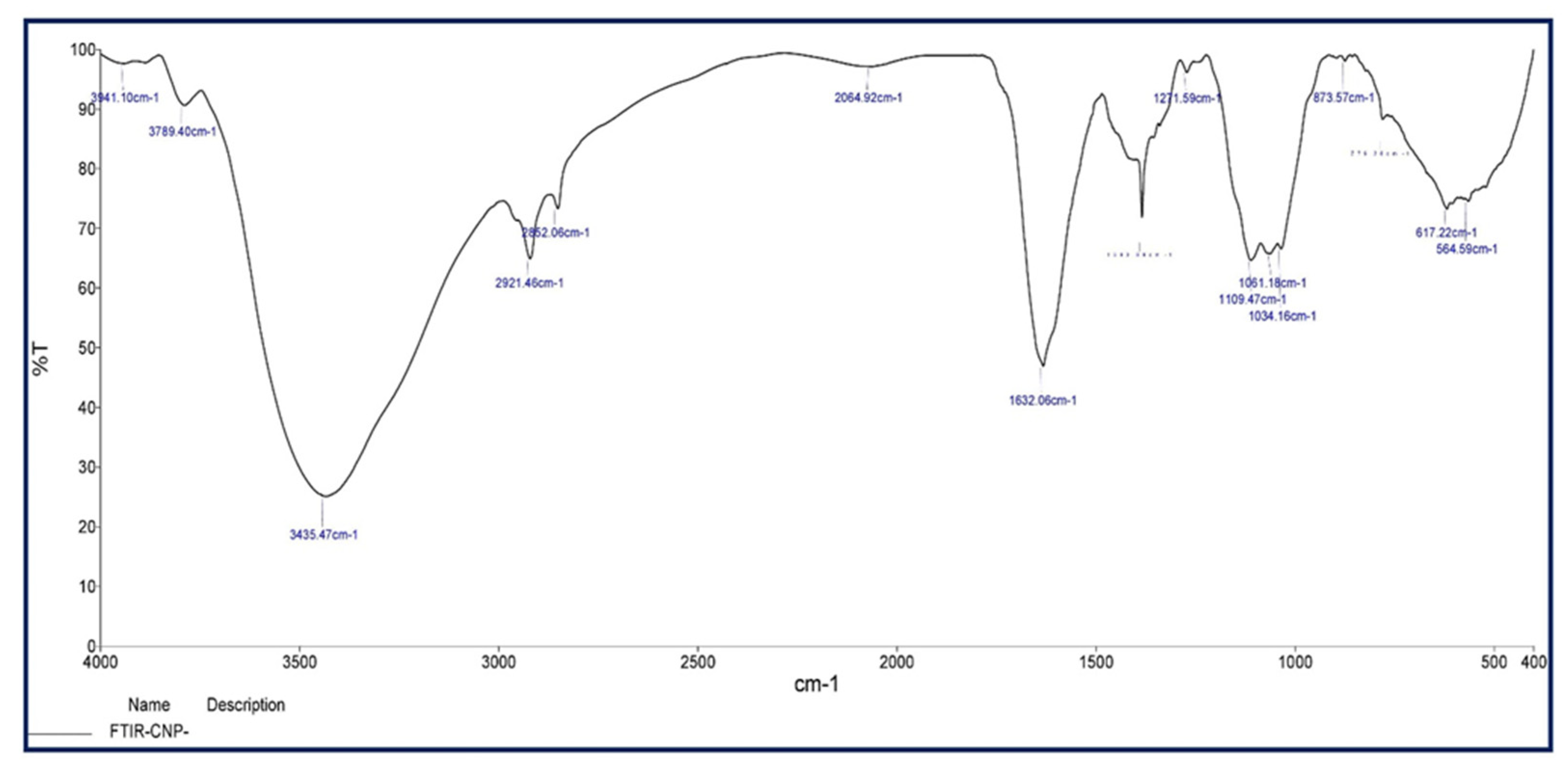
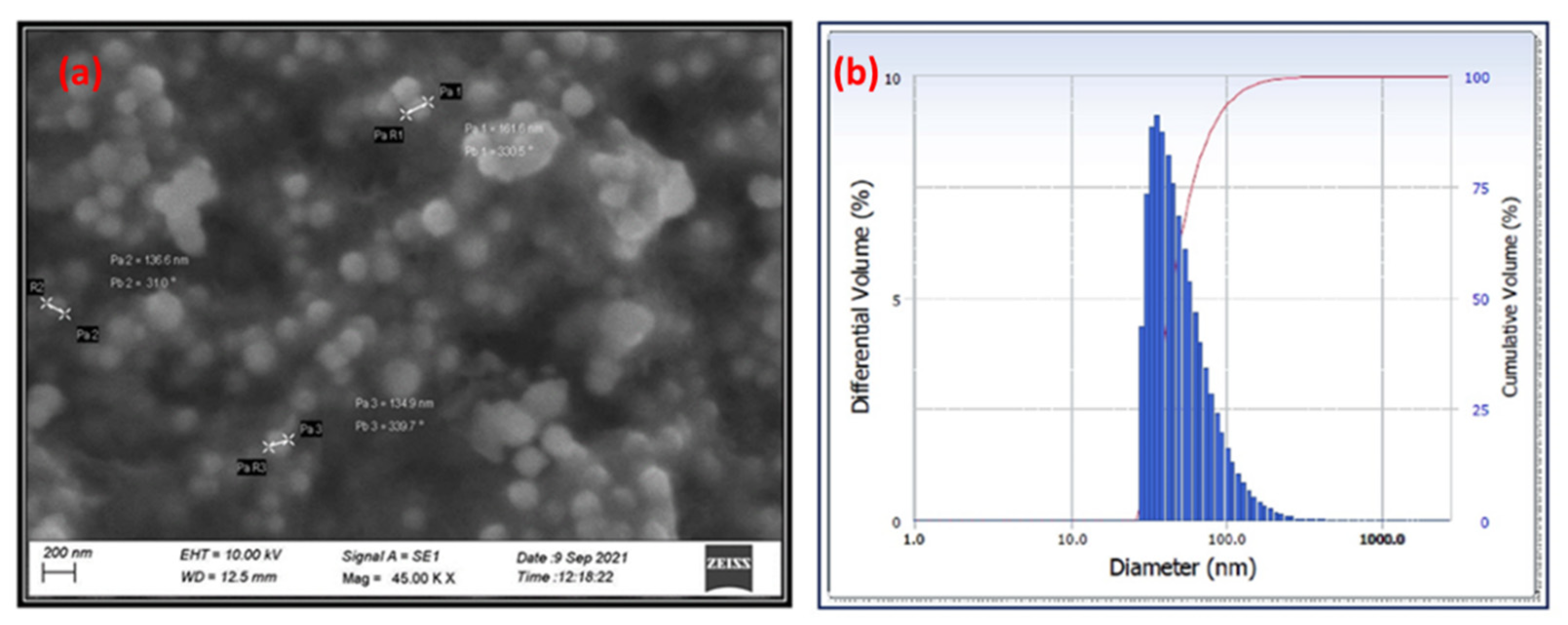

| Sl. No. | Phytochemicals Name | Specific Test | Ethanol Extract |
|---|---|---|---|
| 1 | Carbohydrate | Benedict’s Test | − |
| 2 | Protein & Amino Acids | Millions Test | + |
| 3 | Alkaloid | Dragendorff’s Test | + |
| 4 | Tannin & Phenol | Ferric Chloride Test | + |
| 5 | Flavonoids | Zn–HCl Test | − |
| 6 | Terpenoids | Salkowski Test | − |
| 7 | Saponin | Froth Test | + |
| 8 | Glycosides | Keller-Kilani Test | + |
| 9 | Quinons | NaOH Test | + |
| 10 | Fixed Oil | Paper/spot Test | + |
| 11 | Resins | Acetone Test | + |
| 12 | Coumarins | Fluorescence Test | − |
| 13 | Carotenoids | - | + |
| Bacterial Pathogens | Concentration (µg/mL)/Zone of Inhibition (mm) | ||||
|---|---|---|---|---|---|
| 500 | 250 | 100 | 50 | Gentamycin (Positive Control) | |
| Gram Negative | |||||
| Pseudomonas aeruginosa | 13 ± 1.0 | 9.9 ± 0.9 | 8.5 ± 0.5 | 7.4 ± 0.4 | 22 ± 1.0 |
| Escherichia coli | 12 ± 2.0 | 9 ± 1.0 | 8.5 ± 0.5 | 6.5 ± 0.5 | 21 ± 1.0 |
| Bacteroides fragilis | 14.5 ± 1.5 | 10.5 ± 0.5 | 7.7 ± 0.7 | 6.5 ± 0.5 | 30 ± 2.0 |
| Aeromonas hydrophila | 16 ± 2.5 | 16 ± 1.0 | 14.6 ± 0.6 | 13.5 ± 0.5 | 23.5 ± 0.5 |
| Gram Positive | |||||
| Streptococcus oralis | 12.5 ± 2.0 | 9.5 ± 0.5 | 8.4 ± 0.4 | 7.2 ± 0.2 | 28 ± 1.0 |
| Streptococcus faecalis | 13 ± 1.5 | 10.9 ± 0.9 | 8.5 ± 0.5 | 7.4 ± 0.4 | 32 ± 1.0 |
| Staphylococcus aureus | 16 ± 2.0 | 11 ± 1.5 | 8.4 ± 0.4 | 7.3 ± 0.3 | 27 ± 2.0 |
| Streptococcus mutans | 15.5 ± 2.0 | 12.5 ± 0.5 | 11.4 ± 0.4 | 8.3 ± 0.3 | 32 ± 1.0 |
| Propionibacterium acnes | 12.5 ± 1.5 | 9.7 ± 0.7 | 8.5 ± 0.5 | 7.3 ± 0.3 | 29 ± 1.0 |
| Bacillus cereus | 15 ± 2.0 | 13 ± 1.0 | 10.7 ± 0.7 | 8.5 ± 0.5 | 27 ± 1.0 |
| Tested Bacterial Pathogens | Concentration of MA-CNPs (μg/mL)/Zone of Inhibition (mm) | ||||
|---|---|---|---|---|---|
| 500 | 250 | 100 | 50 | PC (Gentamycin) | |
| Gram Negative | |||||
| Pseudomonas aeruginosa | 17.55 ± 0.07 | 15.15 ± 0.05 | 10.05 ± 0.40 | 10.20 ± 0.28 | 22 ± 1.0 |
| Escherichia coli | 17.05 ± 0.12 | 15.05 ± 0.35 | 12.00 ± 0.35 | 10.20 ± 0.05 | 21 ± 1.0 |
| Bacteroides fragilis | 18.35 ± 0.35 | 14.25 ± 0.60 | 11.50 ± 0.10 | 9.65 ± 0.55 | 30 ± 2.0 |
| Aeromonas hydrophila | 16.50 ± 0.17 | 15.25 ± 0.35 | 14.25 ± 0.30 | 13.25 ± 0.30 | 23.5 ± 0.5 |
| Gram Positive | |||||
| Streptococcus oralis | 18.25 ± 0.35 | 17.25 ± 0.25 | 16.20 ± 0.06 | 15.00 ± 0.35 | 28 ± 1.0 |
| Streptococcus faecalis | 14.25 ± 0.35 | 11.25 ± 0.15 | 10.75 ± 0.25 | 9.25 ± 0.35 | 32 ± 1.0 |
| Staphylococcus aureus | 17.25 ± 0.20 | 16.20 ± 0.20 | 15.25 ± 0.35 | 14.25 ± 0.10 | 27 ± 2.0 |
| Streptococcus mutans | 16.50 ± 0.70 | 11.25 ± 0.16 | 10.50 ± 0.20 | 9.20 ± 0.15 | 32 ± 1.0 |
| Propionibacterium acnes | 18.05 ± 0.15 | 16.55 ± 0.30 | 15.45 ± 0.60 | 14.70 ± 0.45 | 29 ± 1.0 |
| Bacillus cereus | 17.00 ± 0.27 | 16.30 ± 0.29 | 10.15 ± 0.30 | 9.00 ± 1.00 | 27 ± 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duraisamy, N.; Dhayalan, S.; Shaik, M.R.; Shaik, A.H.; Shaik, J.P.; Shaik, B. Green Synthesis of Chitosan Nanoparticles Using of Martynia annua L. Ethanol Leaf Extract and Their Antibacterial Activity. Crystals 2022, 12, 1550. https://doi.org/10.3390/cryst12111550
Duraisamy N, Dhayalan S, Shaik MR, Shaik AH, Shaik JP, Shaik B. Green Synthesis of Chitosan Nanoparticles Using of Martynia annua L. Ethanol Leaf Extract and Their Antibacterial Activity. Crystals. 2022; 12(11):1550. https://doi.org/10.3390/cryst12111550
Chicago/Turabian StyleDuraisamy, Narayanasamy, Sangeetha Dhayalan, Mohammed Rafi Shaik, Althaf Hussain Shaik, Jilani P. Shaik, and Baji Shaik. 2022. "Green Synthesis of Chitosan Nanoparticles Using of Martynia annua L. Ethanol Leaf Extract and Their Antibacterial Activity" Crystals 12, no. 11: 1550. https://doi.org/10.3390/cryst12111550







