Nanocrystal-Based Topical Gels for Improving Wound Healing Efficacy of Curcumin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Curcumin Nanocrystals
2.3. Determination of Morphology by Scanning Electron Microscopy (SEM)
2.4. Determination of Average Particle Size and Zeta Potential
2.5. Percentage Yield
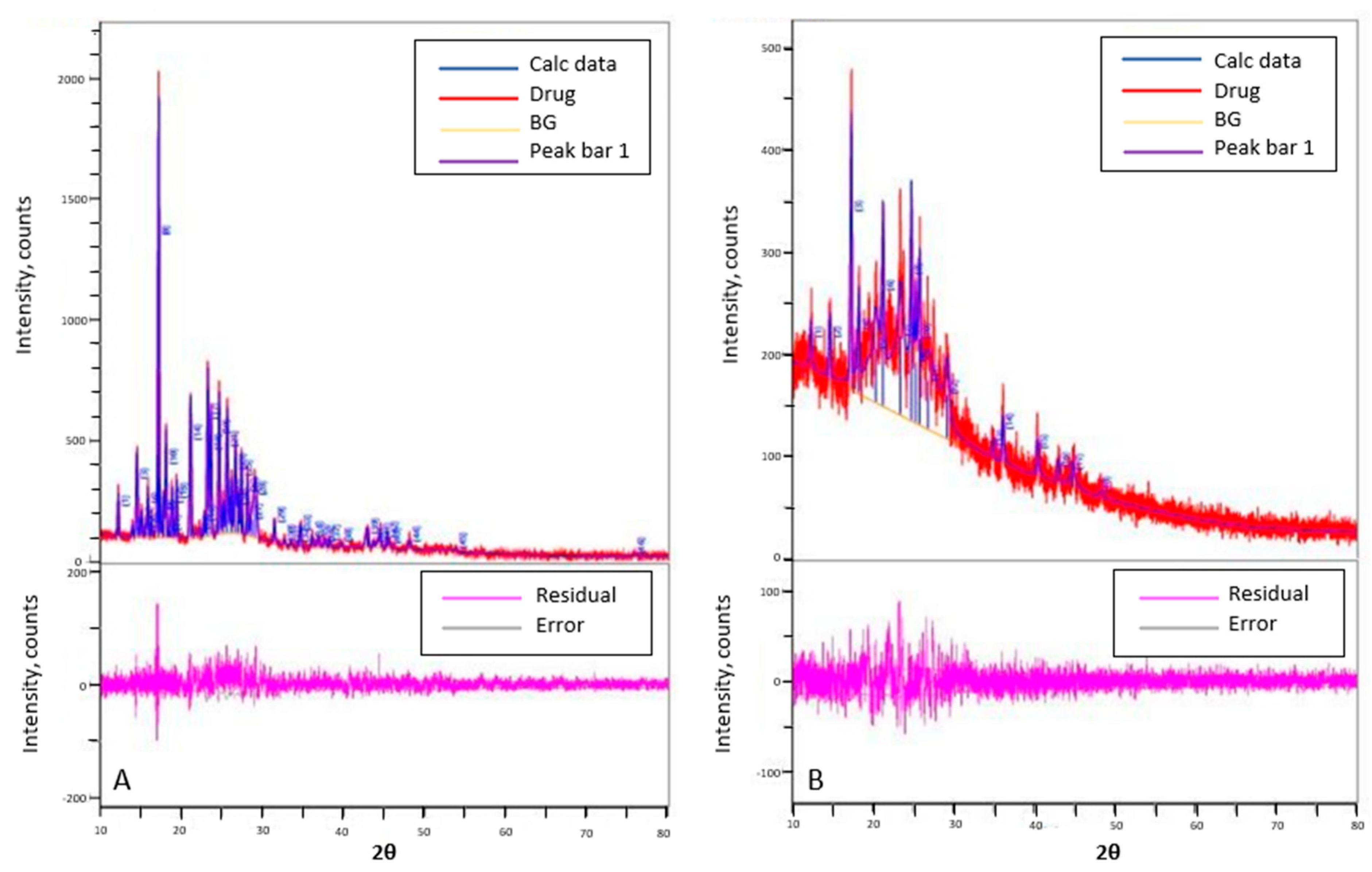
2.6. Powder X-ray Diffraction (PXRD) Studies
2.7. In Vitro Drug Release Studies
Kinetic Analysis of Drug Release
2.8. Formulation of Nanocrystals-Based Gel
2.9. Evaluation of Nanocrystals-Based Gel
2.9.1. Drug Content
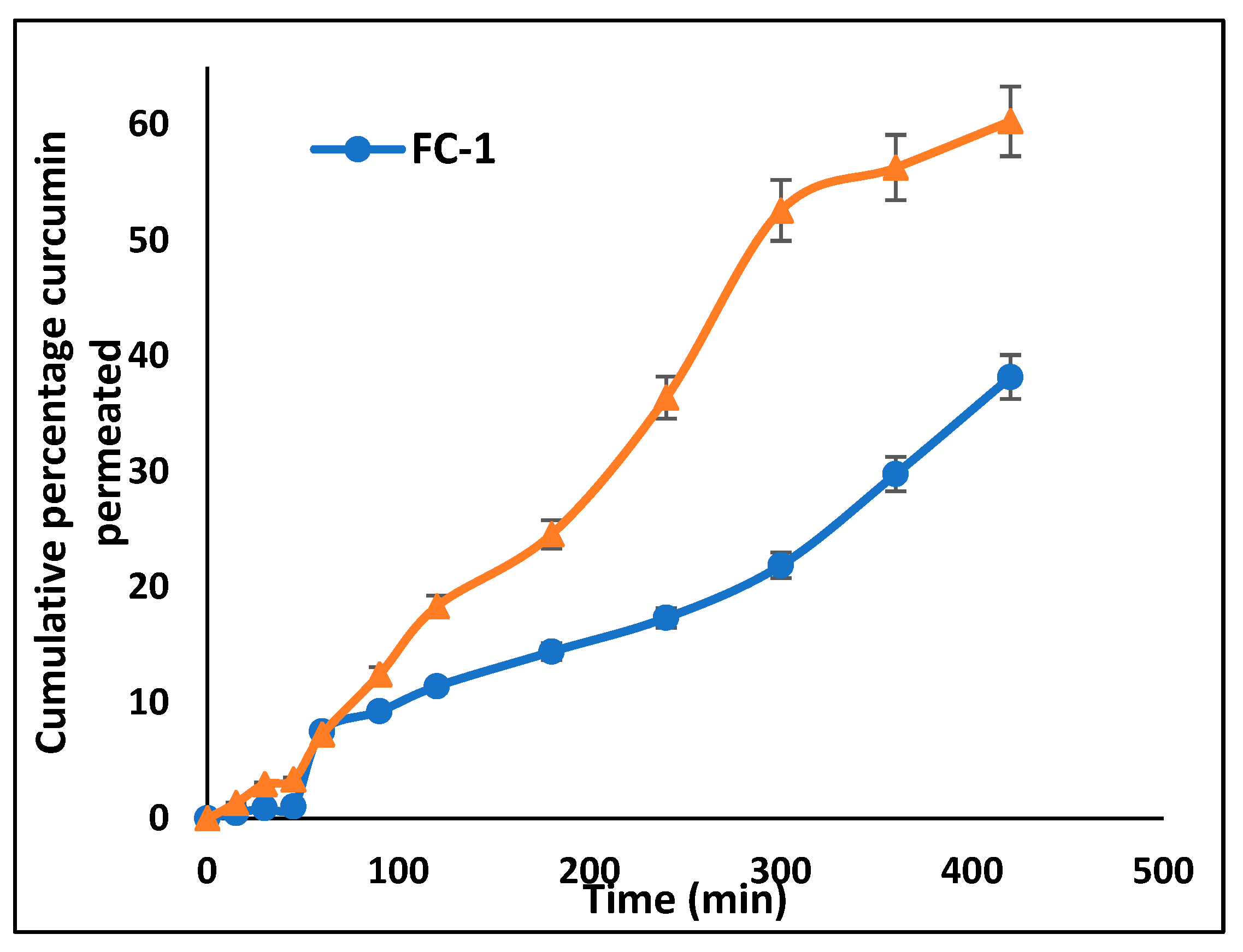
2.9.2. Ex Vivo Drug Permeation Study
2.9.3. In Vivo Skin Irritability Study
- Control (Untreated)
- Test 1 (FC-1 or unprocessed curcumin powder-containing gel, 1.0% w/w)
- Test 2 (FC-2b or optimized curcumin nanocrystal gel, 1.0% w/w) and
- Standard (Povidone Iodine Ointment USP, 5.0% w/w)
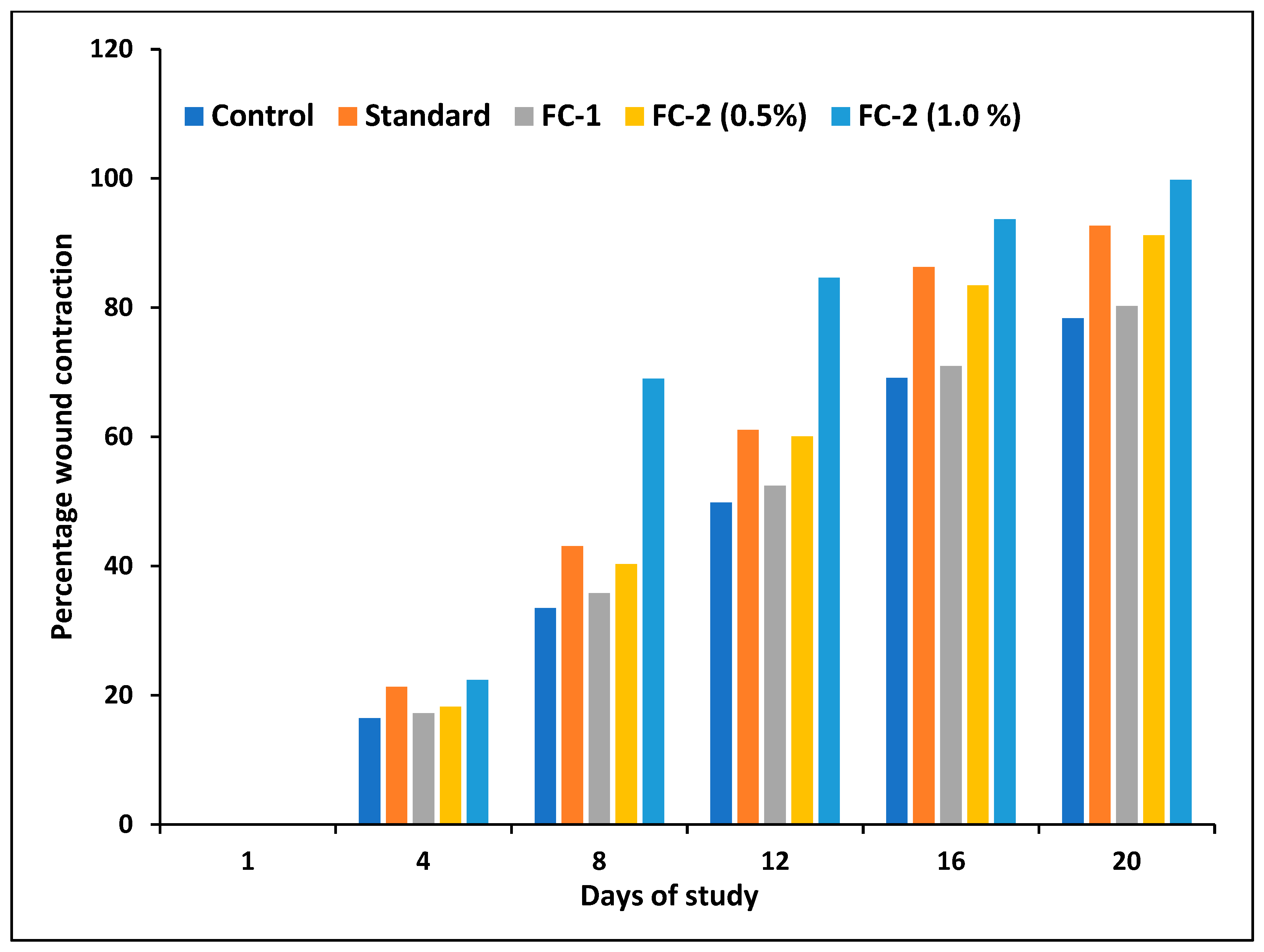
2.9.4. Excision Wound Healing Study
2.9.5. Statistical Analysis
3. Results and Discussion
3.1. Preparation of Curcumin Nanocrystals by Sonoprecipitation Method
Optimization of Curcumin Nanocrystals
- Effect of variables on particle size of nanocrystals
- Effect of variables on practical yield of nanocrystals
- Numerical optimization
3.2. Particle Size, Polydispersity Index (PDI), Zeta Potential
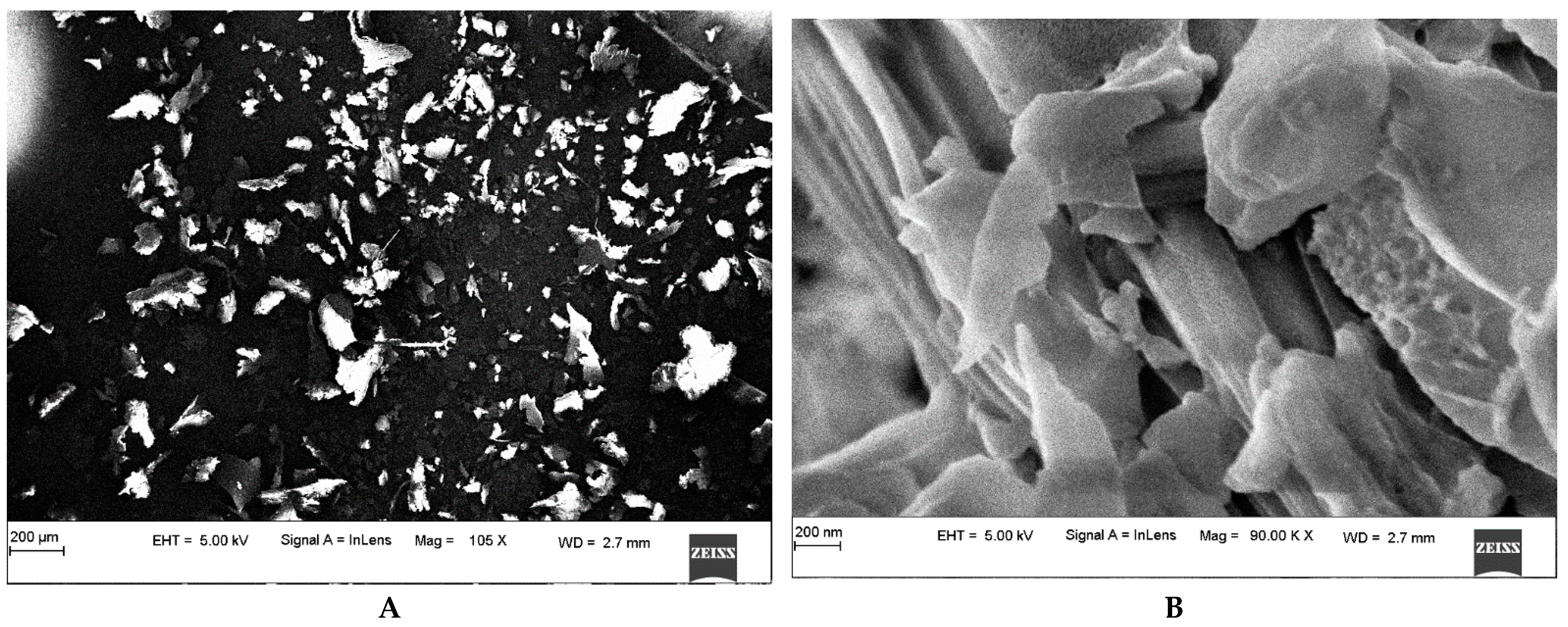
3.3. Morphology Determination
3.4. Powder X-ray Diffraction Studies (PXRD)
3.5. In Vitro Drug Release Studies
Kinetic Analysis of In Vitro Drug Release Studies
3.6. Evaluation of Nanocrystals Based Gel
3.6.1. Drug Content in Nanocrystal-Incorporated Gel
3.6.2. Ex Vivo Drug Permeation Study
3.6.3. In Vivo Skin Irritability Study
3.6.4. Excision Wound Healing Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boer, M.; Duchnik, E.; Maleszka, R.; Marchlewicz, M. Structural and Biophysical Characteristics of Human Skin in Maintaining Proper Epidermal Barrier Function. Postep. Dermatol. I Alergol. 2016, 33, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Akther, S.; Hannan, J.M.A.; Seidel, V.; Nujat, N.J.; Abdel-Wahab, Y.H.A. Pharmacologically Active Phytomolecules Isolated from Traditional Antidiabetic Plants and Their Therapeutic Role for the Management of Diabetes Mellitus. Molecules 2022, 27, 4278. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, P.; Khan, Z.; Chakraverty, N. Soluble Curcumin: A Promising Oral Supplement for Health Management. J. Appl. Pharm. Sci. 2011, 1, 1–7. [Google Scholar]
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a Wound Healing Agent. Life Sci. 2014, 116, 1–7. [Google Scholar] [CrossRef]
- Mohanty, C.; Sahoo, S.K. The in Vitro Stability and in Vivo Pharmacokinetics of Curcumin Prepared as an Aqueous Nanoparticulate Formulation. Biomaterials 2010, 31, 6597–6611. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.; Liversidge, G.G.; Cooper, E.R. Nanosizing: A Formulation Approach for Poorly-Water-Soluble Compounds. Eur. J. Pharm. Sci. 2003, 18, 113–120. [Google Scholar] [CrossRef]
- Fu, Q.; Sun, J.; Ai, X.; Zhang, P.; Li, M.; Wang, Y.; Liu, X.; Sun, Y.; Sui, X.; Sun, L.; et al. Nimodipine Nanocrystals for Oral Bioavailability Improvement: Role of Mesenteric Lymph Transport in the Oral Absorption. Int. J. Pharm. 2013, 448, 290–297. [Google Scholar] [CrossRef]
- Gigliobianco, M.R.; Casadidio, C.; Censi, R.; Di Martino, P. Nanocrystals of Poorly Soluble Drugs: Drug Bioavailability and Physicochemical Stability. Pharmaceutics 2018, 10, 134. [Google Scholar] [CrossRef] [Green Version]
- Kalia, Y.N.; Guy, R.H. Modeling Transdermal Drug Release. Adv. Drug Deliv. Rev. 2001, 48, 159–172. [Google Scholar] [CrossRef]
- Carneiro, R.d.S.; Canuto, M.R.; Ribeiro, L.K.; Ferreira, D.C.L.; Assunção, A.F.C.; Costa, C.A.C.B.; de Freitas, J.D.; Rai, M.; Cavalcante, L.S.; Alves, W.d.S.; et al. Novel Antibacterial Efficacy of ZnO Nanocrystals/Ag Nanoparticles Loaded with Extract of Ximenia Americana L. (Stem Bark) for Wound Healing. S. Afr. J. Bot. 2022, 151, 18–32. [Google Scholar] [CrossRef]
- Ouyang, X.K.; Zhao, L.; Jiang, F.; Ling, J.; Yang, L.Y.; Wang, N. Cellulose Nanocrystal/Calcium Alginate-Based Porous Microspheres for Rapid Hemostasis and Wound Healing. Carbohydr. Polym. 2022, 293, 119688. [Google Scholar] [CrossRef] [PubMed]
- Koseki, Y.; Ikuta, Y.; Taemaitree, F.; Saito, N.; Suzuki, R.; Dao, A.T.N.; Onodera, T.; Oikawa, H.; Kasai, H. Fabrication of Size-Controlled SN-38 Pure Drug Nanocrystals through an Ultrasound-Assisted Reprecipitation Method toward Efficient Drug Delivery for Cancer Treatment. J. Cryst. Growth 2021, 572, 126265. [Google Scholar] [CrossRef]
- Heng, M.C. Topical Curcumin: A Review of Mechanisms and Uses in Dermatology. Int. J. Dermatol. Clin. Res. 2017, 3, 010–017. [Google Scholar] [CrossRef] [Green Version]
- Parmar, P.K.; Wadhawan, J.; Bansal, A.K. Pharmaceutical Nanocrystals: A Promising Approach for Improved Topical Drug Delivery. Drug Discov. Today 2021, 26, 2329–2349. [Google Scholar] [CrossRef] [PubMed]
- Poojary, K.K.; Nayak, G.; Vasani, A.; Kumari, S.; Dcunha, R.; Kunhiraman, J.P.; Gopalan, D.; Rao, R.R.; Mutalik, S.; Kalthur, S.G.; et al. Curcumin Nanocrystals Attenuate Cyclophosphamide-Induced Testicular Toxicity in Mice. Toxicol. Appl. Pharmacol. 2021, 433, 115772. [Google Scholar] [CrossRef]
- Zong, R.; Ruan, H.; Zhu, W.; Zhang, P.; Feng, Z.; Liu, C.; Fan, S.; Liang, H.; Li, J. Curcumin Nanocrystals with Tunable Surface Zeta Potential: Preparation, Characterization and Antibacterial Study. J. Drug. Deliv. Sci. Technol. 2022, 76, 103771. [Google Scholar] [CrossRef]
- Finnegan, S.; Percival, S.L. Clinical and Antibiofilm Efficacy of Antimicrobial Hydrogels. Adv. Wound Care 2015, 4, 398. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.T.; Rodríguez-Hornedo, N.; Ciotti, S.; Ackermann, C. Rheological Characterization of Topical Carbomer Gels Neutralized to Different PH. Pharm. Res. 2004, 21, 1192–1199. [Google Scholar] [CrossRef] [Green Version]
- Shelake, S.S.; Patil, S.V.; Patil, S.S.; Sangave, P. Formulation and Evaluation of Fenofibrate-Loaded Nanoparticles by Precipitation Method. Indian J. Pharm. Sci. 2018, 80, 420–427. [Google Scholar] [CrossRef]
- De Waard, H.; Frijlink, H.W.; Hinrichs, W.L.J. Bottom-Up Preparation Techniques for Nanocrystals of Lipophilic Drugs. Pharm. Res. 2011, 28, 1220. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Matas, M.D.; Zhang, J.; Anwar, J. Nanocrystal Preparation: Low-Energy Precipitation Method Revisited. Crystal Growth and Design 2013, 13, 2766–2777. [Google Scholar] [CrossRef]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-Drying of Nanoparticles: Formulation, Process and Storage Considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Lademann, J.; Keck, C.M.; Müller, R.H. Nanocrystals of Medium Soluble Actives—Novel Concept for Improved Dermal Delivery and Production Strategy. Int. J. Pharm. 2014, 470, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Kaszuba, M.; Corbett, J.; Watson, F.M.N.; Jones, A. High-Concentration Zeta Potential Measurements Using Light-Scattering Techniques. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 4439. [Google Scholar] [CrossRef] [Green Version]
- Marković, Z.M.; Prekodravac, J.R.; Tošić, D.D.; Holclajtner-Antunović, I.D.; Milosavljević, M.S.; Dramićanin, M.D.; Todorović-Marković, B.M. Facile Synthesis of Water-Soluble Curcumin Nanocrystals. J. Serb. Chem. Soc. 2015, 80, 63–72. [Google Scholar] [CrossRef]
- Agrawal, R.; Sandhu, S.K.; Sharma, I.; Kaur, I.P. Development and Evaluation of Curcumin-Loaded Elastic Vesicles as an Effective Topical Anti-Inflammatory Formulation. AAPS PharmSciTech 2015, 16, 364. [Google Scholar] [CrossRef]
- Zhai, X.; Lademann, J.; Keck, C.M.; Müller, R.H. Dermal Nanocrystals from Medium Soluble Actives—Physical Stability and Stability Affecting Parameters. Eur. J. Pharm. Biopharm. 2014, 88, 85–91. [Google Scholar] [CrossRef]
- Zamarioli, C.M.; Martins, R.M.; Carvalho, E.C.; Freitas, L.A.P. Nanoparticles Containing Curcuminoids (Curcuma longa): Development of Topical Delivery Formulation. Rev. Bras. Farmacogn. 2015, 25, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Mutalik, S.; Suthar, N.A.; Managuli, R.S.; Shetty, P.K.; Avadhani, K.; Kalthur, G.; Kulkarni, R.V.; Thomas, R. Development and Performance Evaluation of Novel Nanoparticles of a Grafted Copolymer Loaded with Curcumin. Int. J. Biol. Macromol. 2016, 86, 709–720. [Google Scholar] [CrossRef] [Green Version]
- Gong, C.Y.; Wu, Q.J.; Wang, Y.J.; Zhang, D.D.; Luo, F.; Zhao, X.; Wei, Y.Q.; Qian, Z.Y. A Biodegradable Hydrogel System Containing Curcumin Encapsulated in Micelles for Cutaneous Wound Healing. Biomaterials 2013, 34, 6377–6387. [Google Scholar] [CrossRef]
- Patel, N.A.; Patel, N.J.; Patel, R.P. Formulation and Evaluation of Curcumin Gel for Topical Application. Pharm. Dev. Technol. 2009, 14, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Estevão, L.R.d.M.; Cassini-Vieira, P.; Leite, A.G.B.; Bulhões, A.A.V.d.C.; Barcelos, L.d.S.; Evêncio-Neto, J. Morphological Evaluation of Wound Healing Events in the Excisional Wound Healing Model in Rats. Bio-Protoc. 2019, 9, e3285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, P.K.; Singh, A.; Gaurav, K.; Goel, S.; Khanna, H.D.; Goel, R.K. Evaluation of Wound Healing Activity of Extracts of Plantain Banana (Musa Sapientum Var. Paradisiaca) in Rats. Available online: https://pubmed.ncbi.nlm.nih.gov/19317349/ (accessed on 16 September 2022).
- Jain, S.; Patel, K.; Arora, S.; Reddy, V.A.; Dora, C.P. Formulation, Optimization, and in Vitro-in Vivo Evaluation of Olmesartan Medoxomil Nanocrystals. Drug Deliv. Transl. Res. 2017, 7, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, A.; Gigliobianco, M.R.; Pellitteri, R.; Santonocito, D.; Carbone, C.; Di Martino, P.; Puglisi, G.; Musumeci, T. Optimization of Curcumin Nanocrystals as Promising Strategy for Nose-to-Brain Delivery Application. Pharmaceutics 2020, 12, 476. [Google Scholar] [CrossRef] [PubMed]
- El-Batal, A.I.; Elmenshawi, S.F.; Abdelhaleem Ali, A.M.; Eldbaiky, E.G. Preparation and Characterization of Silymarin Nanocrystals and Phytosomes with Investigation of Their Stability Using Gamma Irradiation. Indian J. Pharm. Educ. Res. 2018, 52, S174–S183. [Google Scholar] [CrossRef] [Green Version]
- Pawar, Y.B.; Purohit, H.; Valicherla, G.R.; Munjal, B.; Lale, S.V.; Patel, S.B.; Bansal, A.K. Novel Lipid Based Oral Formulation of Curcumin: Development and Optimization by Design of Experiments Approach. Int. J. Pharm. 2012, 436, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.; Zhou, W.; Huang, G.; Lei, F.; Chen, Y.; Ma, Z.; Chen, L.; Yang, M. Nanocrystals Based Pulmonary Inhalation Delivery System: Advance and Challenge. Drug Deliv. 2022, 29, 637. [Google Scholar] [CrossRef]
- Homayouni, A.; Amini, M.; Sohrabi, M.; Varshosaz, J.; Nokhodchi, A. Curcumin Nanoparticles Containing Poloxamer or Soluplus Tailored by High Pressure Homogenization Using Antisolvent Crystallization. Int. J. Pharm. 2019, 562, 124–134. [Google Scholar] [CrossRef]
- Yadav, D.; Kumar, N. Nanonization of Curcumin by Antisolvent Precipitation: Process Development, Characterization, Freeze Drying and Stability Performance. Int. J. Pharm. 2014, 477, 564–577. [Google Scholar] [CrossRef]
- Homayouni, A.; Sohrabi, M.; Amini, M.; Varshosaz, J.; Nokhodchi, A. Effect of High Pressure Homogenization on Physicochemical Properties of Curcumin Nanoparticles Prepared by Antisolvent Crystallization Using HPMC or PVP. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Londoño-Restrepo, S.M.; Jeronimo-Cruz, R.; Millán-Malo, B.M.; Rivera-Muñoz, E.M.; Rodriguez-García, M.E. Effect of the Nano Crystal Size on the X-Ray Diffraction Patterns of Biogenic Hydroxyapatite from Human, Bovine, and Porcine Bones. Sci. Rep. 2019, 9, 5915. [Google Scholar] [CrossRef]
- Chu, K.R.; Lee, E.; Jeong, S.H.; Park, E.S. Effect of Particle Size on the Dissolution Behaviors of Poorly Water-Soluble Drugs. Arch. Pharm. Res. 2012, 35, 1187–1195. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Vazifehasl, Z.; Salatin, S.; Adibkia, K.; Javadzadeh, Y. Nanosizing of Drugs: Effect on Dissolution Rate. Res. Pharm. Sci. 2015, 10, 95. [Google Scholar] [PubMed]
- Shi, Z.; Pan, S.; Wang, L.; Li, S. Topical Gel Based Nanoparticles for the Controlled Release of Oleanolic Acid: Design and in Vivo Characterization of a Cubic Liquid Crystalline Anti-Inflammatory Drug. BMC Complement. Med. Ther. 2021, 21, 224. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Shanthi, N.; Mahato, A.K.; Soni, S.; Rajnikanth, P.S. Preparation of Luliconazole Nanocrystals Loaded Hydrogel for Improvement of Dissolution and Antifungal Activity. Heliyon 2019, 5, e01688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pyo, S.M.; Hespeler, D.; Keck, C.M.; Müller, R.H. Dermal Miconazole Nitrate Nanocrystals—Formulation Development, Increased Antifungal Efficacy & Skin Penetration. Int. J. Pharm. 2017, 531, 350–359. [Google Scholar] [CrossRef]
- Pelikh, O.; Keck, C.M. Hair Follicle Targeting and Dermal Drug Delivery with Curcumin Drug Nanocrystals—Essential Influence of Excipients. Nanomaterials 2020, 10, 2323. [Google Scholar] [CrossRef]
- Tirunagari, M.; Jangala, V.R.; Nandagopal, A. Development and Physicochemical, In Vitro and In Vivo Evaluation of Transdermal Patches of Zaleplon. Indian J. Pharm. Educ. Res. 2013, 47, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Sidhu, G.S.; Singh, A.K.; Thaloor, D.; Banaudha, K.K.; Patnaik, G.K.; Srimal, R.C.; Maheshwari, R.K. Enhancement of Wound Healing by Curcumin in Animals. Wound Repair Regen. 1998, 6, 167–177. [Google Scholar] [CrossRef]
- Mehrabani, D.; Farjam, M.; Geramizadeh, B.; Tanideh, N.; Amini, M.; Panjehshahin, M.R. The Healing Effect of Curcumin on Burn Wounds in Rat. World J. Plast. Surg. 2015, 4, 29. [Google Scholar]
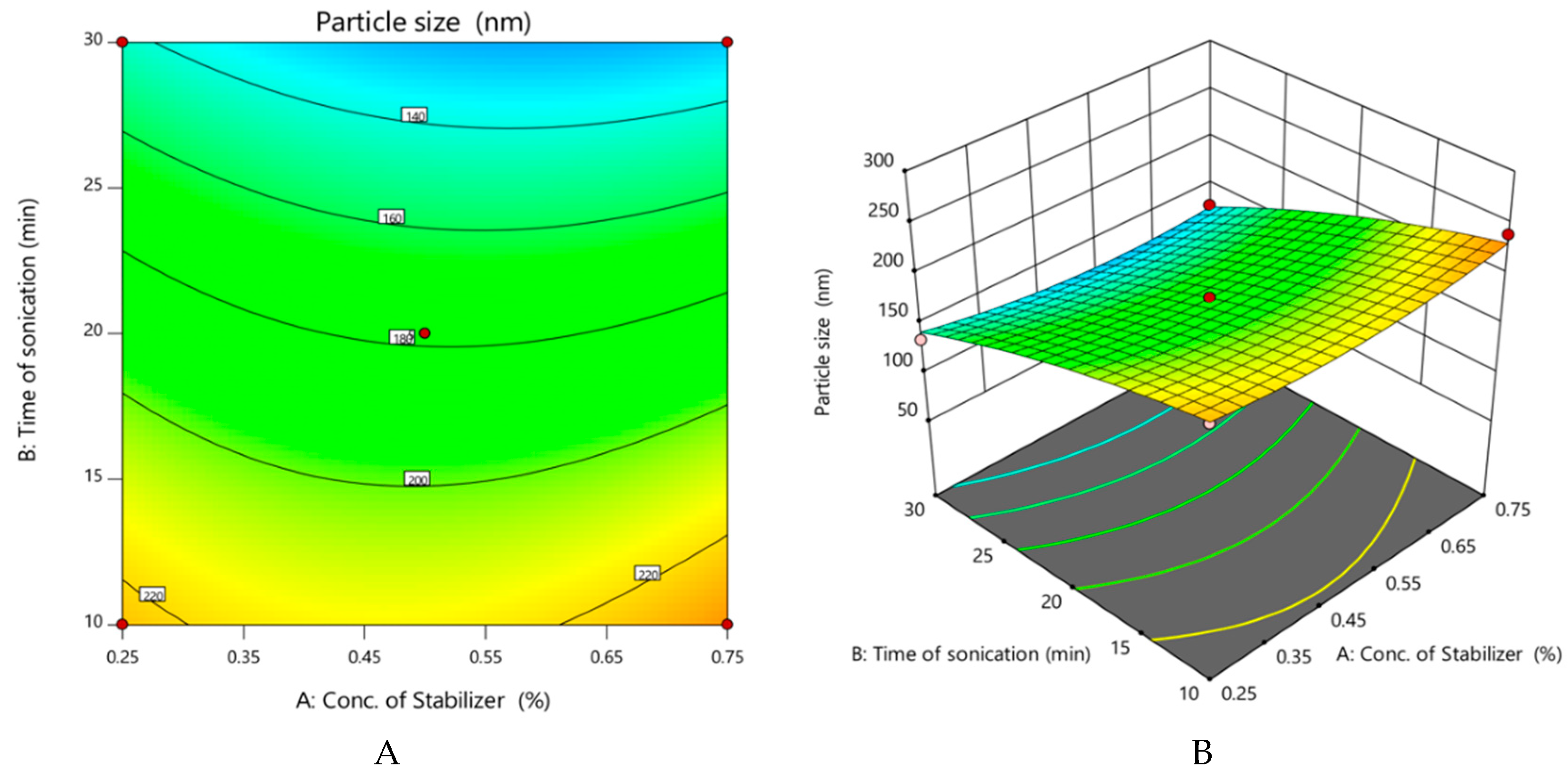
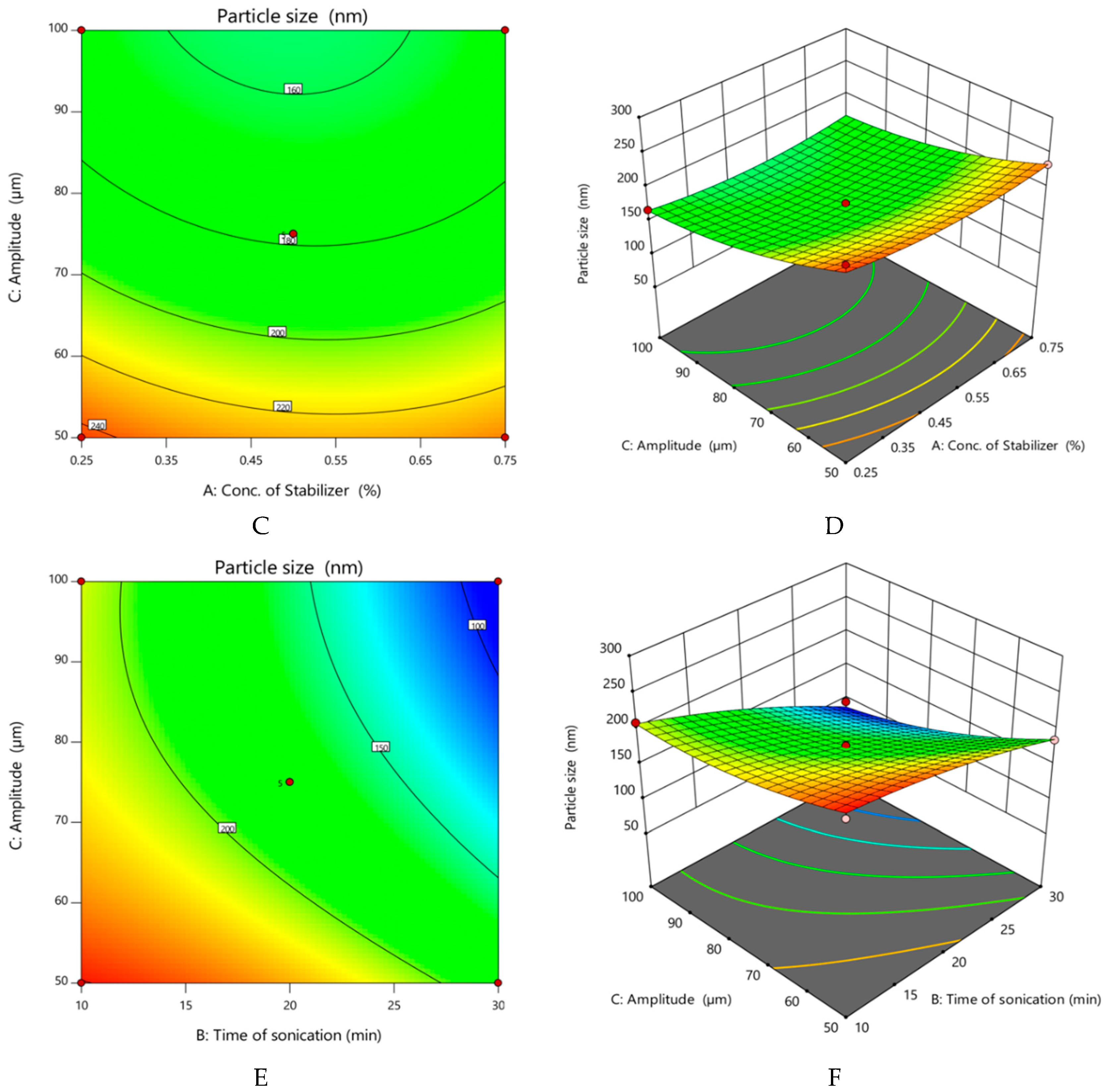
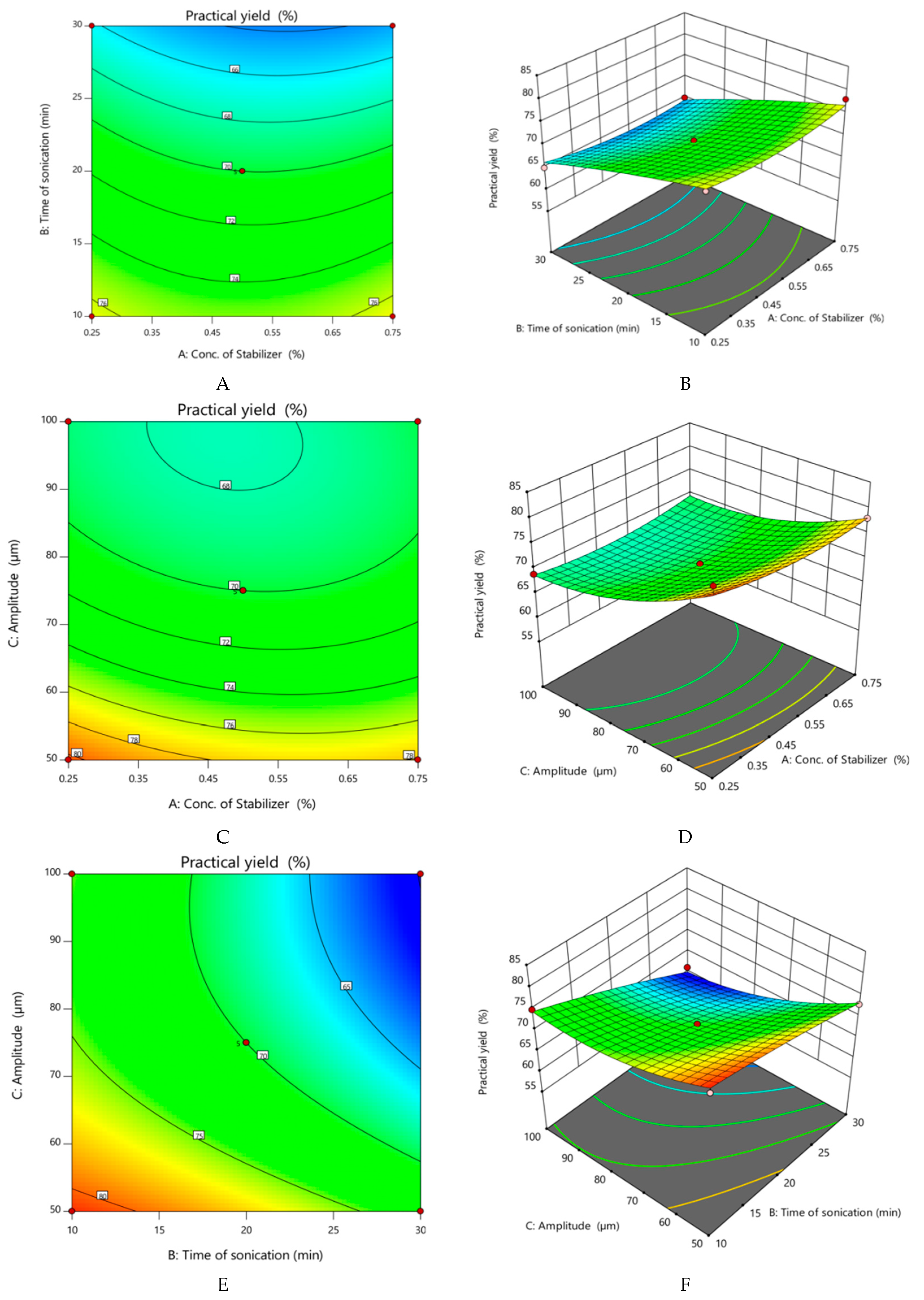




| Run | Factor 1 A: Conc. of Stabilizer (%) | Factor 2 B: Time of Sonication (min) | Factor 3 C: Amplitude (µm) | Response 1 Particle Size (nm) | Response 2 Practical Yield (%) |
|---|---|---|---|---|---|
| 1 | 0.75 | 20 | 100 | 159 | 68 |
| 2 | 0.5 | 20 | 75 | 178 | 70 |
| 3 | 0.75 | 30 | 75 | 128 | 65 |
| 4 | 0.5 | 20 | 75 | 178 | 70 |
| 5 | 0.25 | 20 | 100 | 168 | 69 |
| 6 | 0.5 | 20 | 75 | 178 | 70 |
| 7 | 0.75 | 10 | 75 | 240 | 78 |
| 8 | 0.5 | 20 | 75 | 178 | 70 |
| 9 | 0.25 | 10 | 75 | 222 | 76 |
| 10 | 0.5 | 10 | 100 | 210 | 75 |
| 11 | 0.75 | 20 | 50 | 234 | 78 |
| 12 | 0.25 | 30 | 75 | 135 | 65 |
| 13 | 0.25 | 20 | 50 | 254 | 82 |
| 14 | 0.5 | 30 | 100 | 94 | 61 |
| 15 | 0.5 | 10 | 50 | 243 | 80 |
| 16 | 0.5 | 30 | 50 | 186 | 73 |
| 17 | 0.5 | 20 | 75 | 178 | 70 |
| Factor | Particle Size | Practical Yield | ||||
|---|---|---|---|---|---|---|
| F-Value | p-Value | Coefficient Estimate | F-Value | p-Value | Coefficient Estimate | |
| Model | 52.82 | <0.0001 | 178.00 (Intercept) | 33.98 | <0.0001 | 70.00 (Intercept) |
| A—Conc. of stabilizer (%) | 0.6349 | 0.4517 | −2.250 | 0.6702 | 0.4400 | −0.375 |
| B—Time of sonication (min) | 271.19 | <0.0001 | −46.50 | 150.80 | <0.0001 | −5.620 |
| C—Amplitude (µm) | 160.29 | <0.0001 | −35.75 | 119.15 | <0.0001 | −5.000 |
| AB | 2.45 | 0.1615 | −6.250 | 0.5957 | 0.4655 | −0.500 |
| AC | 0.4742 | 0.5132 | 2.750 | 1.34 | 0.2849 | 0.7500 |
| BC | 13.64 | 0.0077 | −14.750 | 7.30 | 0.0306 | −1.750 |
| A2 | 9.31 | 0.0186 | 11.870 | 5.64 | 0.0492 | 1.500 |
| B2 | 4.91 | 0.0622 | −8.630 | 0.6271 | 0.4544 | −0.500 |
| C2 | 12.71 | 0.0092 | 13.880 | 18.97 | 0.0033 | 2.750 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotian, V.; Koland, M.; Mutalik, S. Nanocrystal-Based Topical Gels for Improving Wound Healing Efficacy of Curcumin. Crystals 2022, 12, 1565. https://doi.org/10.3390/cryst12111565
Kotian V, Koland M, Mutalik S. Nanocrystal-Based Topical Gels for Improving Wound Healing Efficacy of Curcumin. Crystals. 2022; 12(11):1565. https://doi.org/10.3390/cryst12111565
Chicago/Turabian StyleKotian, Vinith, Marina Koland, and Srinivas Mutalik. 2022. "Nanocrystal-Based Topical Gels for Improving Wound Healing Efficacy of Curcumin" Crystals 12, no. 11: 1565. https://doi.org/10.3390/cryst12111565








