Schiff Base Derivatives in Zinc(II) and Cadmium(II) Complexation with the closo-Dodecaborate Anion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Organic Ligands L1–L3
2.2.1. N-(4-Methoxyphenyl)-1-(1-methylbenzimidazol-2-yl)methanimine (L1)
2.2.2. 4-Methoxy-N-[(1-methylbenzimidazol-2-yl)methyl]aniline (L2)
2.2.3. 2-[(E)-(1-Propylbenzimidazol-2-yl)iminomethyl]phenol (L3)
2.3. Synthesis of Zinc(II) and Cadmium(II) Complexes
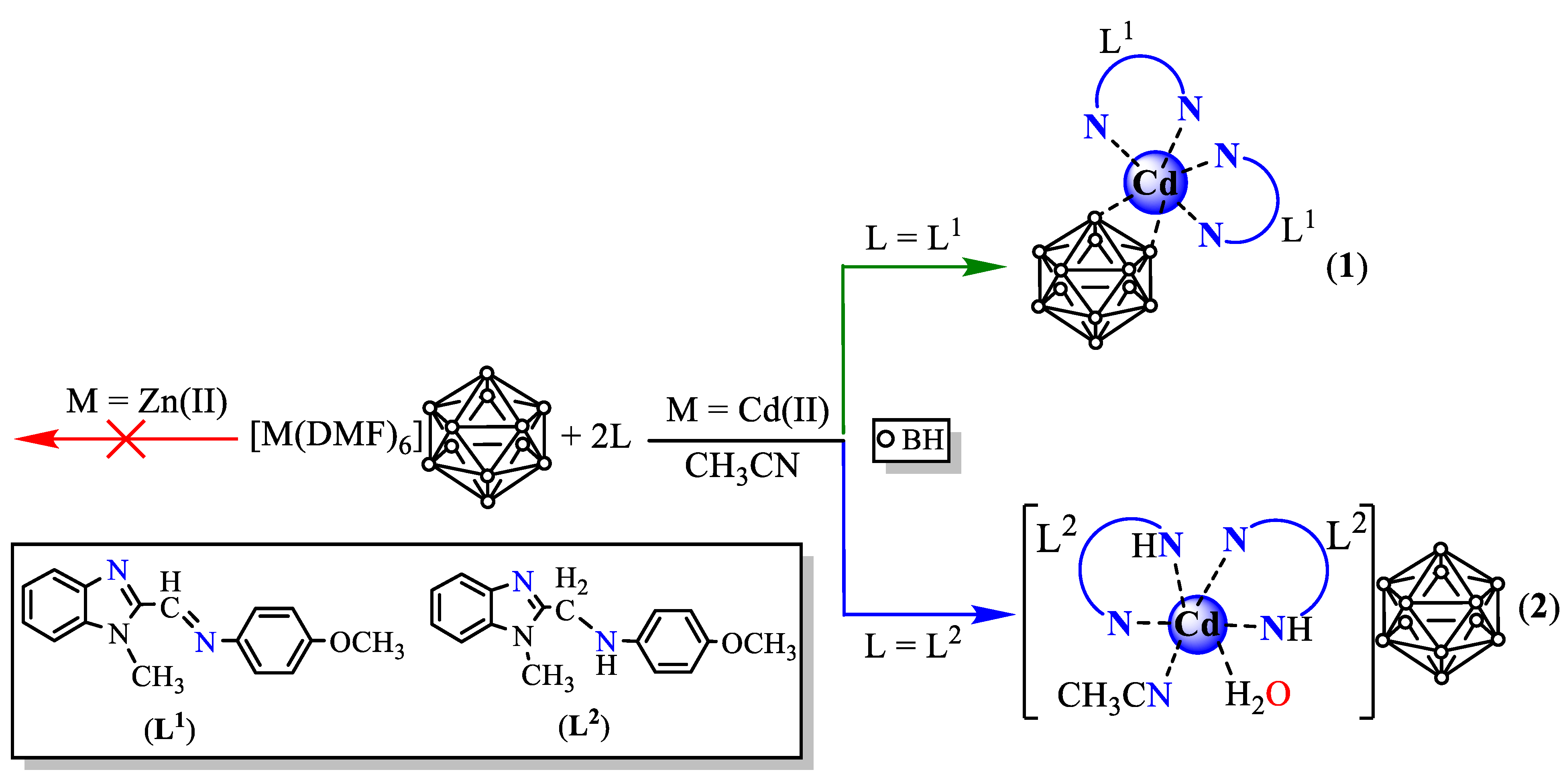
2.3.1. [Cd(L1)2[B12H12]] (Compound 1)
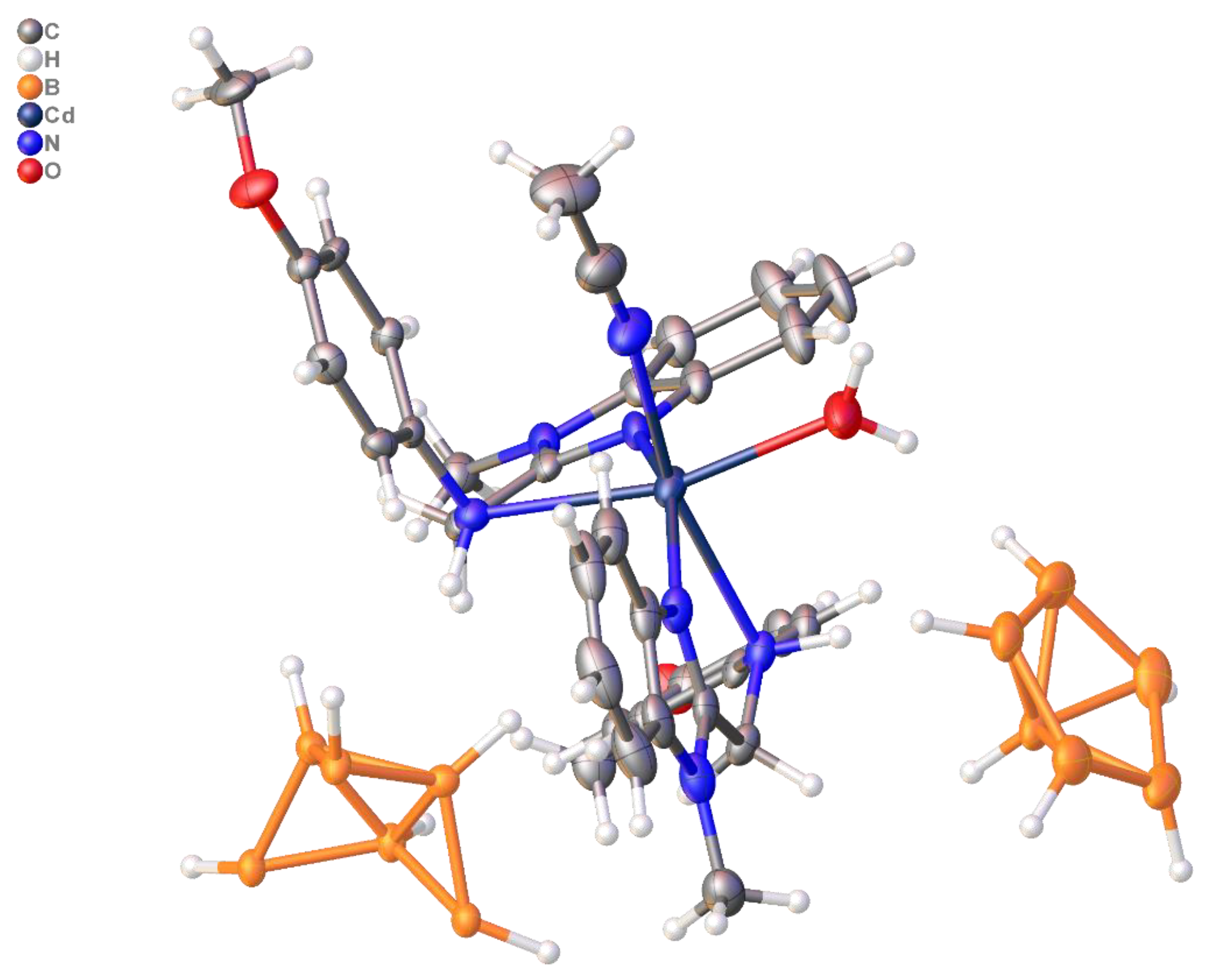
2.3.2. [Cd(L2)2(CH3CN)(H2O)][B12H12] (Compound 2)
2.3.3. [Zn(Bz-NH2)2(CH3COO)2] (Compound 3)
2.3.4. (HBz-NH2)2[B12H12]∙2CH3CN (Compound 4∙2CH3CN) + Cadmium(II) Complex with Salicylaldehyde
2.4. Elemental Analysis
2.5. IR Spectroscopy
2.6. UV Spectroscopy
2.7. 1H and 13C NMR Spectroscopy
2.8. X-ray Diffraction
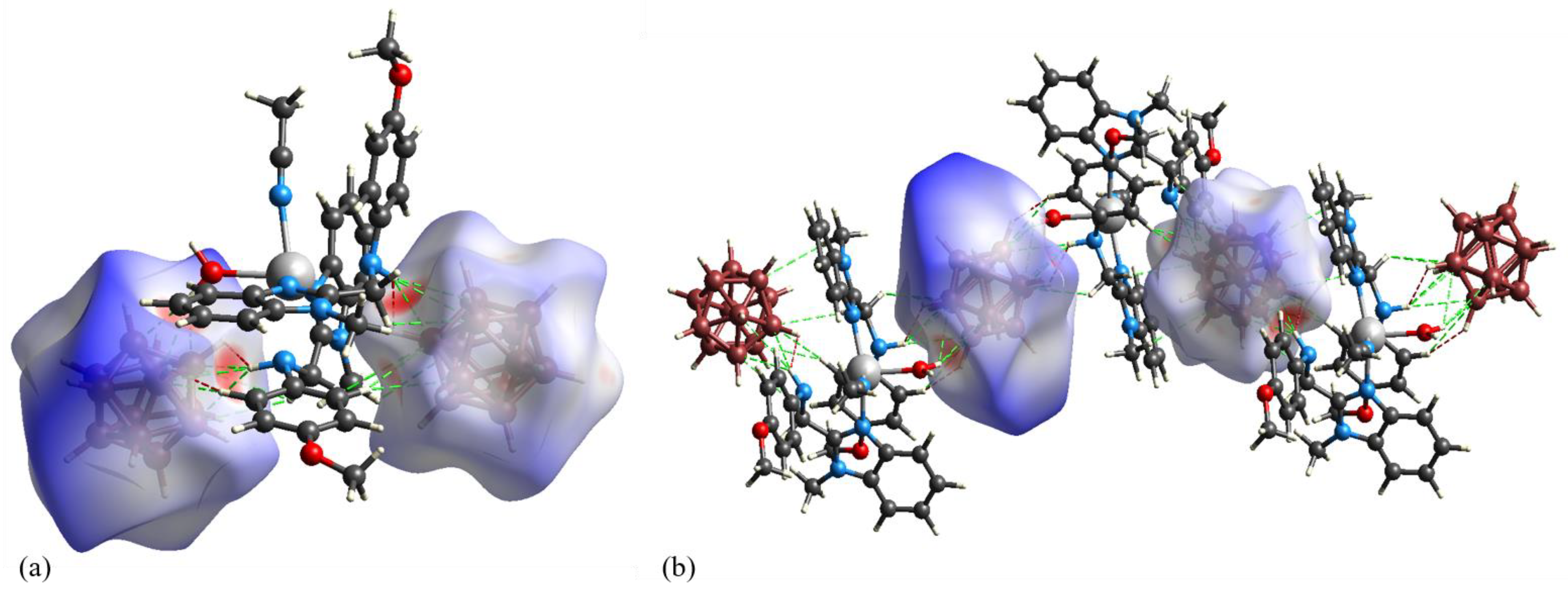
2.9. Hirshfeld Surface Analysis
3. Results and Discussion
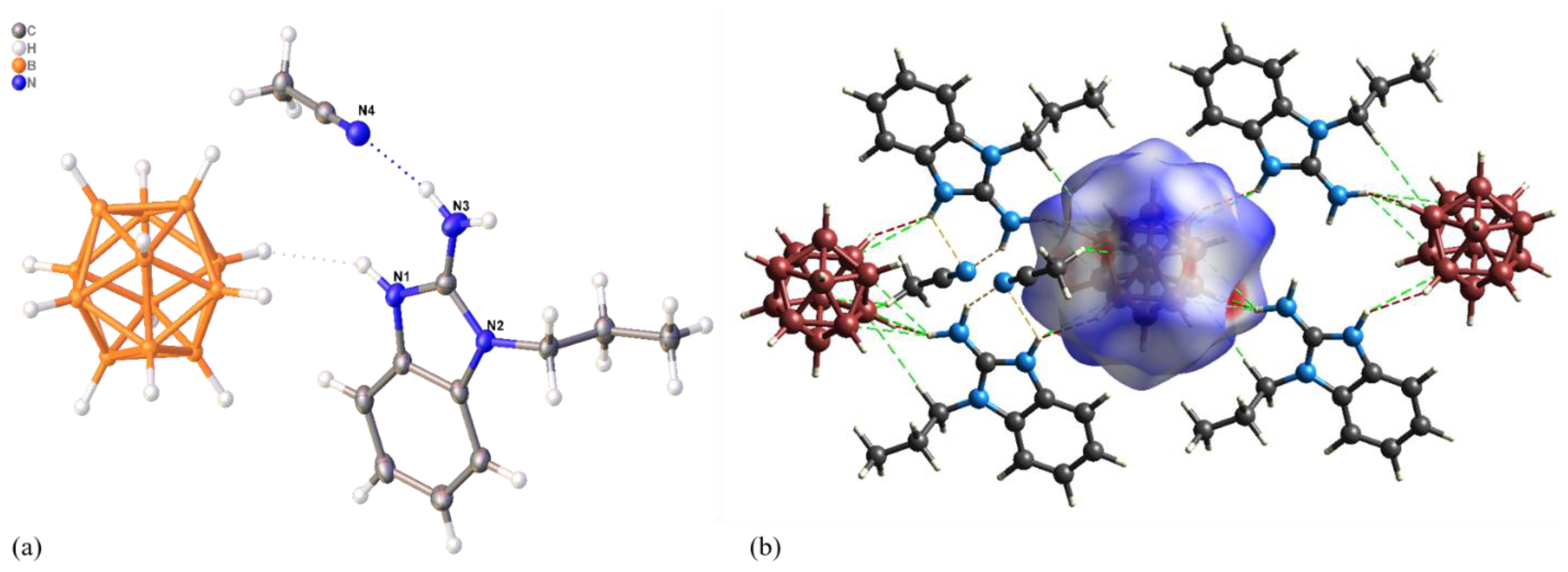
3.1. System [M(DMF)6][B12H12] (M = Zn(II), Cd(II))/L (L = L1, L2)
3.2. System [M(DMF)6][B12H12] (M = Zn(II), Cd(II))/L3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chkirate, K.; Essassi, E.M. Pyrazole and Benzimidazole Derivatives: Chelating Properties Towards Metals Ions and their Applications. Curr. Org. Chem. 2022, 26, 1735–1766. [Google Scholar] [CrossRef]
- Al Awadh, A.A. Biomedical applications of selective metal complexes of indole, benzimidazole, benzothiazole and benzoxazole: A review (From 2015 to 2022). Saudi Pharm. J. 2023, 31, 101698. [Google Scholar] [CrossRef] [PubMed]
- Ugwu, D.I.; Conradie, J. Anticancer properties of complexes derived from bidentate ligands. J. Inorg. Biochem. 2023, 246, 112268. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Moreno, G.V.; Hernández-Romero, D.; García-Barradas, Ó.; Vázquez-Vera, Ó.; Rosete-Luna, S.; Cruz-Cruz, C.A.; López-Monteon, A.; Carrillo-Ahumada, J.; David Morales-Morales, D.; Colorado-Peralta, R. Second and third-row transition metal compounds containing benzimidazole ligands: An overview of their anticancer and antitumour activity. Coord. Chem. Rev. 2022, 472, 214790. [Google Scholar] [CrossRef]
- Mahmood, K.; Hashmi, W.; Ismail, H.; Mirza, B.; Twamley, B.; Akhter, Z.; Rozas, I.; Baker, R.J. Synthesis, DNA binding and antibacterial activity of metal(II) complexes of a benzimidazole Schiff base. Polyhedron 2019, 157, 326–334. [Google Scholar] [CrossRef]
- Šindelář, Z.; Kopel, P. Bis(benzimidazole) Complexes, Synthesis and Their Biological Properties: A Perspective. Inorganics 2023, 11, 113. [Google Scholar] [CrossRef]
- Darwish, N.B.; Kurdi, A.; Alshihri, S.; Tabbakh, T. Organic heterocyclic-based colorimetric and fluorimetric chemosensors for the detection of different analytes: A review (from 2015 to 2022). Mater. Today Chem. 2023, 27, 101347. [Google Scholar] [CrossRef]
- Jindal, G.; Vashisht, P.; Kaur, N. Benzimidazole appended optical sensors for ionic species: Compilation of literature reports from 2017 to 2022. Results Chem. 2022, 4, 100551. [Google Scholar] [CrossRef]
- Cruz-Navarro, A.; Hernández-Romero, D.; Flores-Parra, A.; Rivera, J.M.; Castillo-Blum, S.E.; Colorado-Peralta, R. Structural diversity and luminescent properties of coordination complexes obtained from trivalent lanthanide ions with the ligands: Tris ((1H-benzo[d]imidazol-2-yl)methyl)amine and 2,6-bis(1H-benzo[d]imidazol-2-yl)pyridine. Coord. Chem. Rev. 2021, 427, 213587. [Google Scholar] [CrossRef]
- Nair, P.; Jayaraj, A.; Swamy, C.A. Recent Advances in Benzimidazole Based NHC-Metal Complex Catalysed Cross-Coupling Reactions. ChemistrySelect 2022, 7, e202103517. [Google Scholar] [CrossRef]
- Coghi, P.; Hosmane, N.S.; Zhu, Y. Next generation of boron neutron capture therapy (BNCT) agents for cancer treatment. Med. Res. Rev. 2023, 43, 1809–1830. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Yang, M.; Guo, Y.; Ma, Y.; Guo, M.; Xiao, Y.; Han, G. Synergistic effects of caesium closo-dodecaborate on buried interface for efficient and stable perovskite solar cells. J. Colloid Interface Sci. 2023, 645, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, Z.; Zhang, M.; Zheng, S.; Zhang, W.; Li, S.; Pan, F. Closo-[B12H12]2− Derivatives with Polar Groups As Promising Building Blocks in Metal-Organic Frameworks for Gas Separation. ChemSusChem. 2023, 16, e202300434. [Google Scholar] [CrossRef]
- Corona-López, M.M.; Muñoz-Flores, B.M.; Chaari, M.; Nuñez, R.; Jiménez-Pérez, V.M. Far-Red and Near-Infrared Boron Schiff Bases (BOSCHIBAs) Dyes Bearing Anionic Boron Clusters. Eur. J. Inorg. Chem 2021, 2021, 2047–2054. [Google Scholar] [CrossRef]
- Sun, W.; Jin, Y.; Wu, Y.; Lou, W.; Yuan, Y.; Duttwyler, S.; Wang, L.; Zhang, Y. A new boron cluster anion pillared metal organic framework with ligand inclusion and its selective acetylene capture properties. Inorg. Chem. Front. 2022, 9, 5140–5147. [Google Scholar] [CrossRef]
- Hattori, Y.; Ishimura, M.; Ohta, Y.; Takenaka, H.; Kawabata, S.; Kirihata, M. Dodecaborate Conjugates Targeting Tumor Cell Overexpressing Translocator Protein for Boron Neutron Capture Therapy. ACS Med. Chem. Lett. 2022, 13, 50–54. [Google Scholar] [CrossRef]
- Hagemann, H. Boron Hydrogen Compounds: Hydrogen Storage and Battery Applications. Molecules 2021, 26, 7425. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Z.; Wang, B.; Zhang, J. Boron based hypergolic ionic liquids: A review. Green Energy Environ. 2021, 6, 794–822. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Z.; Chen, H.; Wang, Z.; Zhou, X.; Zhang, H. Progress in three-dimensional aromatic-like closo-dodecaborate. Coord. Chem. Rev. 2021, 444, 214042. [Google Scholar] [CrossRef]
- Tiritiris, I.; Schleid, T. Zu den Kristallstrukturen der Übergangsmetall(II)-Dodekahydro-closo-Dodekaborat-Hydrate Cu(H2O)5,5[B12H12]·2,5 H2O und Zn(H2O)6[B12H12]·6 H2O. Z. Anorg. Allg. Chem. 2008, 634, 317–324. [Google Scholar] [CrossRef]
- Tiritiris, I.; Schield, T. Synthese und Kristallstruktur von Cadmium-Dodekahydro-closo-Dodekaborat-Hexahydrat, Cd(H2O)6[B12H12]. Z. Anorg. Allg. Chem. 2005, 631, 1593–1596. [Google Scholar] [CrossRef]
- Lacroix, M.R.; Bukovsky, E.V.; Lozinsek, M.; Folsom, T.C.; Newell, B.S.; Liu, Y.; Peryshkov, D.V.; Strauss, S.H. Manifestations of Weak O−H···F Hydrogen Bonding in M(H2O)n(B12F12) Salt Hydrates: Unusually Sharp Fourier Transform Infrared ν(OH) Bands and Latent Porosity (M = Mg−Ba, Co, Ni, Zn). Inorg. Chem. 2018, 57, 14983–15000. [Google Scholar] [CrossRef] [PubMed]
- Korolenko, S.E.; Kubasov, A.S.; Goeva, L.V.; Avdeeva, V.V.; Malinina, E.A.; Kuznetsov, N.T. Reactivity of the dodecahydro-closo-dodecaborate anion in zinc(II) and cadmium(II) complexation at the presence of azaheterocyclic ligands. Inorg. Chim. Acta 2021, 527, 120587. [Google Scholar] [CrossRef]
- Groutchik, K.; Jaiswal, K.; Dobrovetsky, R. An air-stable, Zn2+-based catalyst for hydrosilylation of alkenes and alkynes. Org. Biomol. Chem. 2021, 19, 5544–5550. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Li, Z.; Jiao, N.; Liu, L.; Zhang, S. B12H122−-Based Metal (Cu2+, Ni2+, Zn2+) Complexes as Hypergolic Fuels with Superior Hypergolicity. Eur. J. Inorg. Chem. 2018, 2018, 981–986. [Google Scholar] [CrossRef]
- Wehmschulte, R.J.; Bayliss, B.; Reed, S.; Wesenberg, C.; Morgante, P.; Peverati, R.; Neal, S.; Chouinard, C.D.; Tolosa, D.; Powell, D.R. Zinc Ammonio-dodecaborates: Synthesis, Lewis Acid Strength, and Reactivity. Inorg. Chem. 2022, 61, 7032–7042. [Google Scholar] [CrossRef]
- Korolenko, S.E.; Kubasov, A.S.; Khan, N.A.; Goeva, L.V.; Burlov, A.S.; Divaeva, L.N.; Malinina, E.A.; Avdeeva, V.V.; Zhizhin, K.Y.; Kuznetsov, N.T. Boron Cluster Anion [B12H12]2− in Zinc(II) and Cadmium(II) Complexation at the Presence of N-Donor Heterocyclic Ligands. J. Clust. Sci. 2023, 34, 933–942. [Google Scholar] [CrossRef]
- Bruker. SAINT; Bruker AXS Inc.: Madison, WI, USA, 2018. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of Silver and Molybdenum Microfocus X-Ray Sources for Single-Crystal Structure Determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer 17.5; University of Western Australia: Perth, Australia, 2017. [Google Scholar]
- Avdeeva, V.V.; Vologzhanina, A.V.; Malinina, E.A.; Kuznetsov, N.T. Dihydrogen Bonds in Salts of Boron Cluster Anions [BnHn]2− with Protonated Heterocyclic Organic Bases. Crystals 2019, 9, 330. [Google Scholar] [CrossRef]
- Malinina, E.A.; Kubasov, A.S.; Matveev, E.Y.; Nichugovskii, A.I.; Nikiforova, S.E.; Goeva, L.V.; Avdeeva, V.V.; Zhizhin, K.Y.; Kuznetsov, N.T. Hydroxy-substituted closo-dodecaborate anion [B12H11OH]2– in silver(I) and lead(II) complexation in the presence of azaheterocyclic ligands L. Polyhedron 2023, 242, 116516. [Google Scholar] [CrossRef]
- Cao, W.; Zheng, X.-J.; Sun, J.-P.; Wong, W.-T.; Fang, D.-C.; Zhang, J.-X.; Jin, L.-P. A Highly Selective Chemosensor for Al(III) and Zn(II) and Its Coordination with Metal Ions. Inorg. Chem. 2014, 53, 3012–3021. [Google Scholar] [CrossRef] [PubMed]
- Murali, M.; Nayak, S.; Costa, J.S.; Ribas, J.; Mutikainen, I.; Turpeinen, U.; Clémancey, M.; Garcia-Serres, R.; Latour, J.-M.; Gamez, P.; et al. Discrete Tetrairon(III) Cluster Exhibiting a Square-Planar Fe4(μ4-O) Core: Structural and Magnetic Properties. Inorg. Chem. 2010, 49, 2427–2434. [Google Scholar] [CrossRef]
- Booysen, I.N.; Ismail, M.B.; Munro, O.Q. Mono- and dinuclear rhenium(I) compounds with bidentate benzimidazole chelators. Inorg. Chem. Commun. 2013, 30, 168–172. [Google Scholar] [CrossRef]

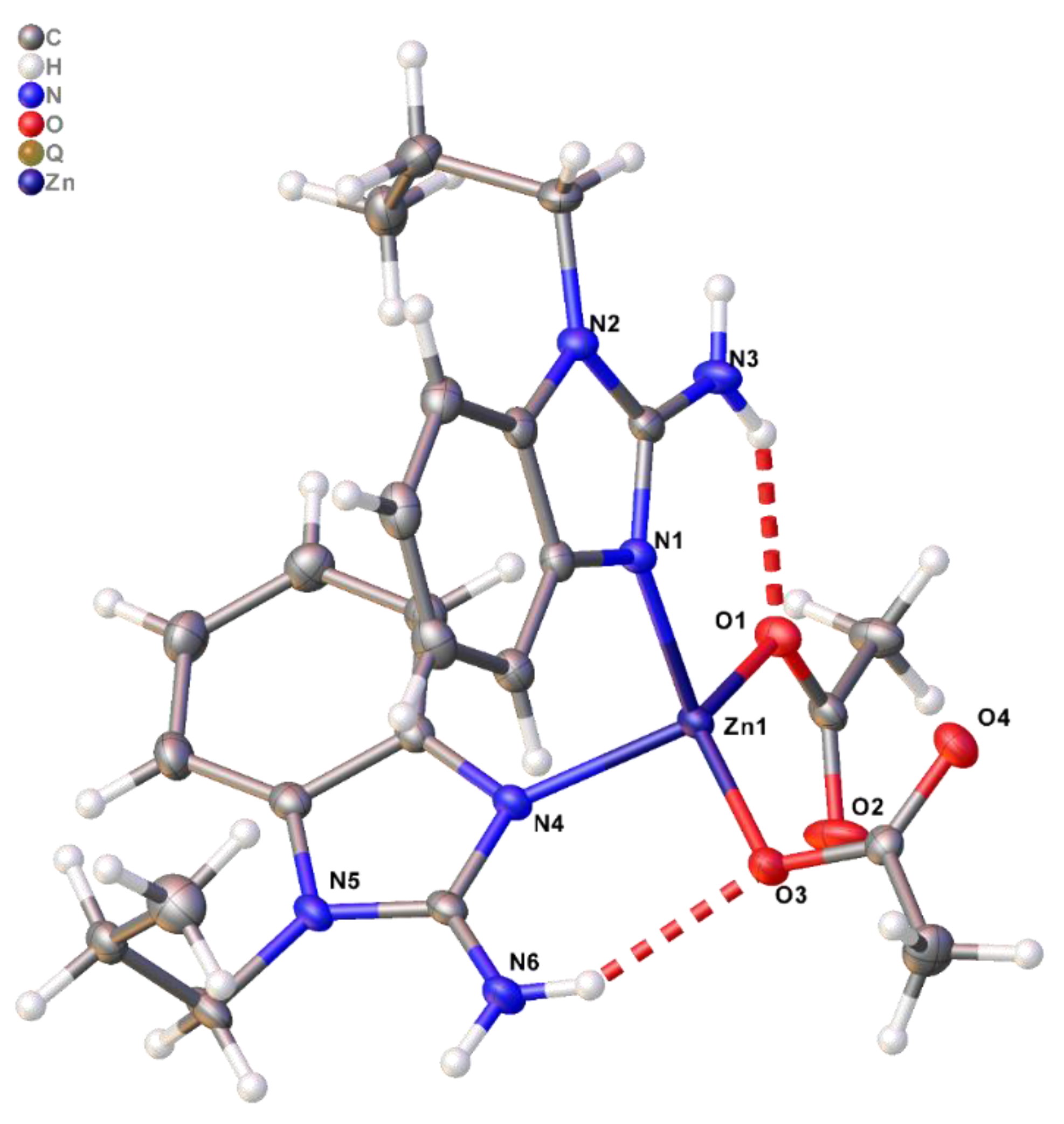

| D–H–A | d(H–A)/Å | d(D–A)/Å | D–H–A/° |
|---|---|---|---|
| N3–H3A–O1 | 2.11 | 2.8771(19) | 145.8 |
| N6–H6A–O3 | 2.11 | 2.8976(18) | 148.4 |
| N6–H6B–O2 1 | 1.95 | 2.8131(18) | 166.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikiforova, S.E.; Khan, N.A.; Kubasov, A.S.; Koshchienko, Y.V.; Burlov, A.S.; Divaeva, L.N.; Goeva, L.V.; Avdeeva, V.V.; Malinina, E.A.; Kuznetsov, N.T. Schiff Base Derivatives in Zinc(II) and Cadmium(II) Complexation with the closo-Dodecaborate Anion. Crystals 2023, 13, 1449. https://doi.org/10.3390/cryst13101449
Nikiforova SE, Khan NA, Kubasov AS, Koshchienko YV, Burlov AS, Divaeva LN, Goeva LV, Avdeeva VV, Malinina EA, Kuznetsov NT. Schiff Base Derivatives in Zinc(II) and Cadmium(II) Complexation with the closo-Dodecaborate Anion. Crystals. 2023; 13(10):1449. https://doi.org/10.3390/cryst13101449
Chicago/Turabian StyleNikiforova, Svetlana E., Nadezhda A. Khan, Alexey S. Kubasov, Yurii V. Koshchienko, Anatolii S. Burlov, Lyudmila N. Divaeva, Lyudmila V. Goeva, Varvara V. Avdeeva, Elena A. Malinina, and Nikolay T. Kuznetsov. 2023. "Schiff Base Derivatives in Zinc(II) and Cadmium(II) Complexation with the closo-Dodecaborate Anion" Crystals 13, no. 10: 1449. https://doi.org/10.3390/cryst13101449






