Rapid and Selective Sensing of 2,4,6-Trinitrophenol via a Nano-Plate Zn(II)-Based MOF Synthesized by Ultrasound Irradiation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Measurements
2.2. Synthesis of TMU-57 Nanostructures
2.3. Measurement of TMU-57 Fluorescence Properties
3. Results and Discussion
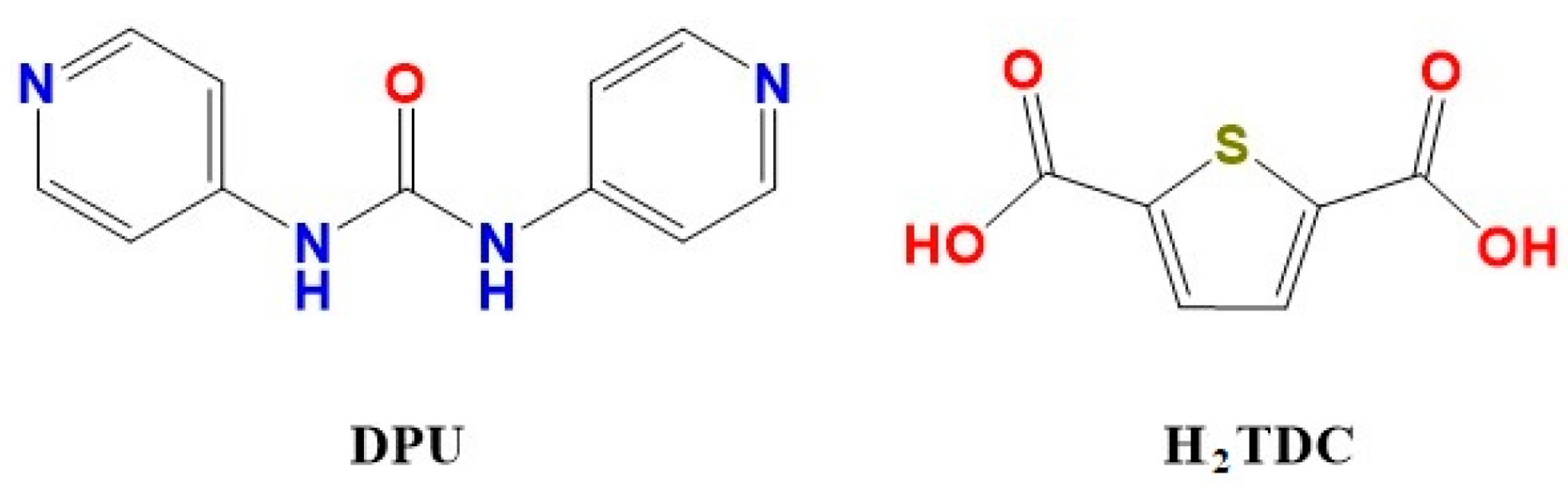
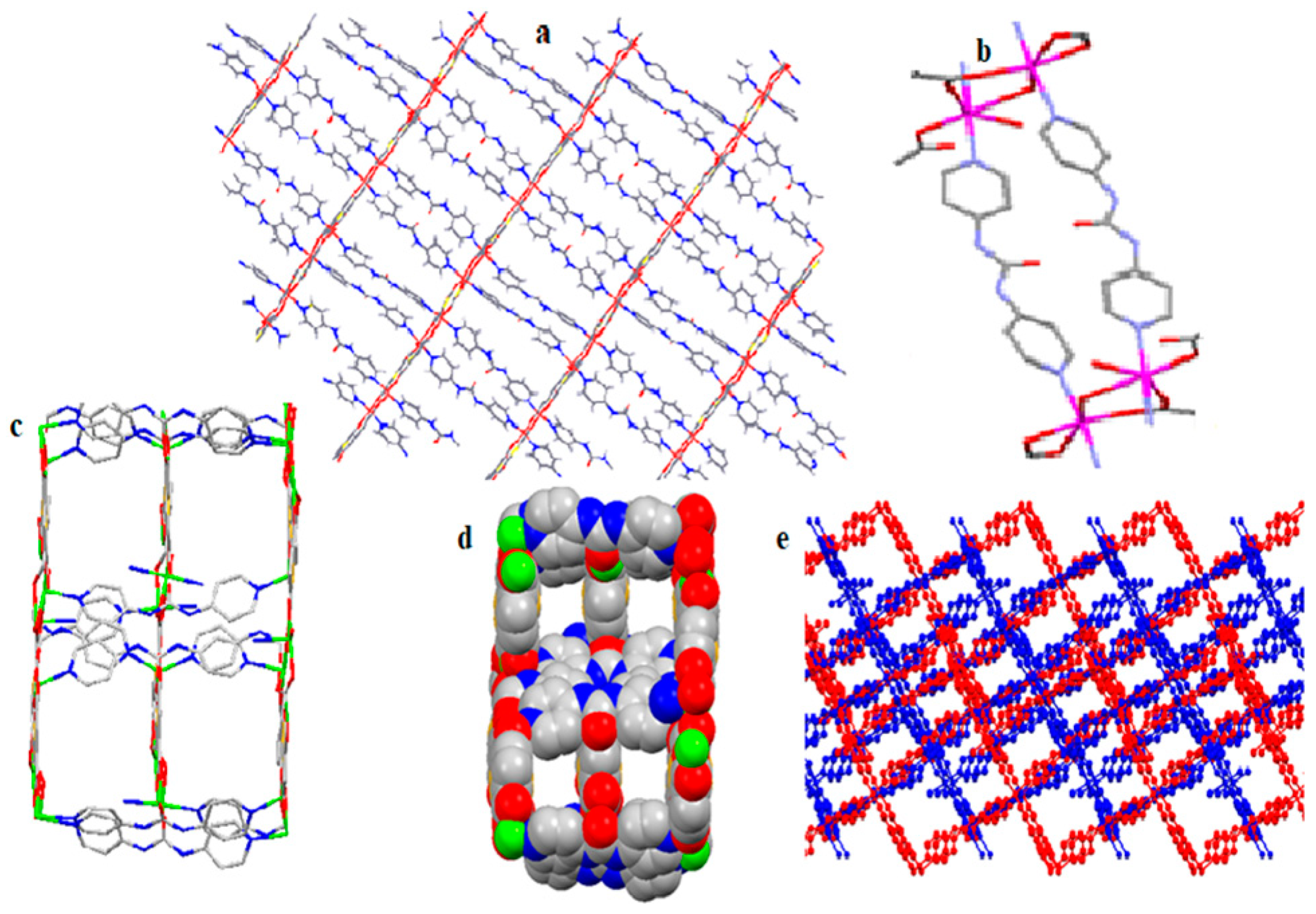
3.1. Structural Description of TMU-57
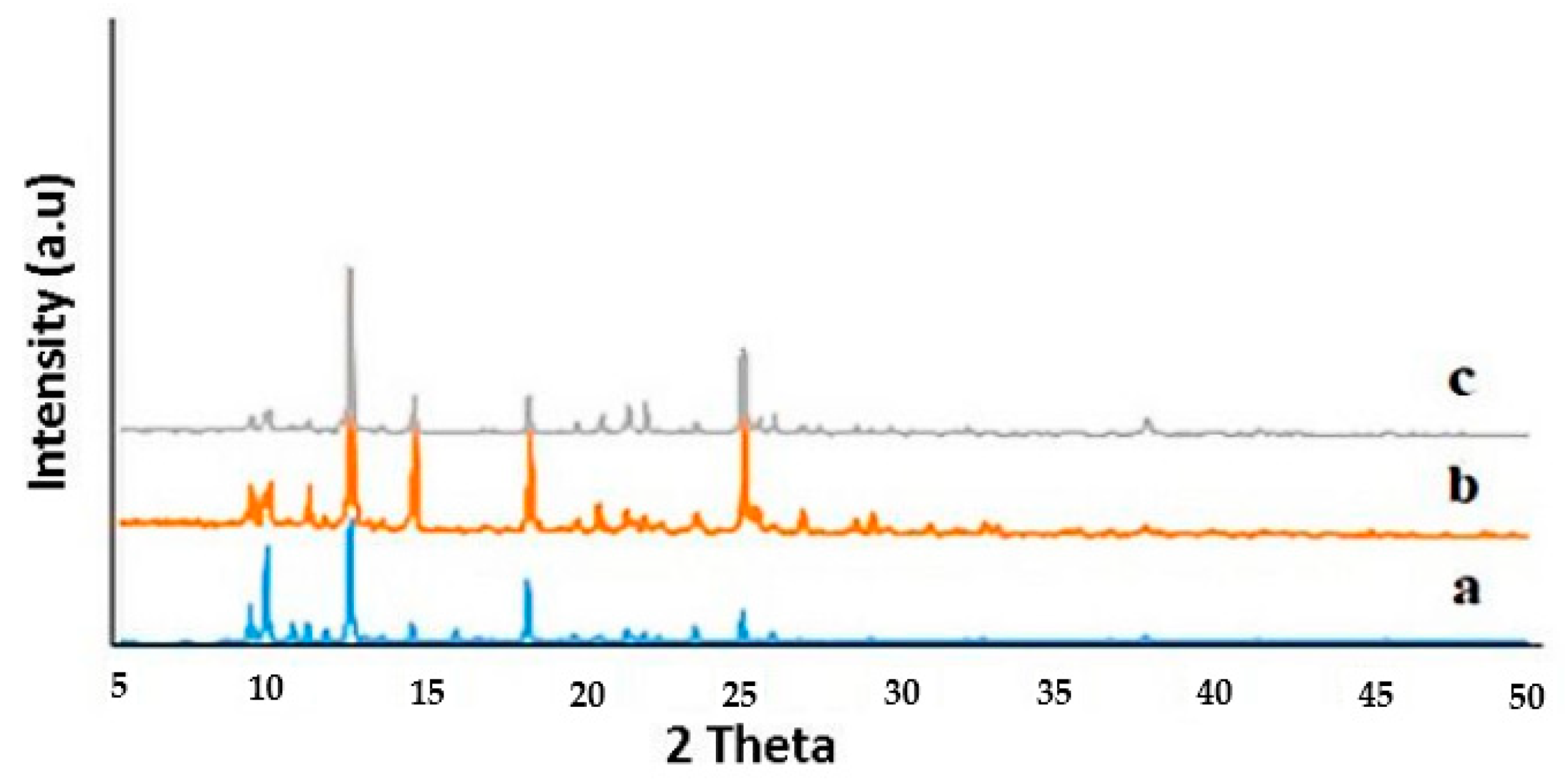
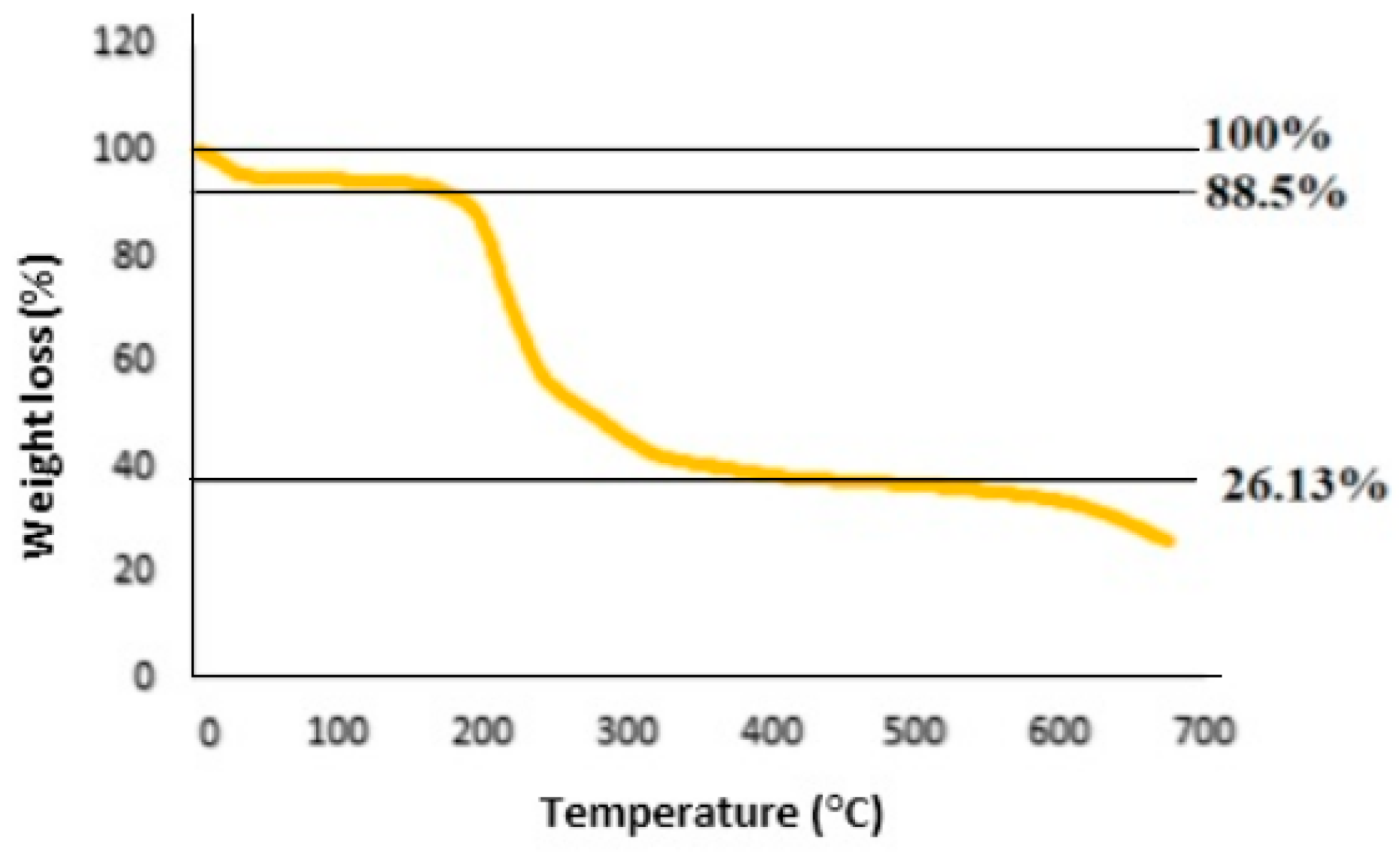
3.2. Nanostructures of TMU-57
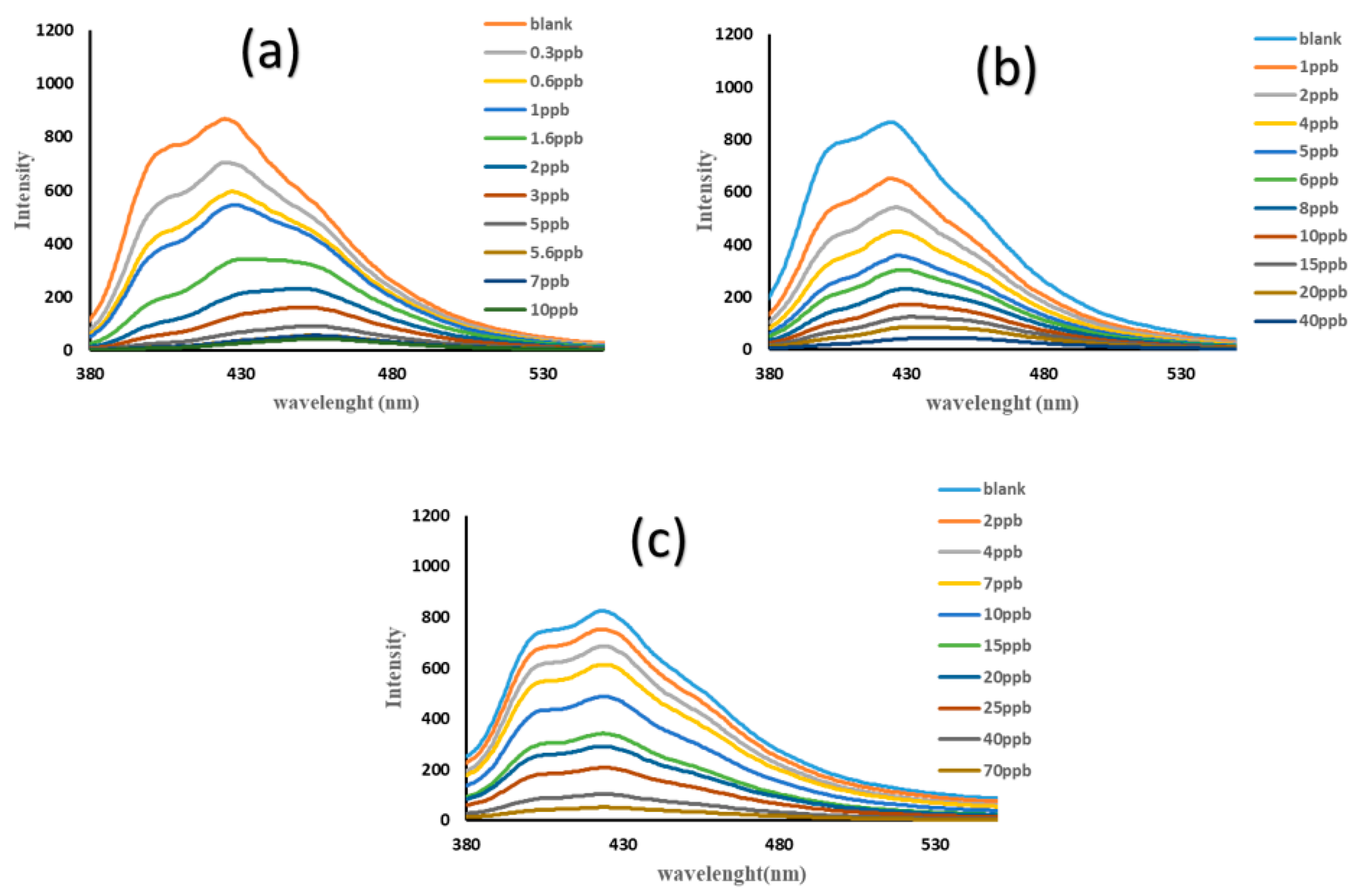
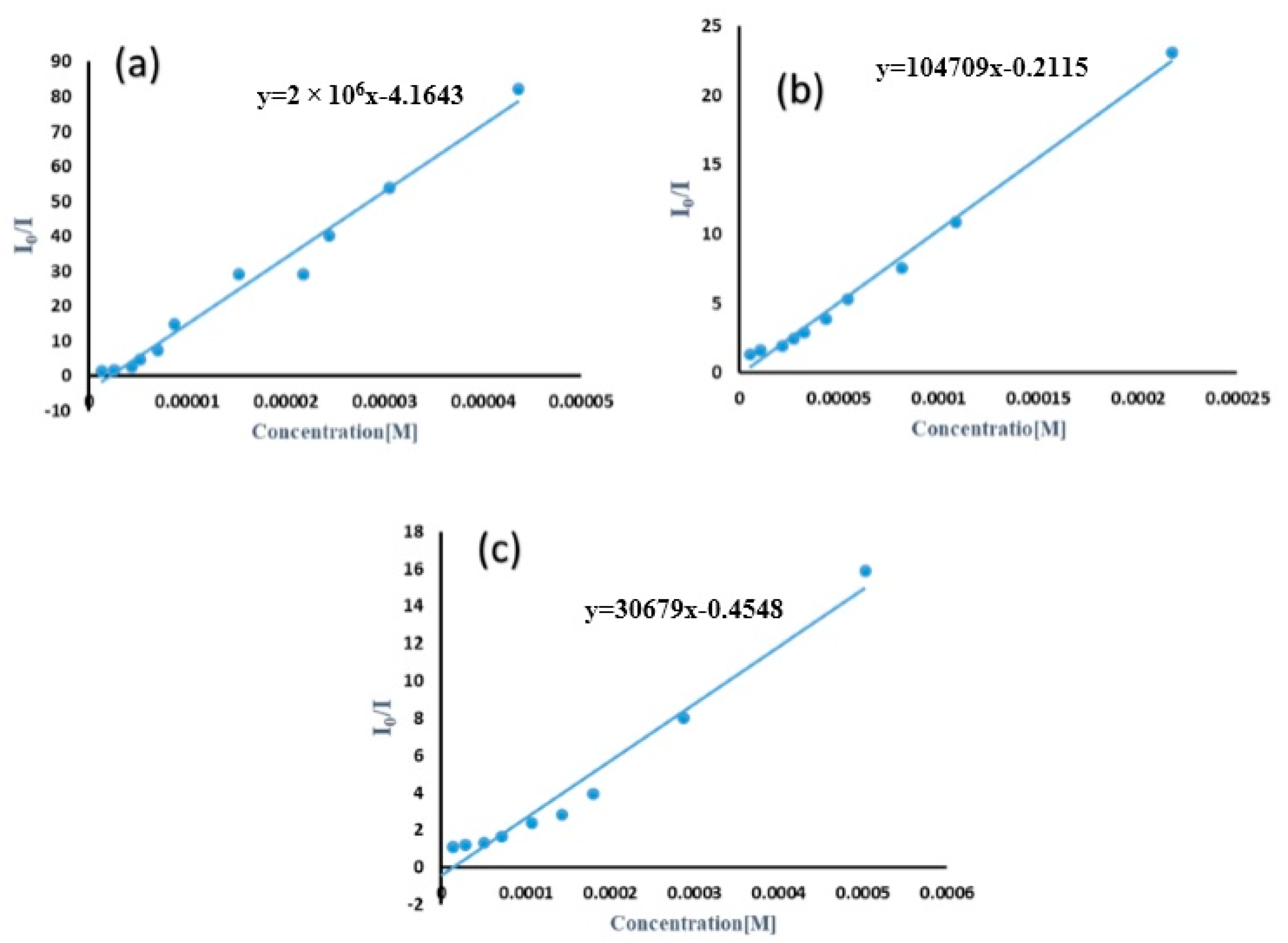
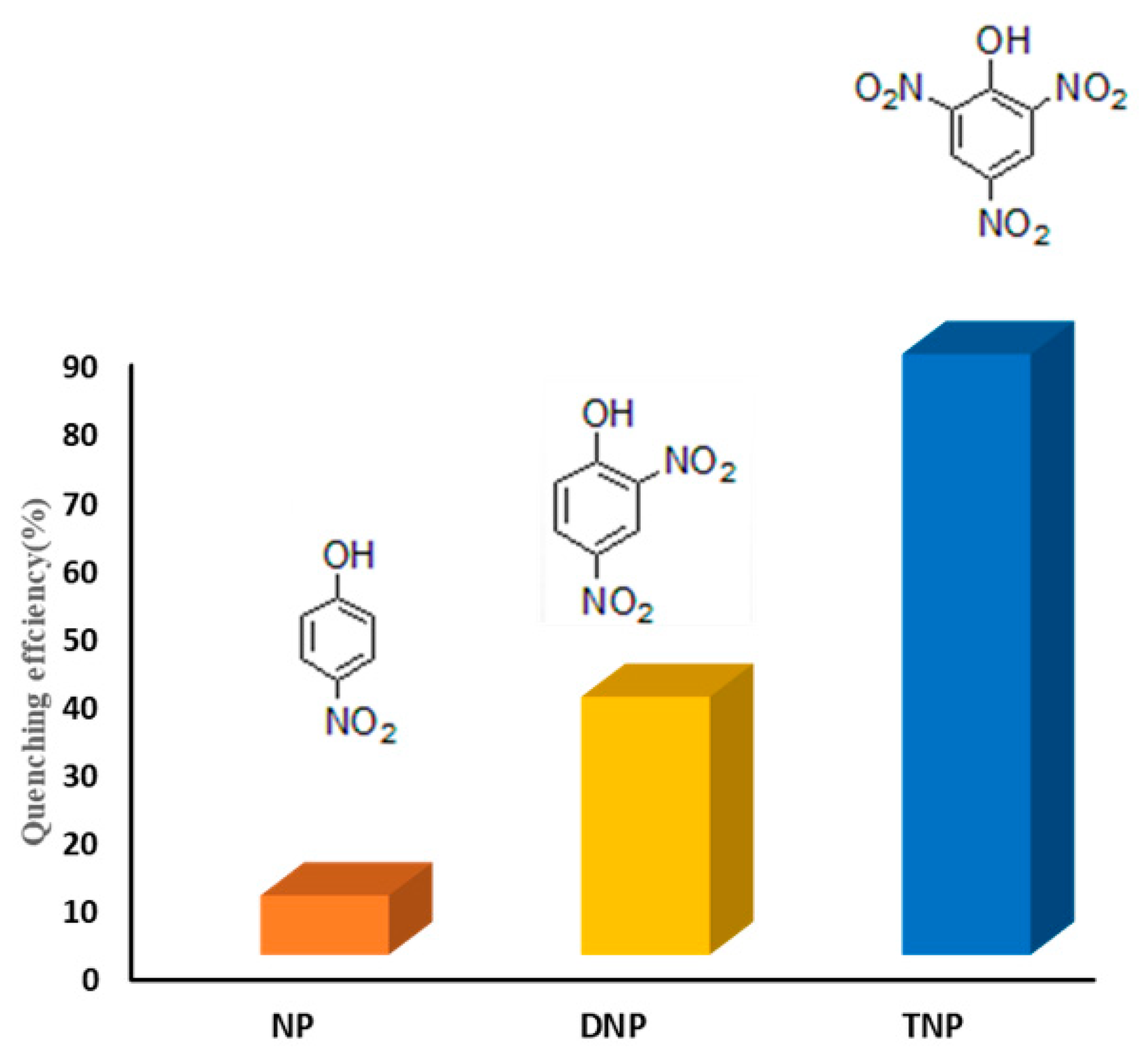
3.3. The Sensing Application of TMU-57 Nanostructures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, H.; Li, Y.; Luo, X.; Xu, Z.; Ge, J. Monodispersed gold nanoparticles supported on a zirconium-based porous metal–organic framework and their high catalytic ability for the reverse water–gas shift reaction. Chem. Commun. 2017, 53, 7953–7956. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, H.; Chen, B. Microporous Metal–Organic Frameworks for Gas Separation. Chem. Asian J. 2014, 9, 1474–1498. [Google Scholar] [CrossRef]
- Hao, S.Y.; Ma, X.G.; Cui, G.H. Ultrasonic synthesis of two nanostructured cadmium(II) coordination supramolecular polymers: Solvent influence, luminescence and photocatalytic properties. Ultrason. Sonochem. 2017, 37, 414–423. [Google Scholar] [CrossRef]
- Li, P.; Moon, S.-Y.; Guelta, M.A.; Harvey, S.P.; Hupp, J.T.; Farha, O.K. Encapsulation of a nerve agent detoxifying enzyme by a mesoporous zirconium metal–organic framework engenders thermal and long-term stability. J. Am. Chem. Soc. 2016, 138, 8052–8055. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Gándara, F.; Zhang, Y.-B.; Jiang, J.; Queen, W.L.; Hudson, M.R.; Yaghi, O.M. Water Adsorption in Porous Metal–Organic Frameworks and Related Materials. J. Am. Chem. Soc. 2014, 136, 4369–4381. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ou, S.; Wu, C.-D. Porous metal–organic frameworks for heterogeneous biomimetic catalysis. Acc. Chem. Res. 2014, 47, 1199–1207. [Google Scholar] [CrossRef]
- Mogale, R.; Conradie, J.; Langner, E.H. Trans–cis kinetic study of azobenzene-4, 4′-dicarboxylic acid and aluminium and zirconium based azobenzene-4, 4′-dicarboxylate MOFs. Molecules 2022, 27, 1370. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Zhong, X.; Dai, W.; Pei, X.; Sun, J.; Zhang, J.; Luo, W.; Zhou, W. Metal-Organic Framework Assembled on Oriented Nanofiber Arrays for Field-Effect Transistor and Gas Sensor-Based Applications. Molecules 2022, 27, 2131. [Google Scholar] [CrossRef]
- Li, P.; Klet, R.C.; Moon, S.-Y.; Wang, T.C.; Deria, P.; Peters, A.W.; Klahr, B.M.; Park, H.-J.; Al-Juaid, S.S.; Hupp, J.T. Synthesis of nanocrystals of Zr-based metal–organic frameworks with csq-net: Significant enhancement in the degradation of a nerve agent simulant. Chem. Commun. 2015, 51, 10925–10928. [Google Scholar] [CrossRef]
- D’Agata, A.; Fasulo, S.; Dallas, L.J.; Fisher, A.S.; Maisano, M.; Readman, J.W.; Jha, A.N. Enhanced toxicity of ‘bulk’titanium dioxide compared to ‘fresh’and ‘aged’nano-TiO2 in marine mussels (Mytilus galloprovincialis). Nanotoxicology 2014, 8, 549–558. [Google Scholar] [CrossRef]
- Soltanzadeh, N.; Morsali, A. Sonochemical synthesis of a new nano-structures bismuth(III) supramolecular compound: New precursor for the preparation of bismuth(III) oxide nano-rods and bismuth(III) iodide nano-wires. Ultrason. Sonochem. 2010, 17, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Mautschke, H.H.; Llabrés i Xamena, F.X. MOF-808 as a Highly Active Catalyst for the Diastereoselective Reduction of Substituted Cyclohexanones. Molecules 2022, 27, 6315. [Google Scholar] [CrossRef] [PubMed]
- Ali Dheyab, M.; Aziz, A.A.; Jameel, M.S. Recent advances in inorganic nanomaterials synthesis using sonochemistry: A comprehensive review on iron oxide, gold and iron oxide coated gold nanoparticles. Molecules 2021, 26, 2453. [Google Scholar] [CrossRef]
- Jameel, M.S.; Aziz, A.A.; Dheyab, M.A.; Mehrdel, B.; Khaniabadi, P.M. Rapid sonochemically-assisted green synthesis of highly stable and biocompatible platinum nanoparticles. Surf. Interfaces 2020, 20, 100635. [Google Scholar] [CrossRef]
- Joharian, M.; Morsali, A. Ultrasound-assisted synthesis of two new fluorinated metal-organic frameworks (F-MOFs) with the high surface area to improve the catalytic activity. J. Solid State Chem. 2019, 270, 135–146. [Google Scholar] [CrossRef]
- Joharian, M.; Abedi, S.; Morsali, A. Sonochemical synthesis and structural characterization of a new nanostructured Co (II) supramolecular coordination polymer with Lewis base sites as a new catalyst for Knoevenagel condensation. Ultrason. Sonochem. 2017, 39, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Negishi, K. Experimental studies on sonoluminescence and ultrasonic cavitation. J. Phys. Soc. Jpn. 1961, 16, 1450–1465. [Google Scholar] [CrossRef]
- Ma, D.; Li, B.; Zhou, X.; Zhou, Q.; Liu, K.; Zeng, G.; Li, G.; Shi, Z.; Feng, S. A dual functional MOF as a luminescent sensor for quantitatively detecting the concentration of nitrobenzene and temperature. Chem. Commun. 2013, 49, 8964–8966. [Google Scholar] [CrossRef]
- Sadeghzadeh, H.; Morsali, A.; Retailleau, P. Ultrasonic-assisted synthesis of two new nano-structured 3D lead(II) coordination polymers: Precursors for preparation of PbO nano-structures. Polyhedron 2010, 29, 925–933. [Google Scholar] [CrossRef]
- Fard-Jahromi, M.J.S.; Morsali, A. Sonochemical synthesis of nanoscale mixed-ligands lead(II) coordination polymers as precursors for preparation of Pb2(SO4)O and PbO nanoparticles; thermal, structural and X-ray powder diffraction studies. Ultrason. Sonochem. 2010, 17, 435–440. [Google Scholar] [CrossRef]
- Nur Atiqah, S. Design and Fabrication of Solar Light Responsive New Metal Organic Frameworks for Photocatalysis/Nur Atiqah Surib; University of Malaya: Kuala Lumpur, Malaya, 2018. [Google Scholar]
- Abbasi, A.R.; Morsali, A. Syntheses and characterization of AgI nano-structures by ultrasonic method: Different morphologies under different conditions. Ultrason. Sonochem. 2010, 17, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Hakimifar, A.; Morsali, A. High-sensitivity detection of nitroaromatic compounds (NACs) by the pillared-layer metal-organic framework synthesized via ultrasonic method. Ultrason. Sonochem. 2019, 52, 62–68. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S.; Khaniabadi, P.M.; Mehrdel, B. Sonochemical-assisted synthesis of highly stable gold nanoparticles catalyst for decoloration of methylene blue dye. Inorg. Chem. Commun. 2021, 127, 108551. [Google Scholar] [CrossRef]
- Zarekarizi, F.; Morsali, A. Ultrasonic-assisted synthesis of nano-sized metal-organic framework; a simple method to explore selective and fast Congo Red adsorption. Ultrason. Sonochem. 2020, 69, 105246. [Google Scholar] [CrossRef] [PubMed]
- Bigdeli, F.; Rouhani, F.; Morsali, A.; Ramazani, A. Ultrasonic-assisted synthesis of the nanostructures of a Co(II) metal organic framework as a highly sensitive fluorescence probe of phenol derivatives. Ultrason. Sonochem. 2020, 62, 104862. [Google Scholar] [CrossRef] [PubMed]
- Vaitsis, C.; Sourkouni, G.; Argirusis, C. Metal Organic Frameworks (MOFs) and ultrasound: A review. Ultrason. Sonochem. 2019, 52, 106–119. [Google Scholar] [CrossRef]
- Liang, J.; Zulkifli, M.Y.B.; Yong, J.; Du, Z.; Ao, Z.; Rawal, A.; Scott, J.A.; Harmer, J.R.; Wang, J.; Liang, K. Locking the Ultrasound-Induced Active Conformation of Metalloenzymes in Metal–Organic Frameworks. J. Am. Chem. Soc. 2022, 144, 17865–17875. [Google Scholar] [CrossRef]
- Sargazi, G.; Afzali, D.; Mostafavi, A.; Ebrahimipour, S.Y. Ultrasound-assisted facile synthesis of a new tantalum(V) metal-organic framework nanostructure: Design, characterization, systematic study, and CO2 adsorption performance. J. Solid State Chem. 2017, 250, 32–48. [Google Scholar] [CrossRef]
- Tanhaei, M.; Mahjoub, A.R.; Safarifard, V. Ultrasonic-assisted synthesis and characterization of nanocomposites from azine-decorated metal-organic framework and graphene oxide layers. Mater. Lett. 2018, 227, 318–321. [Google Scholar] [CrossRef]
- Abazari, R.; Mahjoub, A.R. Ultrasound-assisted synthesis of Zinc(II)-based metal organic framework nanoparticles in the presence of modulator for adsorption enhancement of 2,4-dichlorophenol and amoxicillin. Ultrason. Sonochem. 2018, 42, 577–584. [Google Scholar] [CrossRef]
- Safarifard, V.; Morsali, A. Applications of ultrasound to the synthesis of nanoscale metal–organic coordination polymers. Coord. Chem. Rev. 2015, 292, 1–14. [Google Scholar] [CrossRef]
- Qiu, L.-G.; Li, Z.-Q.; Wu, Y.; Wang, W.; Xu, T.; Jiang, X. Facile synthesis of nanocrystals of a microporous metal–organic framework by an ultrasonic method and selective sensing of organoamines. Chem. Commun. 2008, 3642–3644. [Google Scholar] [CrossRef]
- Howarth, A.J.; Wang, T.C.; Al-Juaid, S.S.; Aziz, S.G.; Hupp, J.T.; Farha, O.K. Efficient extraction of sulfate from water using a Zr-metal–organic framework. Dalton Trans. 2016, 45, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Gole, B.; Bar, A.K.; Mukherjee, P.S. Fluorescent metal–organic framework for selective sensing of nitroaromatic explosives. Chem. Commun. 2011, 47, 12137–12139. [Google Scholar] [CrossRef]
- Nagarkar, S.S.; Desai, A.V.; Ghosh, S.K. Engineering metal–organic frameworks for aqueous phase 2, 4, 6-trinitrophenol (TNP) sensing. CrystEngComm 2016, 18, 2994–3007. [Google Scholar] [CrossRef]
- Yang, J.; Che, J.; Jiang, X.; Fan, Y.; Gao, D.; Bi, J.; Ning, Z. A Novel Turn-On Fluorescence Probe Based on Cu (II) Functionalized Metal–Organic Frameworks for Visual Detection of Uric Acid. Molecules 2022, 27, 4803. [Google Scholar] [CrossRef]
- Chen, M.; Xu, W.-M.; Tian, J.-Y.; Cui, H.; Zhang, J.-X.; Liu, C.-S.; Du, M. A terbium (III) lanthanide–organic framework as a platform for a recyclable multi-responsive luminescent sensor. J. Mater. Chem. C 2017, 5, 2015–2021. [Google Scholar] [CrossRef]
- Han, M.-L.; Wen, G.-X.; Dong, W.-W.; Zhou, Z.-H.; Wu, Y.-P.; Zhao, J.; Li, D.-S.; Ma, L.-F.; Bu, X. A heterometallic sodium–europium-cluster-based metal–organic framework as a versatile and water-stable chemosensor for antibiotics and explosives. J. Mater. Chem. C 2017, 5, 8469–8474. [Google Scholar] [CrossRef]
- Li, B.; Jiang, Y.-Y.; Sun, Y.-Y.; Wang, Y.-J.; Han, M.-L.; Wu, Y.-P.; Ma, L.-F.; Li, D.-S. The highly selective detecting of antibiotics and support of noble metal catalysts by a multifunctional Eu-MOF. Dalton Trans. 2020, 49, 14854–14862. [Google Scholar] [CrossRef]
- Wen, G.-X.; Han, M.-L.; Wu, X.-Q.; Wu, Y.-P.; Dong, W.-W.; Zhao, J.; Li, D.-S.; Ma, L.-F. A multi-responsive luminescent sensor based on a super-stable sandwich-type terbium (III)–organic framework. Dalton Trans. 2016, 45, 15492–15499. [Google Scholar] [CrossRef]
- Nagarkar, S.S.; Joarder, B.; Chaudhari, A.K.; Mukherjee, S.; Ghosh, S.K. Highly selective detection of nitro explosives by a luminescent metal–organic framework. Angew. Chem. 2013, 125, 2953–2957. [Google Scholar] [CrossRef]
- Jurcic, M.; Peveler, W.J.; Savory, C.N.; Scanlon, D.O.; Kenyon, A.J.; Parkin, I.P. The vapour phase detection of explosive markers and derivatives using two fluorescent metal–organic frameworks. J. Mater. Chem. A 2015, 3, 6351–6359. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Insights in the application of stoichiometric and non-stoichiometric titanium oxides for the design of sensors for the determination of gases and VOCs (TiO2−x and TinO2n−1 vs. TiO2). Sensors 2020, 20, 6833. [Google Scholar] [CrossRef] [PubMed]
- Monsef, R.; Ghiyasiyan-Arani, M.; Salavati-Niasari, M. Application of ultrasound-aided method for the synthesis of NdVO4 nano-photocatalyst and investigation of eliminate dye in contaminant water. Ultrason. Sonochem. 2018, 42, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, B. A responsive MOF nanocomposite for decoding volatile organic compounds. Chem. Commun. 2016, 52, 2265–2268. [Google Scholar] [CrossRef]
- Kumar, P.; Deep, A.; Kim, K.-H.; Brown, R.J. Coordination polymers: Opportunities and challenges for monitoring volatile organic compounds. Prog. Polym. Sci. 2015, 45, 102–118. [Google Scholar] [CrossRef]
- Zheng, J.P.; Ou, S.; Zhao, M.; Wu, C.D. A highly sensitive luminescent dye@ MOF composite for probing different volatile organic compounds. ChemPlusChem 2016, 81, 758–763. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Lei, Y. Fluorescence based explosive detection: From mechanisms to sensory materials. Chem. Soc. Rev. 2015, 44, 8019–8061. [Google Scholar] [CrossRef]
- Hawes, C.S.; Nolvachai, Y.; Kulsing, C.; Knowles, G.P.; Chaffee, A.L.; Marriott, P.J.; Batten, S.R.; Turner, D.R. Metal–organic frameworks as stationary phases for mixed-mode separation applications. Chem. Commun. 2014, 50, 3735–3737. [Google Scholar] [CrossRef]
- Miao, Q.; Hakimifar, A.; Akbar Razavi, S.A.; Abbasi, H.; Tehrani, A.A.; Chen, J.-Q.; Hu, M.-L.; Morsali, A.; Retailleau, P. Multi-functionalization strategy for environmental monitoring: A metal-organic framework for high capacity Mercury(II) removal and exceptionally sensitive detection of nitroaromatics. J. Clean. Prod. 2022, 376, 134301. [Google Scholar] [CrossRef]
- Pramanik, S.; Zheng, C.; Zhang, X.; Emge, T.J.; Li, J. New microporous metal–organic framework demonstrating unique selectivity for detection of high explosives and aromatic compounds. J. Am. Chem. Soc. 2011, 133, 4153–4155. [Google Scholar] [CrossRef]
- He, Q.; Guan, T.; He, Y.; Lu, B.; Li, D.; Chen, X.; Feng, G.; Liu, S.; Ji, Y.; Xin, M. Digital encoding based molecular imprinting suspension array for multiplexed label-free sensing of phenol derivatives. Sens. Actuators B Chem. 2018, 271, 367–373. [Google Scholar] [CrossRef]
- Kaur, S.; Bhalla, V.; Vij, V.; Kumar, M. Fluorescent aggregates of hetero-oligophenylene derivative as “no quenching” probe for detection of picric acid at femtogram level. J. Mater. Chem. C 2014, 2, 3936–3941. [Google Scholar] [CrossRef]
- Nagarkar, S.S.; Desai, A.V.; Ghosh, S.K. A fluorescent metal–organic framework for highly selective detection of nitro explosives in the aqueous phase. Chem. Commun. 2014, 50, 8915–8918. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Li, P.; Sui, Q.; Chen, J.; Xu, J.; Gao, E.-Q. A stable electron-deficient metal–organic framework for colorimetric and luminescence sensing of phenols and anilines. J. Mater. Chem. A 2018, 6, 9236–9244. [Google Scholar] [CrossRef]
- Wen, Y.; Li, R.; Liu, J.; Zhang, X.; Wang, P.; Zhang, X.; Zhou, B.; Li, H.; Wang, J.; Li, Z. Promotion effect of Zn on 2D bimetallic NiZn metal organic framework nanosheets for tyrosinase immobilization and ultrasensitive detection of phenol. Anal. Chim. Acta 2020, 1127, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-H.; Xue, L.-P.; Li, S.; Zang, P.-F. Different metal–ligand ratios regulated two Cd (II)-containing viologen-derived photochromic coordination polymers. Dye. Pigment. 2022, 200, 110129. [Google Scholar] [CrossRef]
- Azhdari Tehrani, A.; Esrafili, L.; Abedi, S.; Morsali, A.; Carlucci, L.; Proserpio, D.M.; Wang, J.; Junk, P.C.; Liu, T. Urea metal–organic frameworks for nitro-substituted compounds sensing. Inorg. Chem. 2017, 56, 1446–1454. [Google Scholar] [CrossRef]




| Identification Code | TMU-57 |
|---|---|
| CCDC number | 1,908,579 |
| Formula | C51H36N12O15S3Zn3,2.55 (C3H7NO) |
| Fw | 1535.61 |
| T/K | 293 (2) |
| crystal system | monoclinic |
| space group | P21/c |
| a/Å | 10.5925 (2) |
| b/Å | 18.1702 (4) |
| c/Å | 34.2901 (7) |
| α/° | 90.0 |
| β/° | 98.665 (2) |
| γ/° | 90.0 |
| V/Å3 | 6524.4 (2) |
| Z | 4 |
| Dcalc/g cm−3 | 1.563 |
| μ (mm−1) | 1.272 |
| F (000) | 3144 |
| (h k l) max | (14 24 45) |
| R1 (I > 2σ (I)) | 0.0358 |
| wR2 (I > 2σ (I)) | 0.0993 |
| Sample Name | Molar Ratio (TDC:L:Zn(OAc)2) mmol | Concentration [TDC]/[L]/[Zn(OAc)2] (M) | Time (min) | Morphology |
|---|---|---|---|---|
| A | 1:1:1 | [0.02]/[0.02]/[0.02] | 30 | Nano-plates |
| B | 1:1:1 | [0.02]/[0.02]/[0.02] | 60 | Nano-plates |
| C | 1:1:1 | [0.01]/[0.01]/[0.01] | 30 | Nano-plates |
| D | 1:1:1 | [0.01]/[0.01]/[0.01] | 60 | Nanoparticle |
| E | 1:1:1 | [0.005]/[0.005]/[0.005] | 30 | Nano-plates |
| F | 1:1:1 | [0.005]/[0.005]/[0.005] | 60 | Nano-plates |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.-W.; Hakimifar, A.; Bigdeli, F.; Hanifehpour, Y.; Wang, S.-J.; Liu, K.-G.; Morsali, A.; Joo, S.W. Rapid and Selective Sensing of 2,4,6-Trinitrophenol via a Nano-Plate Zn(II)-Based MOF Synthesized by Ultrasound Irradiation. Crystals 2023, 13, 1344. https://doi.org/10.3390/cryst13091344
Yan X-W, Hakimifar A, Bigdeli F, Hanifehpour Y, Wang S-J, Liu K-G, Morsali A, Joo SW. Rapid and Selective Sensing of 2,4,6-Trinitrophenol via a Nano-Plate Zn(II)-Based MOF Synthesized by Ultrasound Irradiation. Crystals. 2023; 13(9):1344. https://doi.org/10.3390/cryst13091344
Chicago/Turabian StyleYan, Xiao-Wei, Azar Hakimifar, Fahime Bigdeli, Younes Hanifehpour, Su-Juan Wang, Kuan-Guan Liu, Ali Morsali, and Sang Woo Joo. 2023. "Rapid and Selective Sensing of 2,4,6-Trinitrophenol via a Nano-Plate Zn(II)-Based MOF Synthesized by Ultrasound Irradiation" Crystals 13, no. 9: 1344. https://doi.org/10.3390/cryst13091344







