Investigations of Electrochemical Characteristics of Mg-Al-Ca Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
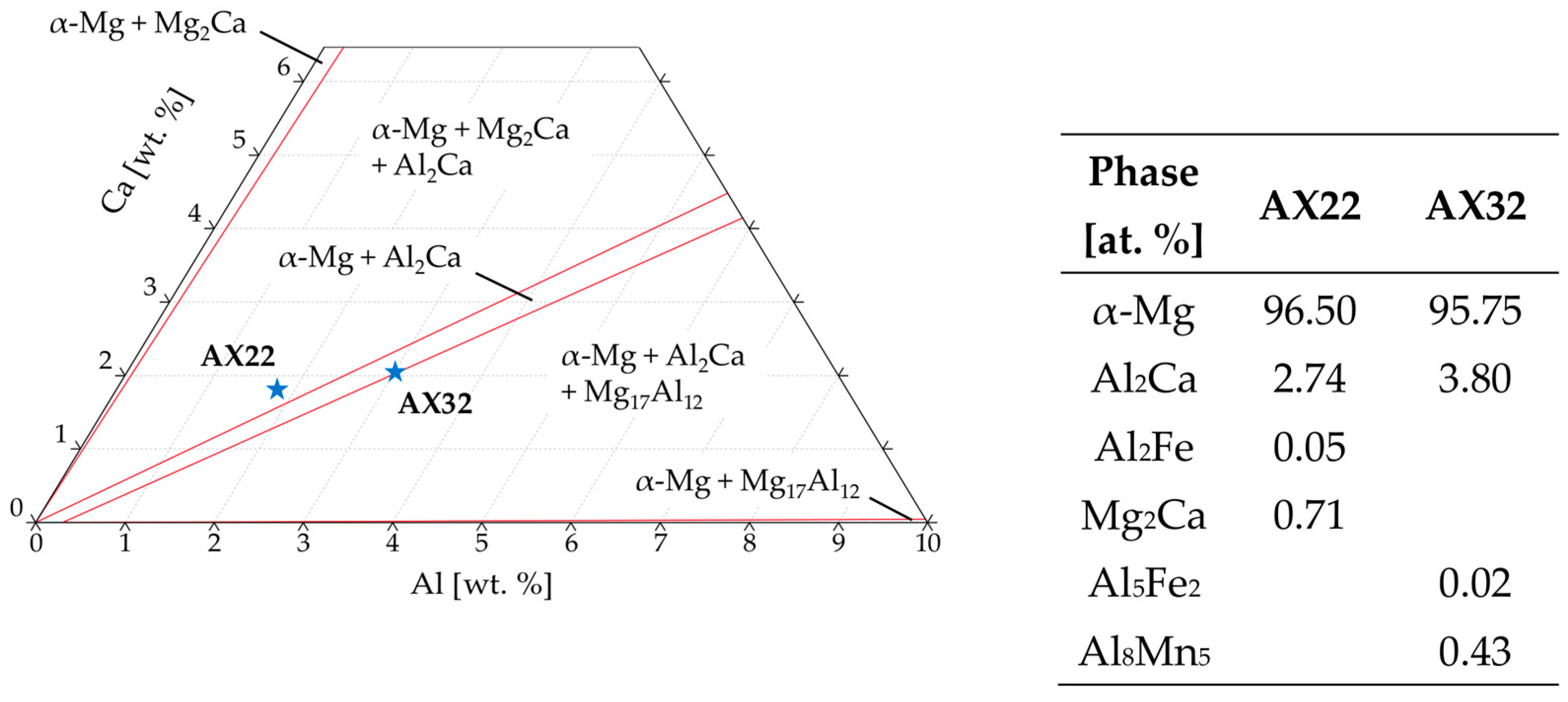
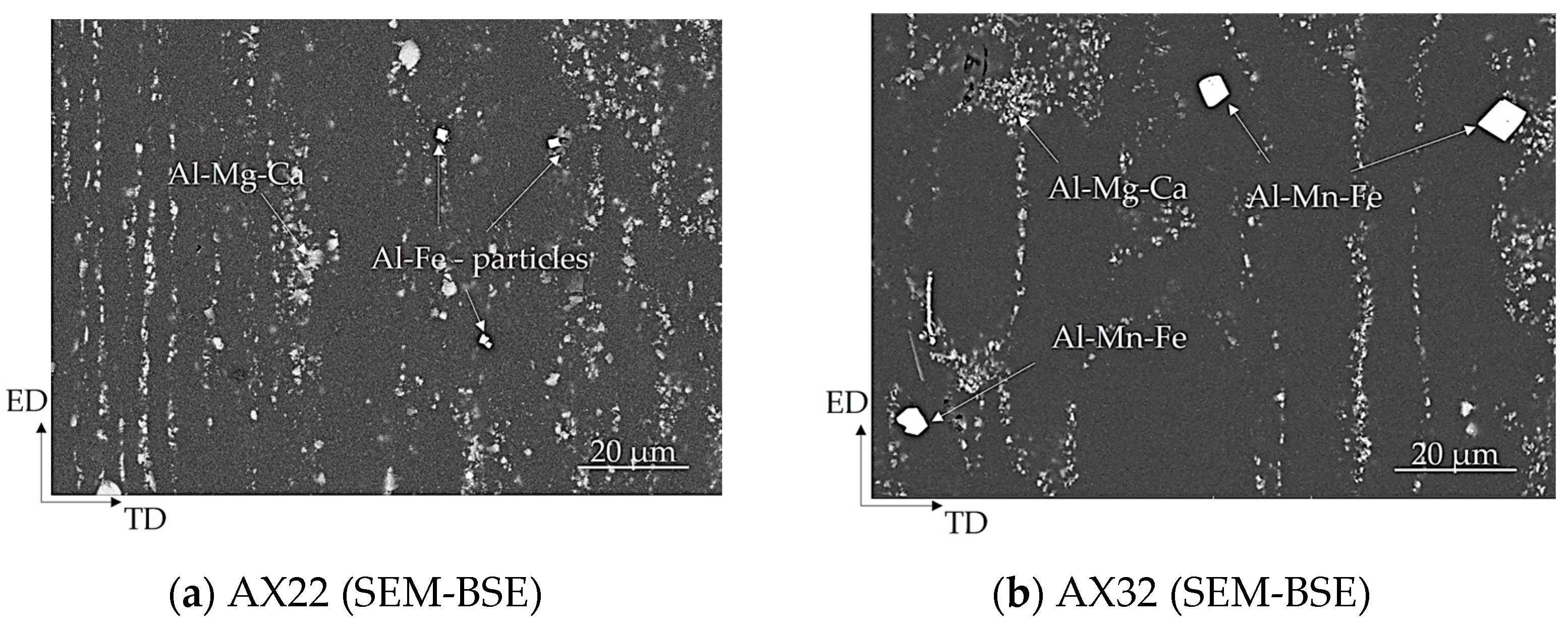
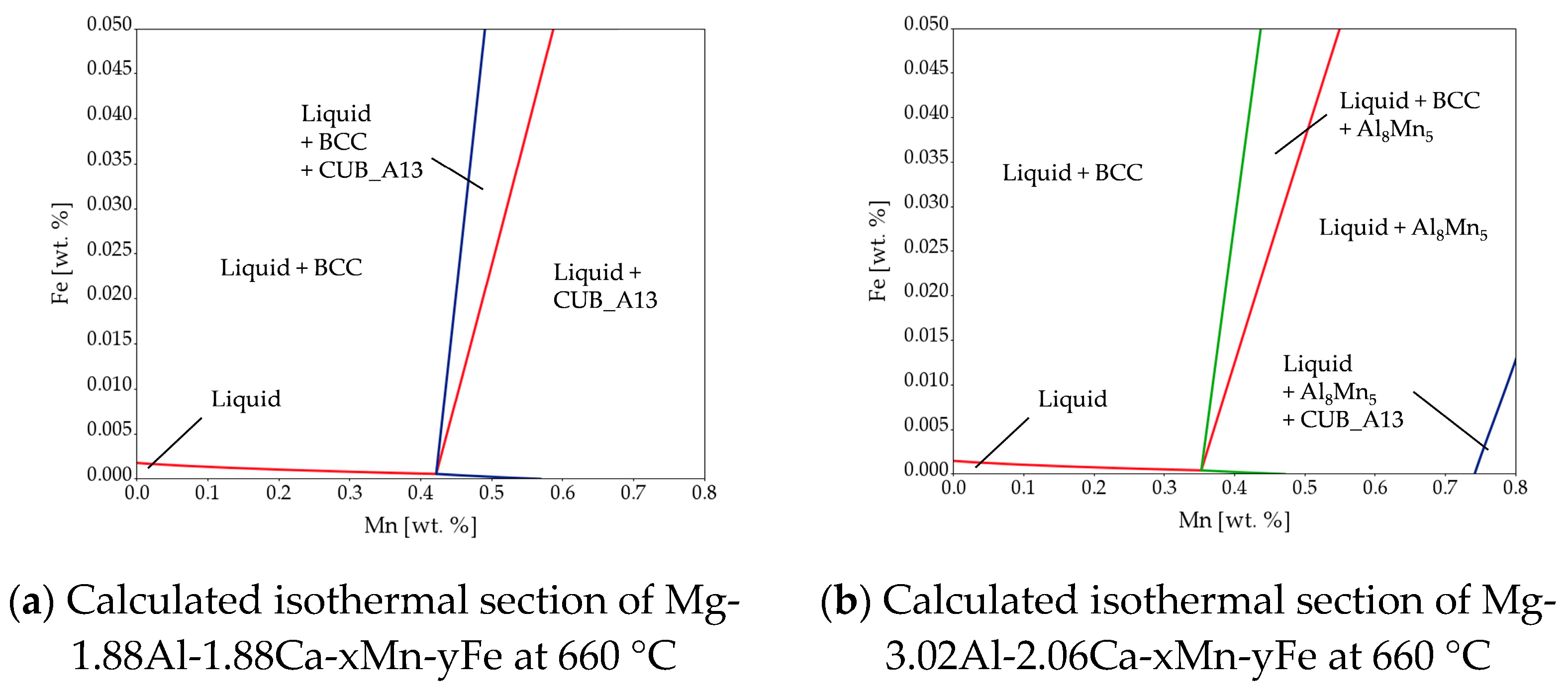
3.1. Microstructure
3.2. Corrosion Testing
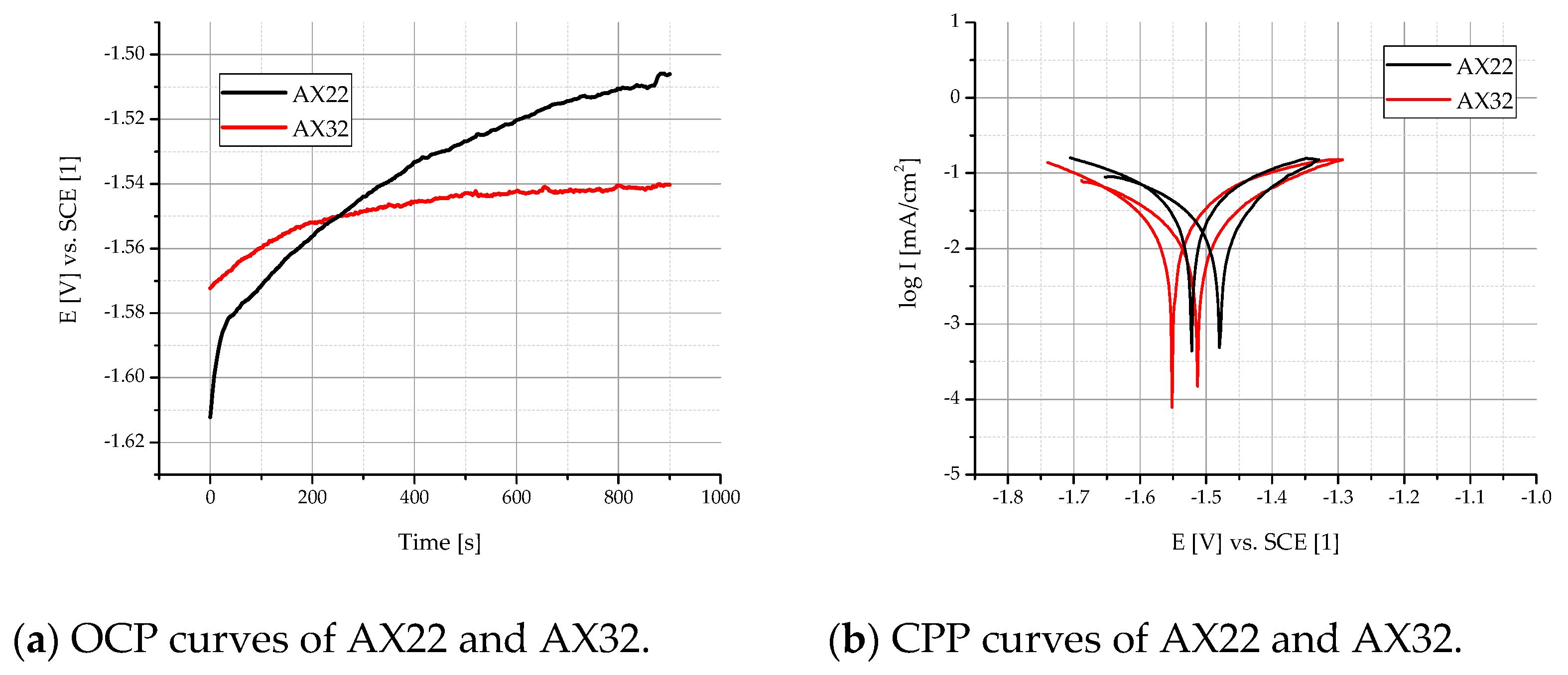
3.2.1. Potentiodynamic Polarization Tests
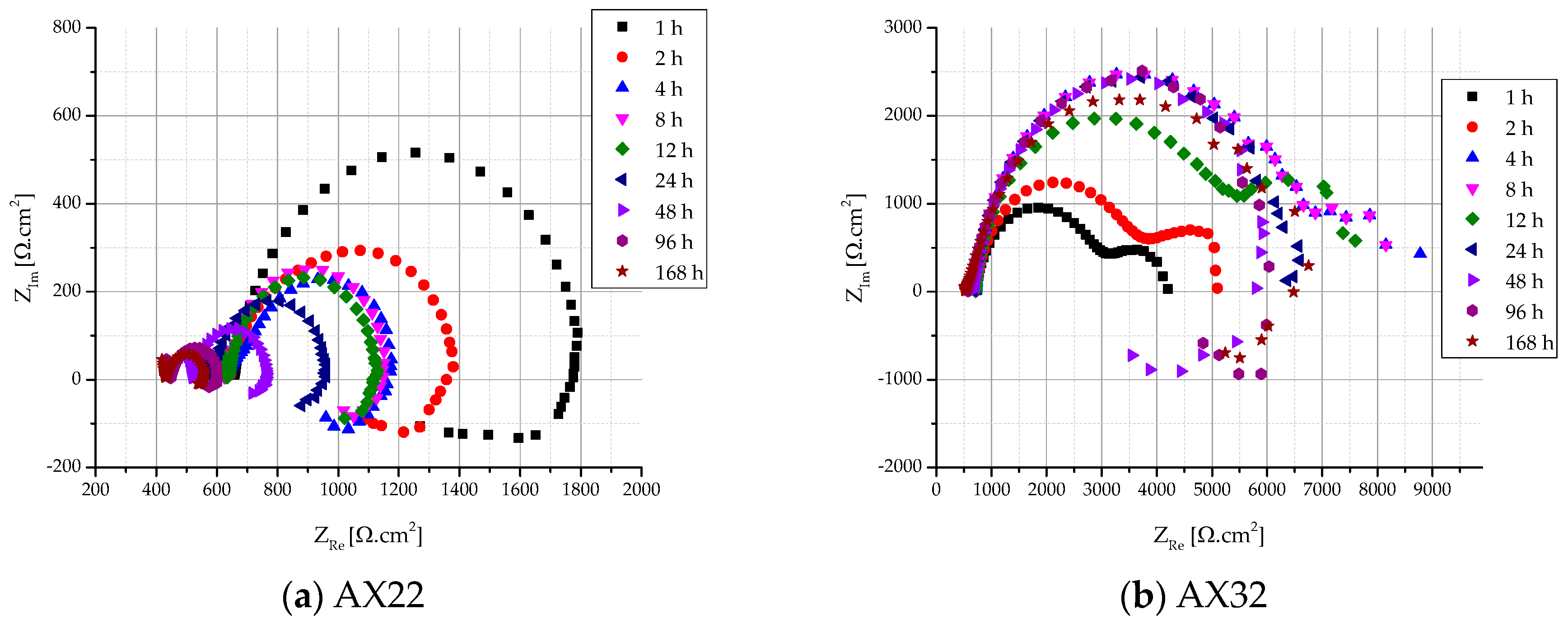
3.2.2. Electrochemical Impedance Spectroscopy
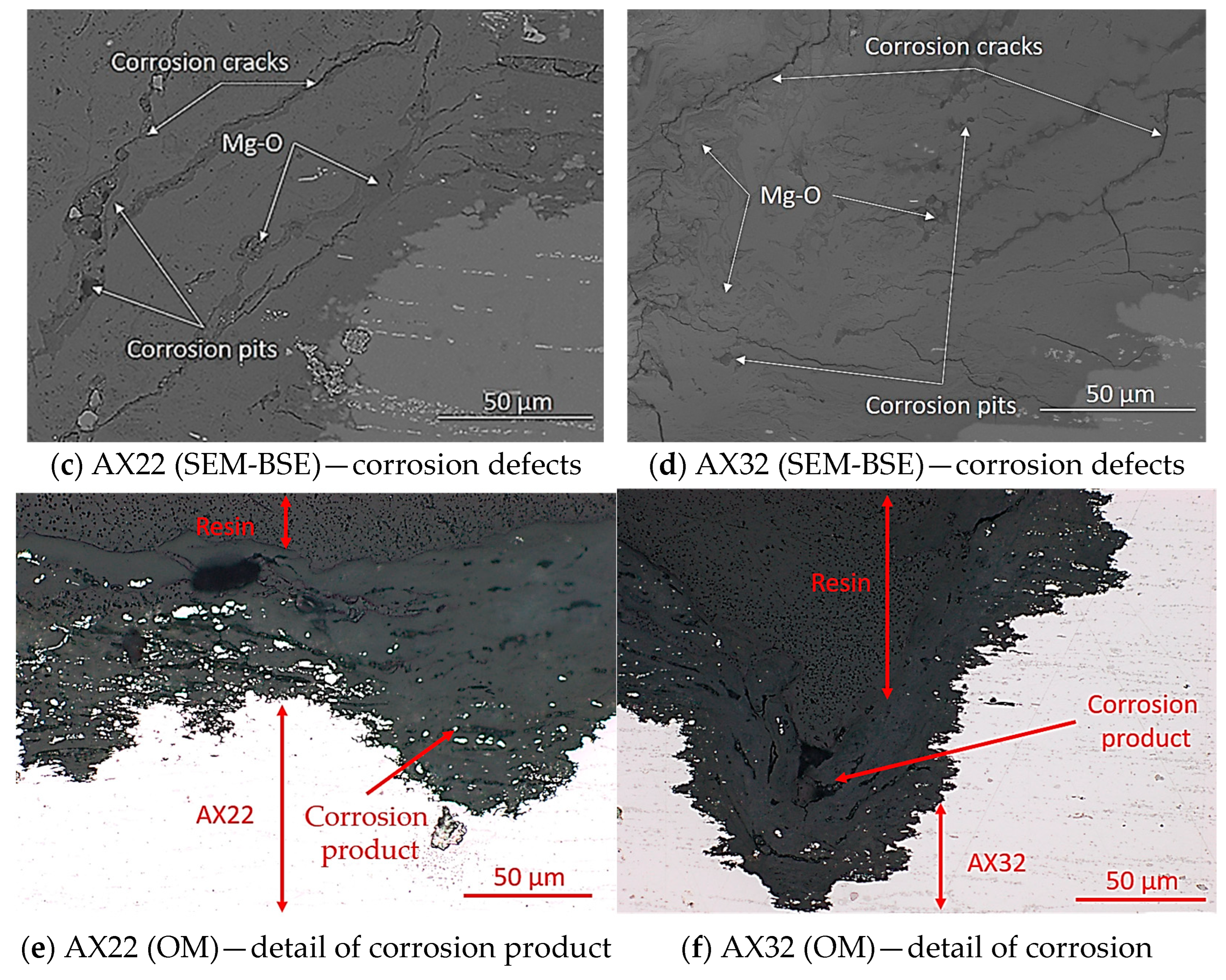
3.2.3. Corrosion Morphology Characterization
4. Conclusions
- ➢
- The microstructure of as-extruded AX22 and AX32 alloys is characterized by a fine-grain structure with Mg-Al-Ca phases arranged along the extrusion direction.
- ➢
- Intermetallic phases of the types Al-Mg-Ca, Al-Fe, and Al-Mn-Fe were found, while Mg17Al12 and Mg2Ca were not detected.
- ➢
- In AX32, the Mn transforms the Al-Fe phases to Al-Mn-Fe phases.
- ➢
- AX32 showed better corrosion resistance with values of icorr (9.81 μA cm−2) and rcorr (0.23 mm/y) in comparison with the AX22 alloy. This can be mainly attributed to the addition of Mn, binding the Fe in Al-Mn-Fe phases, and the higher content of Al and the slightly higher content of Ca.
- ➢
- Rp values measured via EIS confirmed that the AX32 alloy has better corrosion resistance compared to AX22.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, J.; She, J.; Chen, D.; Pan, F. Latest Research Advances on Magnesium and Magnesium Alloys Worldwide. J. Magnes. Alloys 2020, 8, 1–41. [Google Scholar] [CrossRef]
- Xu, S.W.; Oh-ishi, K.; Kamado, S.; Uchida, F.; Homma, T.; Hono, K. High-Strength Extruded Mg–Al–Ca–Mn Alloy. Scr. Mater. 2011, 65, 269–272. [Google Scholar] [CrossRef]
- Li, Z.T.; Zhang, X.D.; Zheng, M.Y.; Qiao, X.G.; Wu, K.; Xu, C.; Kamado, S. Effect of Ca/Al Ratio on Microstructure and Mechanical Properties of Mg-Al-Ca-Mn Alloys. Mater. Sci. Eng. A 2017, 682, 423–432. [Google Scholar] [CrossRef]
- Zeng, Z.R.; Zhu, Y.M.; Nie, J.F.; Xu, S.W.; Davies, C.H.J.; Birbilis, N. Effects of Calcium on Strength and Microstructural Evolution of Extruded Alloys Based on Mg-3Al-1Zn-0.3Mn. Metall. Mater. Trans. A 2019, 50, 4344–4363. [Google Scholar] [CrossRef]
- Nie, J.F.; Shin, K.S.; Zeng, Z.R. Microstructure, Deformation, and Property of Wrought Magnesium Alloys. Met. Mater. Trans. A 2020, 51, 6045–6109. [Google Scholar] [CrossRef]
- Gneiger, S.; Papenberg, N.P.; Arnoldt, A.R.; Schlögl, C.M.; Fehlbier, M. Investigations of High-Strength Mg–Al–Ca–Mn Alloys with a Broad Range of Ca+Al Contents. Materials 2021, 14, 5439. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and Advances in Magnesium Alloy Corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Liang, S.M.; Chen, R.S.; Blandin, J.J.; Suery, M.; Han, E.H. Thermal Analysis and Solidification Pathways of Mg–Al–Ca System Alloys. Mater. Sci. Eng. A 2008, 480, 365–372. [Google Scholar] [CrossRef]
- Huang, X.; Chino, Y.; Ueda, H.; Inoue, M.; Kido, F.; Matsumoto, T. Enhanced Mechanical Properties of Extruded Mg–9mass%Al–1mass%Zn–2mass%Ca Alloy. In Proceedings of the Magnesium Technology 2017; Solanki, K.N., Orlov, D., Singh, A., Neelameggham, N.R., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 269–274. [Google Scholar]
- Suzuki, A.; Saddock, N.D.; Jones, J.W.; Pollock, T.M. Solidification Paths and Eutectic Intermetallic Phases in Mg–Al–Ca Ternary Alloys. Acta Mater. 2005, 53, 2823–2834. [Google Scholar] [CrossRef]
- Zubair, M.; Felten, M.; Hallstedt, B.; Vega Paredes, M.; Abdellaoui, L.; Bueno Villoro, R.; Lipinska-Chwalek, M.; Ayeb, N.; Springer, H.; Mayer, J.; et al. Laves Phases in Mg-Al-Ca Alloys and Their Effect on Mechanical Properties. Mater. Des. 2023, 225, 111470. [Google Scholar] [CrossRef]
- Sanyal, S.; Paliwal, M.; Bandyopadhyay, T.K.; Mandal, S. Evolution of Microstructure, Phases and Mechanical Properties in Lean as-Cast Mg–Al–Ca–Mn Alloys under the Influence of a Wide Range of Ca/Al Ratio. Mater. Sci. Eng. A 2021, 800, 140322. [Google Scholar] [CrossRef]
- Zubair, M.; Sandlöbes, S.; Wollenweber, M.A.; Kusche, C.F.; Hildebrandt, W.; Broeckmann, C.; Korte-Kerzel, S. On the Role of Laves Phases on the Mechanical Properties of Mg-Al-Ca Alloys. Mater. Sci. Eng. A 2019, 756, 272–283. [Google Scholar] [CrossRef]
- Yim, C.D.; Kim, Y.M.; You, B.S. Effect of Ca Addition on the Corrosion Resistance of Gravity Cast AZ31 Magnesium Alloy. Mater. Trans. 2007, 48, 1023–1028. [Google Scholar] [CrossRef]
- Yang, J.; Peng, J.; Nyberg, E.A.; Pan, F. Effect of Ca Addition on the Corrosion Behavior of Mg–Al–Mn Alloy. Appl. Surf. Sci. 2016, 369, 92–100. [Google Scholar] [CrossRef]
- Wu, P.; Xu, F.; Deng, K.; Han, F.; Zhang, Z.; Gao, R. Effect of Extrusion on Corrosion Properties of Mg-2Ca-ΧAl (χ = 0, 2, 3, 5) Alloys. Corros. Sci. 2017, 127, 280–290. [Google Scholar] [CrossRef]
- Chaudry, U.M.; Farooq, A.; bin Tayyab, K.; Malik, A.; Kamran, M.; Kim, J.-G.; Li, C.; Hamad, K.; Jun, T.-S. Corrosion Behavior of AZ31 Magnesium Alloy with Calcium Addition. Corros. Sci. 2022, 199, 110205. [Google Scholar] [CrossRef]
- Liu, M.; Song, G.-L. Impurity Control and Corrosion Resistance of Magnesium–Aluminum Alloy. Corros. Sci. 2013, 77, 143–150. [Google Scholar] [CrossRef]
- ASM. Specialty Handbook: Magnesium and Magnesium Alloys. Available online: https://www.asminternational.org/asm-specialty-handbook-magnesium-and-magnesium-alloys/results/-/journal_content/56/06770G/PUBLICATION/ (accessed on 12 July 2023).
- Song, G.-L. Corrosion Behavior and Prevention Strategies for Magnesium (Mg) Alloys. In Corrosion Prevention of Magnesium Alloys; Elsevier: Amsterdam, The Netherlands, 2013; pp. 3–37. ISBN 978-0-85709-437-7. [Google Scholar]
- Morończyk, B.; Ura-Bińczyk, E.; Kuroda, S.; Jaroszewicz, J.; Molak, R.M. Microstructure and Corrosion Resistance of Warm Sprayed Titanium Coatings with Polymer Sealing for Corrosion Protection of AZ91E Magnesium Alloy. Surf. Coat. Technol. 2019, 363, 142–151. [Google Scholar] [CrossRef]
- Cesiulis, H.; Tsyntsaru, N.; Ramanavicius, A.; Ragoisha, G. The Study of Thin Films by Electrochemical Impedance Spectroscopy. In Nanostructures and Thin Films for Multifunctional Applications; Tiginyanu, I., Topala, P., Ursaki, V., Eds.; NanoScience and Technology; Springer International Publishing: Cham, Switzerland, 2016; pp. 3–42. ISBN 978-3-319-30197-6. [Google Scholar]
- Tkacz, J.; Slouková, K.; Minda, J.; Drábiková, J.; Fintová, S.; Doležal, P.; Wasserbauer, J. Influence of the Composition of the Hank’s Balanced Salt Solution on the Corrosion Behavior of AZ31 and AZ61 Magnesium Alloys. Metals 2017, 7, 465. [Google Scholar] [CrossRef]
- Amirudin, A.; Thieny, D. Application of Electrochemical Impedance Spectroscopy to Study the Degradation of Polymer-Coated Metals. Prog. Org. Coat. 1995, 26, 1–28. [Google Scholar] [CrossRef]
- Olivier, M.-G.; Poelm, M. Use of Electrochemical Impedance Spectroscopy (EIS) for the Evaluation of Electrocoatings Performances. In Recent Researches in Corrosion Evaluation and Protection; Shoja Razavi, R., Ed.; InTech: London, UK, 2012; ISBN 978-953-307-920-2. [Google Scholar]
- Liu, X.; Xue, J.; Liu, S. Discharge and Corrosion Behaviors of the α-Mg and β-Li Based Mg Alloys for Mg-Air Batteries at Different Current Densities. Mater. Des. 2018, 160, 138–146. [Google Scholar] [CrossRef]
- Kajánek, D.; Pastorek, F.; Hadzima, B.; Bagherifard, S.; Jambor, M.; Belány, P.; Minárik, P. Impact of Shot Peening on Corrosion Performance of AZ31 Magnesium Alloy Coated by PEO: Comparison with Conventional Surface Pre-Treatments. Surf. Coat. Technol. 2022, 446, 128773. [Google Scholar] [CrossRef]
- Guadarrama-Muñoz, F.; Mendoza-Flores, J.; Duran-Romero, R.; Genesca, J. Electrochemical Study on Magnesium Anodes in NaCl and CaSO4–Mg(OH)2 Aqueous Solutions. Electrochim. Acta 2006, 51, 1820–1830. [Google Scholar] [CrossRef]
- Han, L.; Li, X.; Bai, J.; Xue, F.; Zheng, Y.; Chu, C. Effects of Flow Velocity and Different Corrosion Media on the in Vitro Bio-Corrosion Behaviors of AZ31 Magnesium Alloy. Mater. Chem. Phys. 2018, 217, 300–307. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, C.; Liu, S.; Guan, K.; Li, M.-X.; Zhang, L.-Y.; Wang, H.-Y. New Insights on Corrosion Behavior of Aging Precipitates in Dilute Mg-Al-Ca Alloy by Experiments and First-Principles Calculations. Corros. Sci. 2023, 220, 111254. [Google Scholar] [CrossRef]
- Guo, Y.; Rogov, A.; Hird, A.; Mingo, B.; Matthews, A.; Yerokhin, A. Plasma Electrolytic Oxidation of Magnesium by Sawtooth Pulse Current. Surf. Coat. Technol. 2022, 429, 127938. [Google Scholar] [CrossRef]
- Feliu, S. Electrochemical Impedance Spectroscopy for the Measurement of the Corrosion Rate of Magnesium Alloys: Brief Review and Challenges. Metals 2020, 10, 775. [Google Scholar] [CrossRef]
- Peng, L.; Zeng, G.; Su, T.C.; Yasuda, H.; Nogita, K.; Gourlay, C.M. Al8Mn5 Particle Settling and Interactions with Oxide Films in Liquid AZ91 Magnesium Alloys. JOM 2019, 71, 2235–2244. [Google Scholar] [CrossRef]
- Peng, L.; Zeng, G.; Xian, J.; Gourlay, C.M. Al–Mn–Fe Intermetallic Formation in AZ91 Magnesium Alloys: Effects of Impurity Iron. Intermetallics 2022, 142, 107465. [Google Scholar] [CrossRef]
- Jeong, Y.S.; Kim, W.J. Enhancement of Mechanical Properties and Corrosion Resistance of Mg–Ca Alloys through Microstructural Refinement by Indirect Extrusion. Corros. Sci. 2014, 82, 392–403. [Google Scholar] [CrossRef]
- Eliezer, D.; Uzan, P.; Aghion, E. Effect of Second Phases on the Corrosion Behavior of Magnesium Alloys. MSF 2003, 419–422, 857–866. [Google Scholar] [CrossRef]
- Veys-Renaux, D.; Rocca, E.; Martin, J.; Henrion, G. Initial Stages of AZ91 Mg Alloy Micro-Arc Anodizing: Growth Mechanisms and Effect on the Corrosion Resistance. Electrochim. Acta 2014, 124, 36–45. [Google Scholar] [CrossRef]
- Mingo, B.; Mohedano, M.; Blawert, C.; Del Olmo, R.; Hort, N.; Arrabal, R. Role of Ca on the Corrosion Resistance of Mg–9Al and Mg–9Al–0.5Mn Alloys. J. Alloys Compd. 2019, 811, 151992. [Google Scholar] [CrossRef]
- Bahmani, A.; Arthanari, S.; Shin, K.S. Corrosion Behavior of Mg–Mn–Ca Alloy: Influences of Al, Sn and Zn. J. Magnes. Alloys 2019, 7, 38–46. [Google Scholar] [CrossRef]
- Chen, T.; Yuan, Y.; Liu, T.; Li, D.; Tang, A.; Chen, X.; Schmid-Fetzer, R.; Pan, F. Effect of Mn Addition on Melt Purification and Fe Tolerance in Mg Alloys. JOM 2021, 73, 892–902. [Google Scholar] [CrossRef]
- Liu, B.-C.; Zhang, S.; Xiong, H.-W.; Dai, W.-H.; Ma, Y.-L. Effect of Al Content on the Corrosion Behavior of Extruded Dilute Mg–Al–Ca–Mn Alloy. Acta Metall. Sin. 2023, 36, 77–90. [Google Scholar] [CrossRef]
- Yin, T.; Sun, X.; Wang, Y.; Zhao, Y.; Wang, S.; Liu, L.; Chen, H. Corrosion Characteristics of Anchor Cables in Electrolytic Corrosion Test and the Applicability of the Test Method in Study of Anchor Cable Corrosion. Adv. Civ. Eng. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Gomes, M.P.; Costa, I.; Pébère, N.; Rossi, J.L.; Tribollet, B.; Vivier, V. On the Corrosion Mechanism of Mg Investigated by Electrochemical Impedance Spectroscopy. Electrochim. Acta 2019, 306, 61–70. [Google Scholar] [CrossRef]
- Samir, A.; Salem, H.; Abdelkawy, M. Optimization of Two Charge Transfer Reactions for Colorimetric Determination of Two Beta 2 Agonist Drugs, Salmeterol Xinafoate and Salbutamol, in Pharmaceutical and Biological Samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 269, 120747. [Google Scholar] [CrossRef]
- Yin, S.; Duan, W.; Liu, W.; Wu, L.; Yu, J.; Zhao, Z.; Liu, M.; Wang, P.; Cui, J.; Zhang, Z. Influence of Specific Second Phases on Corrosion Behaviors of Mg-Zn-Gd-Zr Alloys. Corros. Sci. 2020, 166, 108419. [Google Scholar] [CrossRef]
- Mandal, M.; Moon, A.P.; Deo, G.; Mendis, C.L.; Mondal, K. Corrosion Behavior of Mg–2.4Zn Alloy Micro-Alloyed with Ag and Ca. Corros. Sci. 2014, 78, 172–182. [Google Scholar] [CrossRef]
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The Development of Binary Mg–Ca Alloys for Use as Biodegradable Materials within Bone. Biomaterials 2008, 29, 1329–1344. [Google Scholar] [CrossRef]


| Alloy | Mg [wt. %] | Al [wt. %] | Ca [wt. %] | Mn [wt. %] | Fe [ppm] | Ni [ppm] |
|---|---|---|---|---|---|---|
| AX22 | balance | 1.88 | 1.88 | 0.00 | 340 | 30 |
| AX32 | balance | 3.02 | 2.06 | 0.46 | 150 | 20 |
| Alloy | Ecorr [mV vs. SCE] | icorr [μA cm−2] | βc [mV dec−1] | βa [mV dec−1] | Corrosion Rate [mm y−1] |
|---|---|---|---|---|---|
| AX22 | −1 475 ± 12 | 20.40 ± 2.34 | 226 ± 7 | 158 ± 7 | 0.47 ± 0.05 |
| AX32 | −1 482 ± 11 | 9.81 ± 1.02 | 195 ± 11 | 149 ± 9 | 0.23 ± 0.03 |
| Time | Rs (Ω·cm2) | R1 (Ω·cm2) | RL (Ω·cm2) | Rp (Ω·cm2) | L (H·cm2) | CPE1(F·sn−1·10−6) | n1 |
|---|---|---|---|---|---|---|---|
| 1 h | 657 ± 12 | 1120 ± 102 | 1820 ± 132 | 693 ± 58 | 10,501 ± 136 | 11.3 ± 0.2 | 0.9 |
| 2 h | 644 ± 18 | 726 ± 33 | 1087 ± 115 | 435 ± 26 | 5215 ± 99 | 24.9 ± 4.3 | 0.9 |
| 4 h | 636 ± 15 | 551 ± 41 | 735 ± 44 | 315 ± 21 | 3383 ± 123 | 76.3 ± 0.1 | 0.8 |
| 8 h | 628 ± 11 | 532 ± 42 | 1163 ± 112 | 365 ± 31 | 1163 ± 55 | 87.4 ± 1.5 | 1 |
| 12 h | 634 ± 20 | 493 ± 47 | 1100 ± 159 | 340 ± 36 | 11,649 ± 63 | 91.5 ± 3.4 | 1 |
| 24 h | 584 ± 13 | 377 ± 38 | 983 ± 76 | 273 ± 25 | 10,755 ± 42 | 80.9 ± 3.1 | 1 |
| 48 h | 522 ± 14 | 243 ± 24 | 829 ± 23 | 188 ± 12 | 7885 ± 49 | 76.5 ± 5.2 | 1 |
| 96 h | 448 ± 15 | 155 ± 12 | 536 ± 41 | 120 ± 9 | 5563 ± 21 | 77.2 ± 1.1 | 1 |
| 168 h | 432 ± 23 | 128 ± 18 | 659 ± 28 | 107 ± 11 | 3728 ± 35 | 85.8 ± 7.2 | 0.9 |
| Time | Rs (Ω·cm2) | R1 (Ω·cm2) | R2 (Ω·cm2) | RL (Ω·cm2) | Rp (Ω·cm2) | L (H·cm2) | CPE1 (F·sn−1·10−6) | CPE2 (F·sn−1·10−6) | n1 | n2 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 h | 731 ± 20 | 2283 ± 125 | 1120 ± 182 | - | 3403 ± 307 | - | - | 950.9 ± 10.2 | 0.9 | 0.9 |
| 2 h | 727 ± 19 | 2946 ± 150 | 1552 ± 175 | - | 4498 ± 325 | - | - | 804.2 ± 15.3 | 0.9 | 0.9 |
| 4 h | 714 ± 24 | 5326 ± 77 | 3434 ± 163 | - | 8760 ± 240 | - | 9.5 ± 0.1 | 495.7 ± 7.8 | 0.9 | 0.7 |
| 8 h | 707 ± 20 | 5012 ± 111 | 4215 ± 192 | - | 9227 ± 303 | - | 11.3 ± 1.2 | 514.1 ± 6.1 | 0.9 | 0.8 |
| 12 h | 703 ± 18 | 4541 ± 114 | 3064 ± 120 | - | 7605 ± 234 | - | 12.1 ± 0.7 | 666.2 ± 7.3 | 0.9 | 0.8 |
| 24 h | 674 ± 22 | 5835 ± 102 | - | - | 5835 ± 102 | - | 17.1 ± 0.1 | - | 0.9 | - |
| 48 h | 638 ± 15 | 5449 ± 67 | - | 6 548 ± 62 | 2974 ± 32 | 38,460 ± 420 | 23.4 ± 0.4 | - | 0.9 | - |
| 96 h | 566 ± 13 | 5962 ± 78 | - | 9 602 ± 85 | 3678 ± 41 | 77,624 ± 231 | 42.2 ± 2.3 | - | 0.8 | - |
| 168 h | 532 ± 14 | 5455 ± 82 | - | - | 5455 ± 82 | - | 37.4 ± 1.8 | - | 0.9 | - |
| Alloy | Mg [wt. %] | O [wt. %] | Al [wt. %] | Ca [wt. %] | C [wt. %] |
|---|---|---|---|---|---|
| AX22 | 22.2 | 59.8 | 2.1 | 0.9 | 15.1 |
| AX32 | 76.0 | 1.3 | 0.5 | 0.4 | 21.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sovík, J.; Hadzima, B.; Papenberg, N.P.; Arnoldt, A.R.; Gneiger, S. Investigations of Electrochemical Characteristics of Mg-Al-Ca Alloys. Crystals 2023, 13, 1684. https://doi.org/10.3390/cryst13121684
Sovík J, Hadzima B, Papenberg NP, Arnoldt AR, Gneiger S. Investigations of Electrochemical Characteristics of Mg-Al-Ca Alloys. Crystals. 2023; 13(12):1684. https://doi.org/10.3390/cryst13121684
Chicago/Turabian StyleSovík, Ján, Branislav Hadzima, Nikolaus Peter Papenberg, Aurel Ramon Arnoldt, and Stefan Gneiger. 2023. "Investigations of Electrochemical Characteristics of Mg-Al-Ca Alloys" Crystals 13, no. 12: 1684. https://doi.org/10.3390/cryst13121684






