Nano Nickel-Zirconia: An Effective Catalyst for the Production of Biodiesel from Waste Cooking Oil
Abstract
:1. Introduction
2. Experimental Section
2.1. Catalyst Preparation
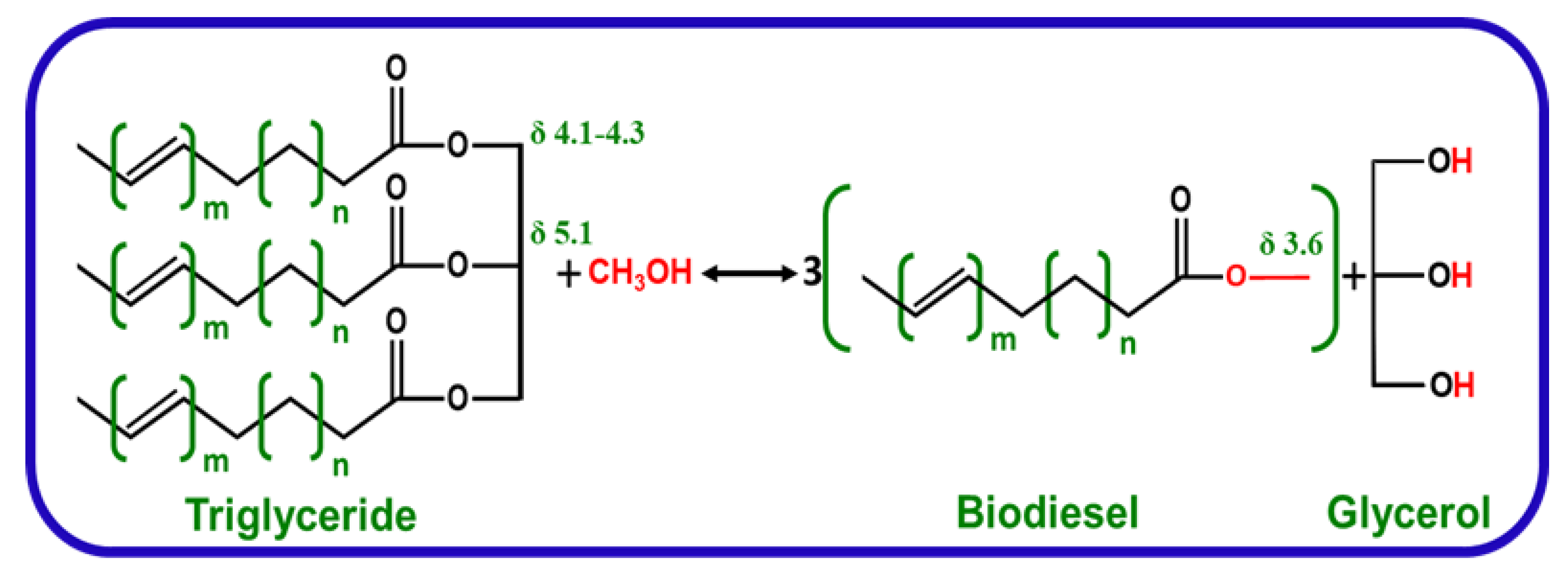
2.2. Transesterification Experiment
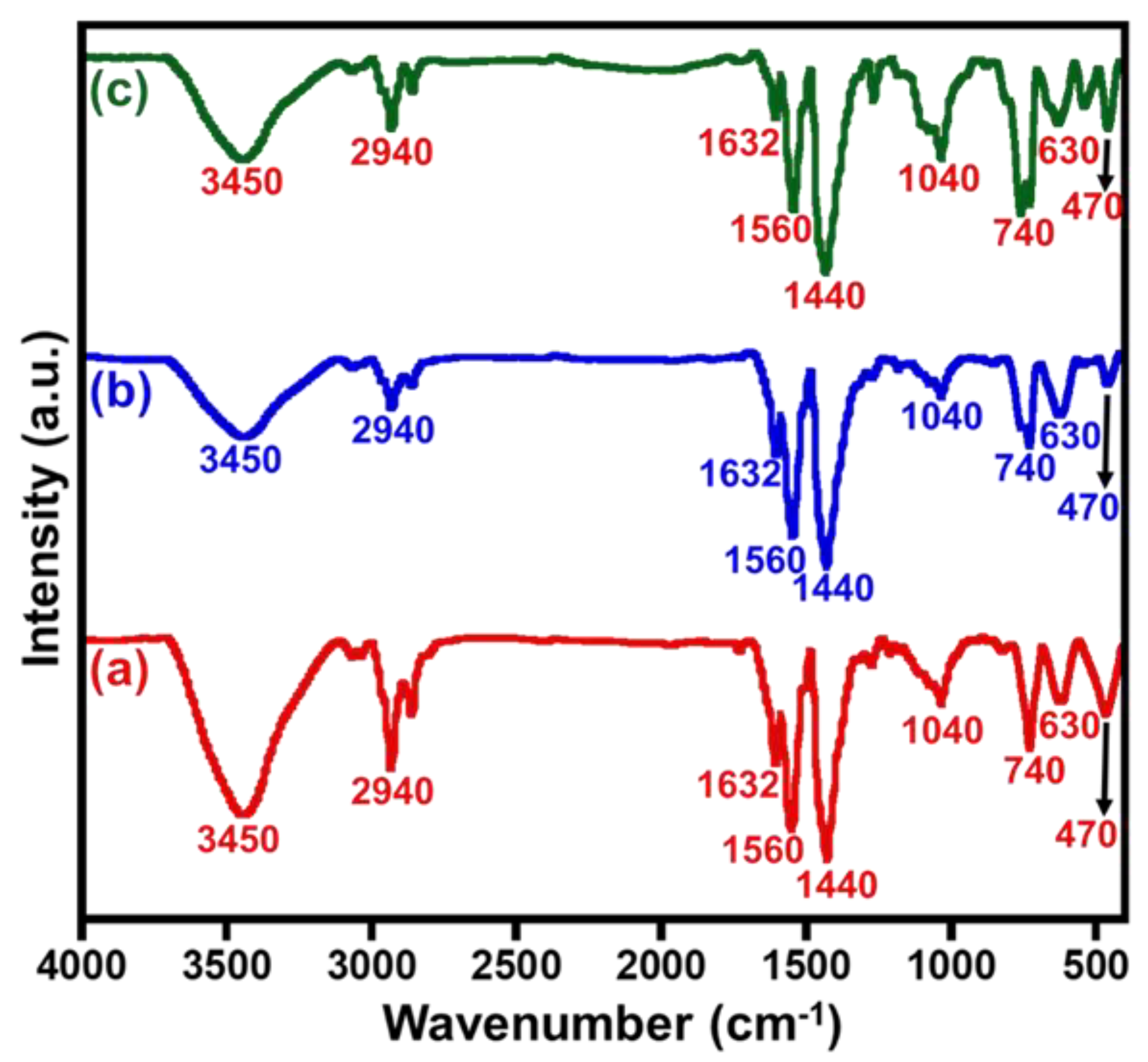
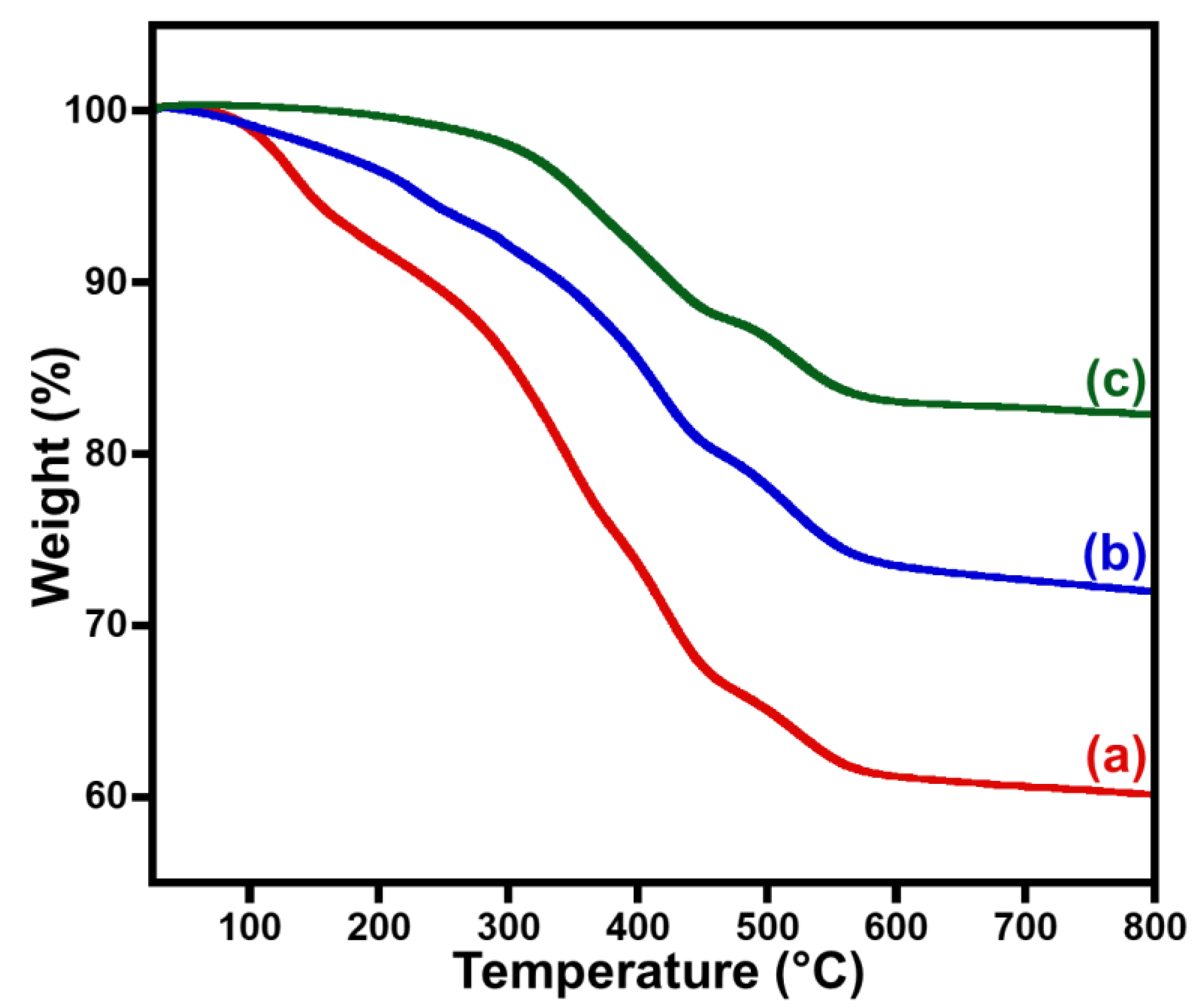
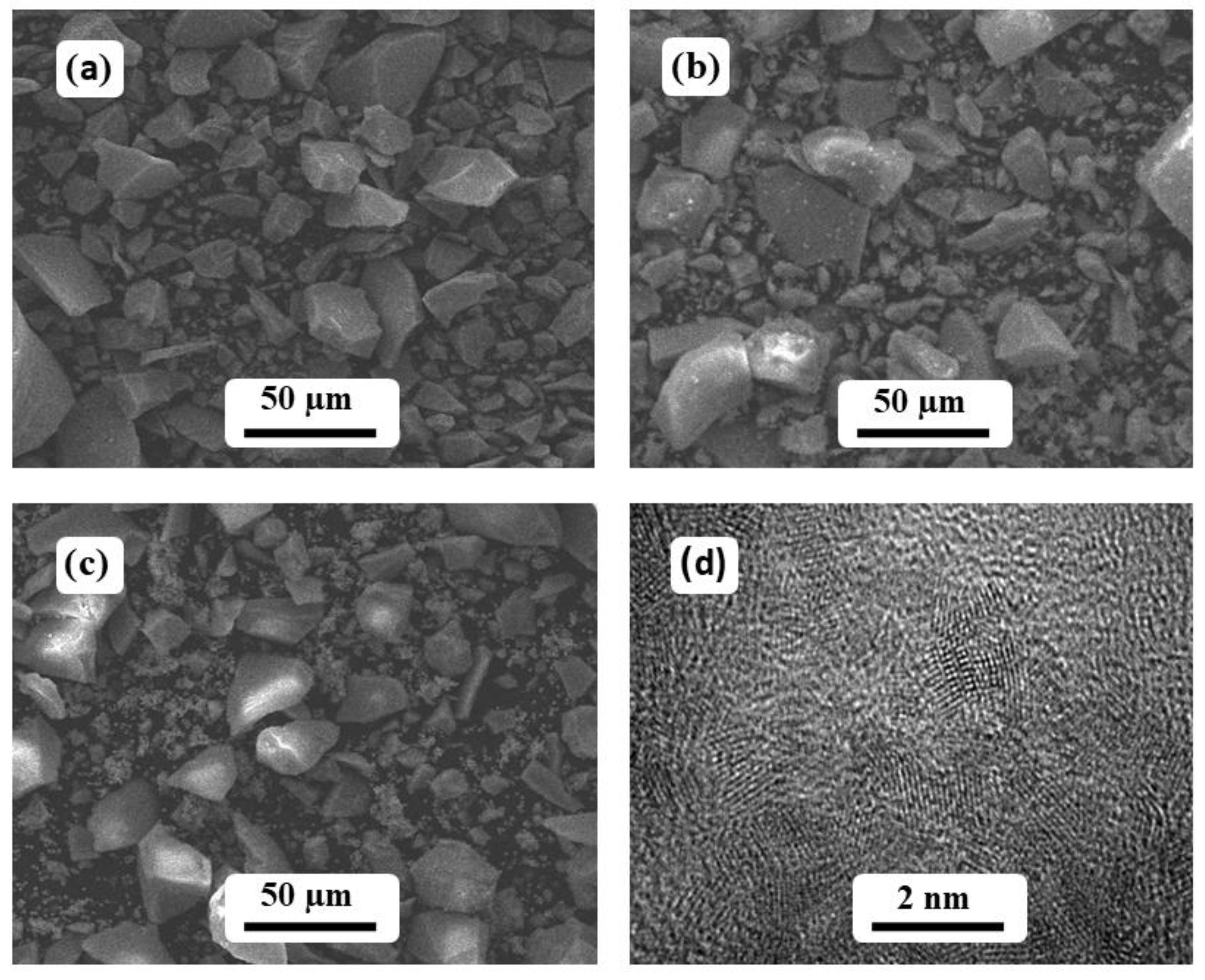
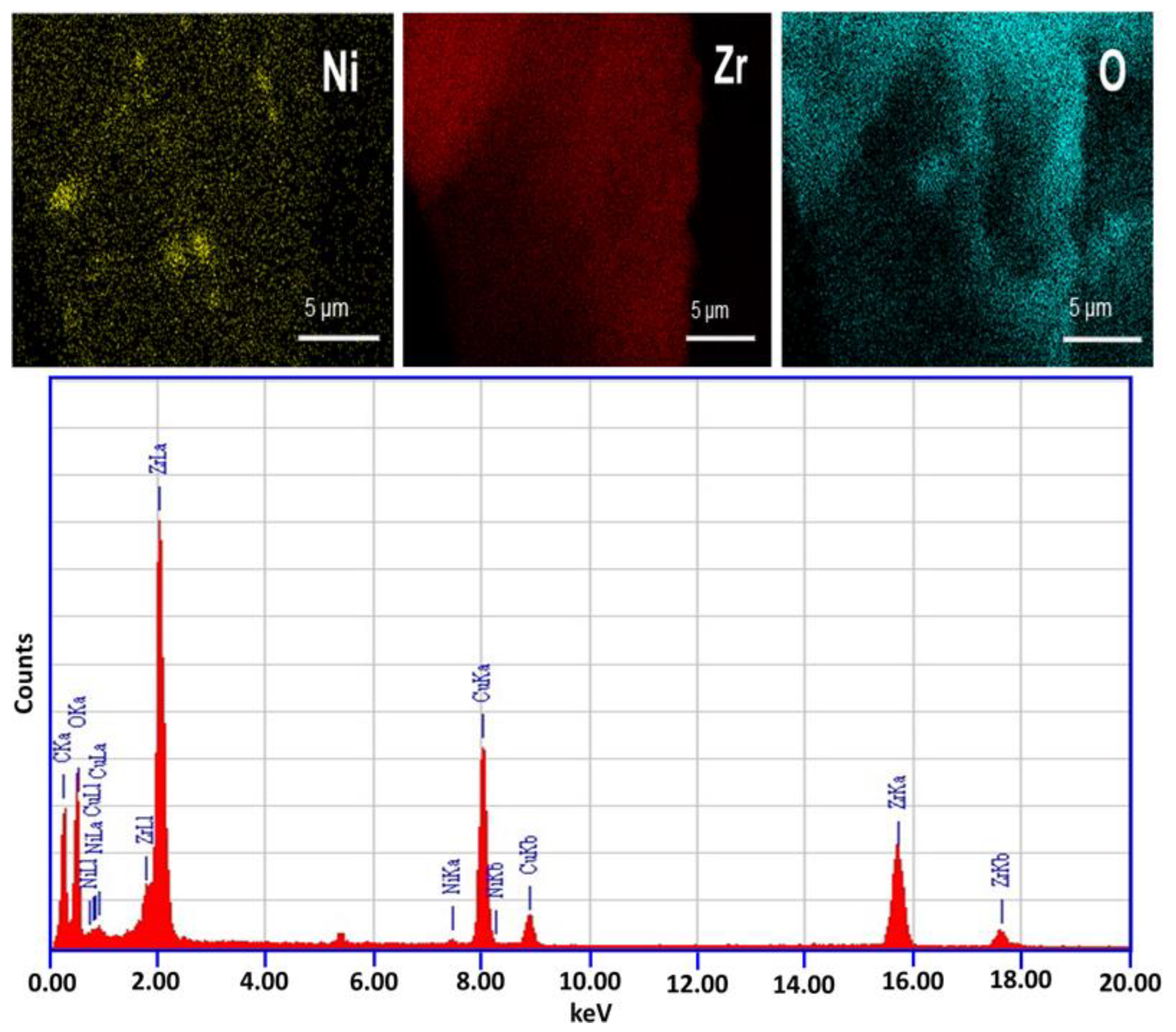
3. Results and Discussion
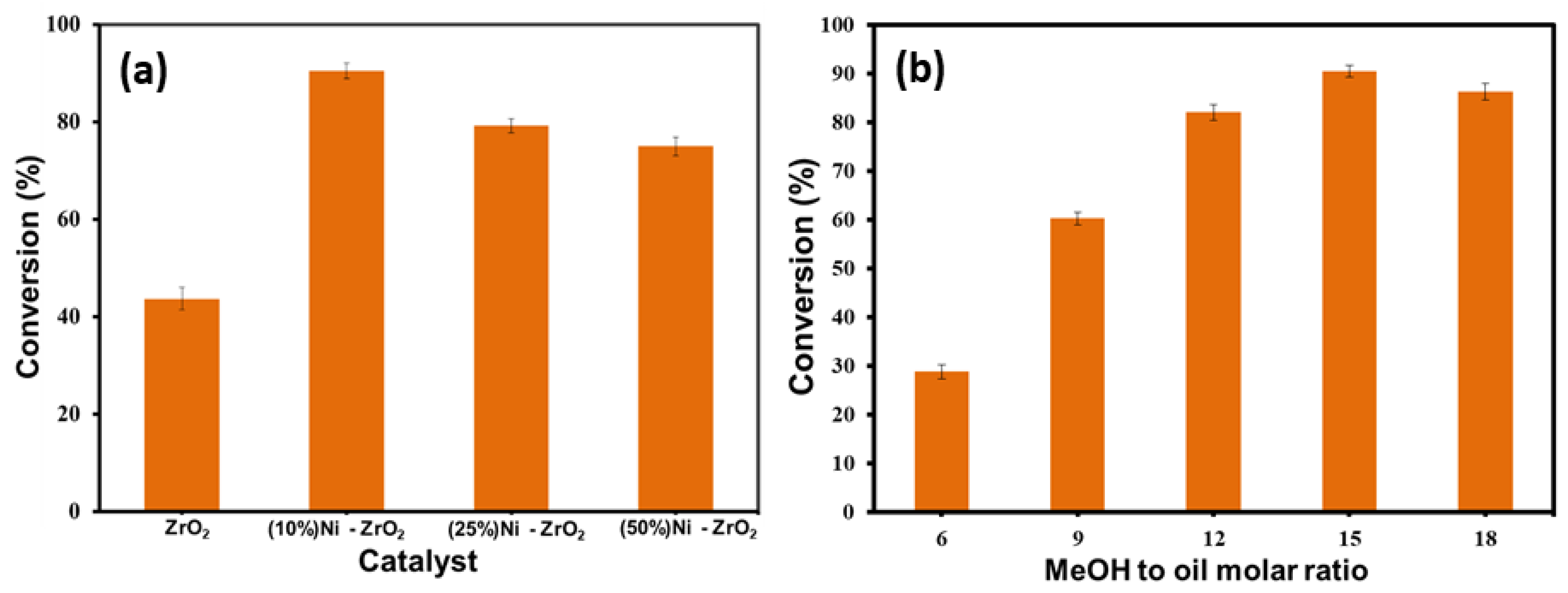
3.1. Catalytic Investigation
3.2. Impact of Catalyst Loading
3.3. Impact of Molar Ratio
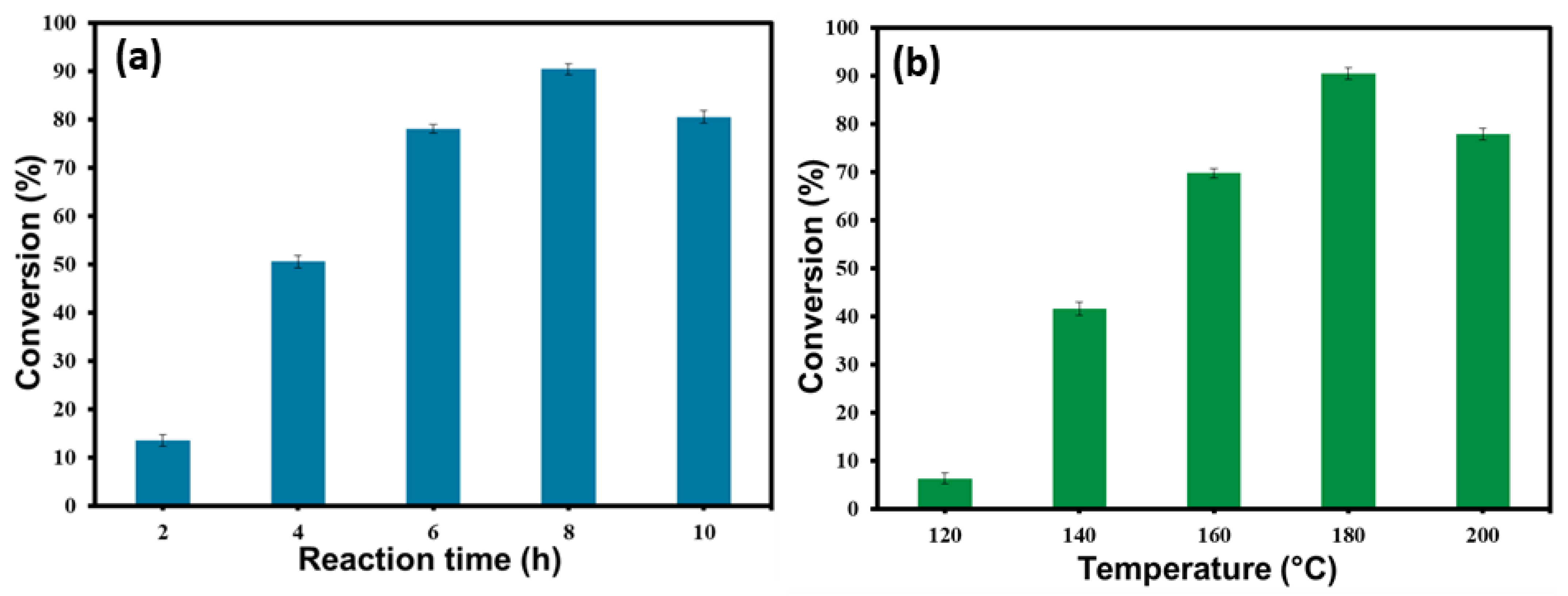
3.4. Impact of Reaction Time
3.5. Impact of Reaction Temperature
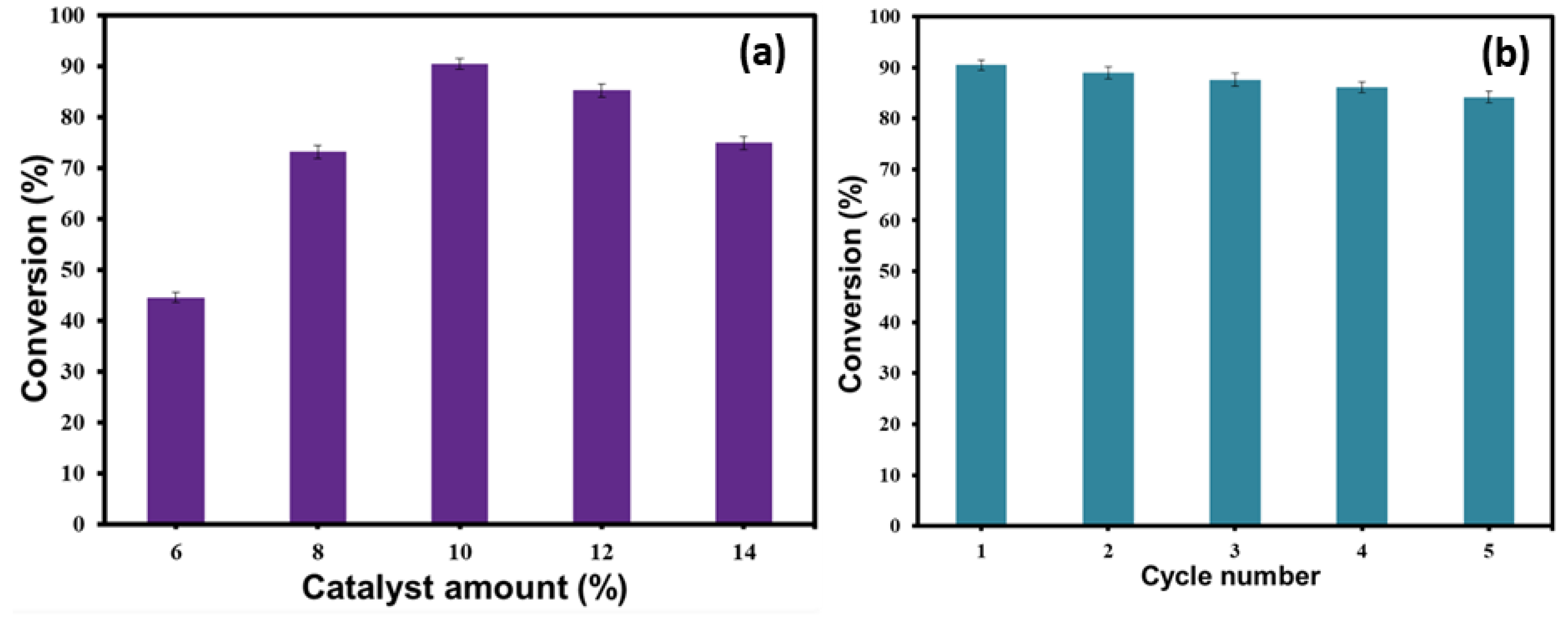
3.6. Impact of Catalyst to Oil Ratio
3.7. Catalyst Recovery Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, C.H.; Qureshi, A.S.; Mbadinga, S.M.; Liu, J.-F.; Yang, S.-Z.; Mu, B.-Z. Biodiesel production from waste cooking oil using onsite produced purified lipase from Pseudomonas aeruginosa FW_SH-1: Central composite design approach. Renew. Energy 2017, 109, 93–100. [Google Scholar] [CrossRef]
- Ahmad, T.; Danish, M.; Kale, P.; Geremew, B.; Adeloju, S.B.; Nizami, M.; Ayoub, M. Optimization of process variables for biodiesel production by transesterification of flaxseed oil and produced biodiesel characterizations. Renew. Energy 2019, 139, 1272–1280. [Google Scholar] [CrossRef]
- Macina, A.; de Medeiros, T.V.; Naccache, R. A carbon dot-catalyzed transesterification reaction for the production of biodiesel. J. Mater. Chem. A 2019, 7, 23794–23802. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Uprety, B.K.; Chaiwong, W.; Ewelike, C.; Rakshit, S.K. Biodiesel production using heterogeneous catalysts including wood ash and the importance of enhancing byproduct glycerol purity. Energy Convers. Manag. 2016, 115, 191–199. [Google Scholar] [CrossRef]
- Hamze, H.; Akia, M.; Yazdani, F. Optimization of biodiesel production from the waste cooking oil using response surface methodology. Process Saf. Environ. Prot. 2015, 94, 1–10. [Google Scholar] [CrossRef]
- Lee, A.F.; Bennett, J.A.; Manayil, J.C.; Wilson, K. Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification. Chem. Soc. Rev. 2014, 43, 7887–7916. [Google Scholar] [CrossRef] [Green Version]
- Ganjehkaviri, A.; Jaafar, M.; Nazri, M.; Hosseini, S.E.; Musthafa, A.B. Performance evaluation of palm oil-based biodiesel combustion in an oil burner. Energies 2016, 9, 97. [Google Scholar] [CrossRef]
- Singh, S.; Singh, D. Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: A review. Renew. Sustain. Energy Rev. 2010, 14, 200–216. [Google Scholar] [CrossRef]
- Mofijur, M.; Masjuki, H.H.; Kalam, M.; Atabani, A.E.; Fattah, I.R.; Mobarak, H. Comparative evaluation of performance and emission characteristics of Moringa oleifera and Palm oil based biodiesel in a diesel engine. Ind. Crops Prod. 2014, 53, 78–84. [Google Scholar] [CrossRef]
- Mihaela, P.; Josef, R.; Monica, N.; Rudolf, Z. Perspectives of safflower oil as biodiesel source for South Eastern Europe (comparative study: Safflower, soybean and rapeseed). Fuel 2013, 111, 114–119. [Google Scholar] [CrossRef]
- Onoji, S.E.; Iyuke, S.E.; Igbafe, A.I.; Nkazi, D.B. Rubber seed oil: A potential renewable source of biodiesel for sustainable development in sub-Saharan Africa. Energy Convers. Manag. 2016, 110, 125–134. [Google Scholar] [CrossRef]
- Teo, S.H.; Rashid, U.; Taufiq-Yap, Y.H. Biodiesel production from crude Jatropha Curcas oil using calcium based mixed oxide catalysts. Fuel 2014, 136, 244–252. [Google Scholar] [CrossRef]
- Dueso, C.; Muñoz, M.; Moreno, F.; Arroyo, J.; Gil-Lalaguna, N.; Bautista, A.; Gonzalo, A.; Sánchez, J.L. Performance and emissions of a diesel engine using sunflower biodiesel with a renewable antioxidant additive from bio-oil. Fuel 2018, 234, 276–285. [Google Scholar] [CrossRef] [Green Version]
- Veljković, V.B.; Biberdžić, M.O.; Banković-Ilić, I.B.; Djalović, I.G.; Tasić, M.B.; Nježić, Z.B.; Stamenković, O.S. Biodiesel production from corn oil: A review. Renew. Sustain. Energy Rev. 2018, 91, 531–548. [Google Scholar] [CrossRef]
- Mansir, N.; Teo, S.H.; Rabiu, I.; Taufiq-Yap, Y.H. Effective biodiesel synthesis from waste cooking oil and biomass residue solid green catalyst. Chem. Eng. J. 2018, 347, 137–144. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H. Progress in biodiesel processing. Appl. Energy 2010, 87, 1815–1835. [Google Scholar] [CrossRef]
- Sánchez-Arreola, E.; Bach, H.; Hernández, L.R. Biodiesel production from Cascabela ovata seed oil. Bioresour. Technol. Rep. 2019, 7, 100220. [Google Scholar] [CrossRef]
- Verziu, M.; Coman, S.M.; Richards, R.; Parvulescu, V.I. Transesterification of vegetable oils over CaO catalysts. Catal. Today 2011, 167, 64–70. [Google Scholar] [CrossRef]
- Lacome, T.; Hillion, G.; Delfort, B.; Revel, R.; Leporq, S.; Acakpo, G. Process for Transesterification of Vegetable or Animal Oils Using Heterogeneous Catalysts Based on Titanium, Zirconium or Antimony and Aluminium. U.S. Patent 7592470B2, 26 May 2004. [Google Scholar]
- Cho, H.J.; Kim, S.H.; Hong, S.W.; Yeo, Y.-K. A single step non-catalytic esterification of palm fatty acid distillate (PFAD) for biodiesel production. Fuel 2012, 93, 373–380. [Google Scholar] [CrossRef]
- Zou, H.; Lei, M. Optimum process and kinetic study of Jatropha curcas oil pre-esterification in ultrasonical field. J. Taiwan Inst. Chem. Eng. 2012, 43, 730–735. [Google Scholar] [CrossRef]
- Wang, Z.-M.; Lee, J.-S.; Park, J.-Y.; Wu, C.-Z.; Yuan, Z.-H. Optimization of biodiesel production from trap grease via acid catalysis. Korean J. Chem. Eng. 2008, 25, 670–674. [Google Scholar] [CrossRef]
- Ni, J.; Meunier, F. Esterification of free fatty acids in sunflower oil over solid acid catalysts using batch and fixed bed-reactors. Appl. Catal. A Gen. 2007, 333, 122–130. [Google Scholar] [CrossRef]
- Chung, Z.L.; Tan, Y.H.; San Chan, Y.; Kansedo, J.; Mubarak, N.; Ghasemi, M.; Abdullah, M.O. Life cycle assessment of waste cooking oil for biodiesel production using waste chicken eggshell derived CaO as catalyst via transesterification. Biocatal. Agric. Biotechnol. 2019, 21, 101317. [Google Scholar] [CrossRef]
- Asri, N.P.; Machmudah, S.; Budikarjono, K.; Roesyadi, A.; Goto, M. Palm oil transesterification in sub-and supercritical methanol with heterogeneous base catalyst. Chem. Eng. Process. 2013, 72, 63–67. [Google Scholar] [CrossRef]
- Wong, Y.; Tan, Y.; Taufiq-Yap, Y.; Ramli, I. Effect of calcination temperatures of CaO/Nb2O5 mixed oxides catalysts on biodiesel production. Sains Malays. 2014, 43, 783–790. [Google Scholar]
- Gurunathan, B.; Ravi, A. Biodiesel production from waste cooking oil using copper doped zinc oxide nanocomposite as heterogeneous catalyst. Bioresour. Technol. 2015, 188, 124–127. [Google Scholar] [CrossRef]
- Mohadesi, M.; Aghel, B.; Maleki, M.; Ansari, A. Production of biodiesel from waste cooking oil using a homogeneous catalyst: Study of semi-industrial pilot of microreactor. Renew. Energy 2019, 136, 677–682. [Google Scholar] [CrossRef]
- Putra, M.D.; Irawan, C.; Ristianingsih, Y.; Nata, I.F. A cleaner process for biodiesel production from waste cooking oil using waste materials as a heterogeneous catalyst and its kinetic study. J. Clean. Prod. 2018, 195, 1249–1258. [Google Scholar] [CrossRef]
- Sadaf, S.; Iqbal, J.; Ullah, I.; Bhatti, H.N.; Nouren, S.; Nisar, J.; Iqbal, M. Biodiesel production from waste cooking oil: An efficient technique to convert waste into biodiesel. Sustain. Cities Soc. 2018, 41, 220–226. [Google Scholar]
- Asri, N.P.; Sari, D.A.P.; Poedjojono, B.; Suprapto, A. Utilization of waste cooking oil for biodiesel production using alumina supported Base catalyst. In Proceedings of the 3rd International Conference on Biological, Chemical and Environmental Sciences, (BCES-2015), Kuala Lumpur, Malaysia, 21–22 September 2015. [Google Scholar]
- Zabeti, M.; Daud, W.M.A.W.; Aroua, M.K. Activity of solid catalysts for biodiesel production: A review. Fuel Process. Technol. 2009, 90, 770–777. [Google Scholar] [CrossRef]
- Chung, K.-H.; Chang, D.-R.; Park, B.-G. Removal of free fatty acid in waste frying oil by esterification with methanol on zeolite catalysts. Bioresour. Technol. 2008, 99, 7438–7443. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.C.C.; Ribeiro, N.F.; Souza, M.M.; Aranda, D.A. Biodiesel production from soybean oil and methanol using hydrotalcites as catalyst. Fuel Process. Technol. 2010, 91, 205–210. [Google Scholar] [CrossRef]
- Braos-Garcıa, P.; Maireles-Torres, P.; Rodrıguez-Castellón, E.; Jiménez-López, A. Gas-phase hydrogenation of acetonitrile on zirconium-doped mesoporous silica-supported nickel catalysts. J. Mol. Catal. A Chem. 2003, 193, 185–196. [Google Scholar] [CrossRef]
- Znak, L.; Stołecki, K.; Zieliński, J. The effect of cerium, lanthanum and zirconium on nickel/alumina catalysts for the hydrogenation of carbon oxides. Catal. Today 2005, 101, 65–71. [Google Scholar] [CrossRef]
- Rodrıguez-Castellón, E.; Dıaz, L.; Braos-Garcıa, P.; Mérida-Robles, J.; Maireles-Torres, P.; Jiménez-López, A.; Vaccari, A. Nickel-impregnated zirconium-doped mesoporous molecular sieves as catalysts for the hydrogenation and ring-opening of tetralin. Appl. Catal. A Gen. 2003, 240, 83–94. [Google Scholar] [CrossRef]
- Jalowiecki-Duhamel, L.; Zarrou, H.; D’Huysser, A. Low temperature hydrogen production from methane on cerium nickel-and zirconium-based oxyhydrides. Catal. Today 2008, 138, 124–129. [Google Scholar] [CrossRef]
- Assal, M.E.; Shaik, M.R.; Kuniyil, M.; Khan, M.; Al-Warthan, A.; Siddiqui, M.R.H.; Khan, S.M.; Tremel, W.; Tahir, M.N.; Adil, S.F. A highly reduced graphene oxide/ZrOx–MnCO3 or–Mn2O3 nanocomposite as an efficient catalyst for selective aerial oxidation of benzylic alcohols. RSC Adv. 2017, 7, 55336–55349. [Google Scholar] [CrossRef] [Green Version]
- Gohel, V.D.; Rajput, A.; Gahlot, S.; Kulshrestha, V. Removal of Toxic Metal Ions From Potable Water by Graphene Oxide Composites. Macromol. Symp. 2017, 376, 1700050. [Google Scholar] [CrossRef]
- Blair, A.C.; Weston, L.A.; Nissen, S.J.; Brunk, G.R.; Hufbauer, R.A. The importance of analytical techniques in allelopathy studies with the reported allelochemical catechin as an example. Biol. Invasions 2009, 11, 325–332. [Google Scholar] [CrossRef]
- Chen, H.; Meng, Y.; Jia, S.; Hua, W.; Cheng, Y.; Lu, J.; Wang, H. Graphene oxide modified waste newspaper for removal of heavy metal ions and its application in industrial wastewater. Mater. Chem. Phys. 2020, 244, 122692. [Google Scholar] [CrossRef]
- Smirnov, A.; Pinargote, S.; Washington, N.; Peretyagin, N.; Pristinskiy, Y.; Peretyagin, P.; Bartolomé, J.F. Zirconia Reduced Graphene Oxide Nano-Hybrid Structure Fabricated by the Hydrothermal Reaction Method. Materials 2020, 13, 687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurushantha, K.; Anantharaju, K.; Renuka, L.; Sharma, S.; Nagaswarupa, H.; Prashantha, S.; Vidya, Y.; Nagabhushana, H. New green synthesized reduced graphene oxide–ZrO2 composite as high performance photocatalyst under sunlight. RSC Adv. 2017, 7, 12690–12703. [Google Scholar] [CrossRef] [Green Version]
- Adil, S.F.; Assal, M.E.; Shaik, M.R.; Kuniyil, M.; AlOtaibi, N.M.; Khan, M.; Sharif, M.; Alam, M.M.; Al-Warthan, A.; Mohammed, J.A. A Facile Synthesis of ZrOx-MnCO3/Graphene Oxide (GRO) Nanocomposites for the Oxidation of Alcohols using Molecular Oxygen under Base Free Conditions. Catalysts 2019, 9, 759. [Google Scholar] [CrossRef] [Green Version]
- Elshazly, E.S.; Abdelal, O.A. Nickel stabilized zirconia for SOFCs: Synthesis and characterization. Int. J. Metall. Eng. 2012, 1, 130–134. [Google Scholar] [CrossRef] [Green Version]
- Kanthimathi, M.; Dhathathreyan, A.; Nair, B. Nanosized nickel oxide using bovine serum albumin as template. Mater. Lett. 2004, 58, 2914–2917. [Google Scholar] [CrossRef]
- Sun, H.; Ding, Y.; Duan, J.; Zhang, Q.; Wang, Z.; Lou, H.; Zheng, X. Transesterification of sunflower oil to biodiesel on ZrO2 supported La2O3 catalyst. Bioresour. Technol. 2010, 101, 953–958. [Google Scholar] [CrossRef]
- Dahdah, E.; Estephane, J.; Haydar, R.; Youssef, Y.; El Khoury, B.; Gennequin, C.; Aboukaïs, A.; Abi-Aad, E.; Aouad, S. Biodiesel production from refined sunflower oil over Ca–Mg–Al catalysts: Effect of the composition and the thermal treatment. Renew. Energy 2020, 146, 1242–1248. [Google Scholar] [CrossRef]
- Knothe, G. Analytical methods used in the production and fuel quality assessment of biodiesel. Trans. ASAE 2001, 44, 193. [Google Scholar] [CrossRef] [Green Version]
- Gelbard, G.; Bres, O.; Vargas, R.; Vielfaure, F.; Schuchardt, U. 1H nuclear magnetic resonance determination of the yield of the transesterification of rapeseed oil with methanol. J. Am. Oil Chem. Soc. 1995, 72, 1239–1241. [Google Scholar] [CrossRef]
- Srinivas, D.; Satyarthi, J.K. Biodiesel Production from Vegetable Oils and Animal Fat over Solid Acid Double-Metal Cyanide Catalysts. Catal. Surv. Asia 2011, 15, 145–160. [Google Scholar] [CrossRef]
- Jacobson, K.; Gopinath, R.; Meher, L.C.; Dalai, A.K. Solid acid catalyzed biodiesel production from waste cooking oil. Appl. Catal. B Environ. 2008, 85, 86–91. [Google Scholar] [CrossRef]
- Đặng, T.-H.; Chen, B.-H.; Lee, D.-J. Optimization of biodiesel production from transesterification of triolein using zeolite LTA catalysts synthesized from kaolin clay. J. Taiwan Inst. Chem. Eng. 2017, 79, 14–22. [Google Scholar] [CrossRef]
- Amani, H.; Ahmad, Z.; Asif, M.; Hameed, B. Transesterification of waste cooking palm oil by MnZr with supported alumina as a potential heterogeneous catalyst. J. Ind. Eng. Chem. 2014, 20, 4437–4442. [Google Scholar] [CrossRef]
- Feyzi, M.; Shahbazi, Z. Preparation, kinetic and thermodynamic studies of Al–Sr nanocatalysts for biodiesel production. J. Taiwan Inst. Chem. Eng. 2017, 71, 145–155. [Google Scholar] [CrossRef]
- Ayoub, M.; Bhat, A.H.; Ullah, S.; Ahmad, M.; Uemura, Y. Optimization of biodiesel production over alkaline modified clay catalyst. J. Jpn. Inst. Energy 2017, 96, 456–462. [Google Scholar] [CrossRef] [Green Version]
- Teo, S.H.; Islam, A.; Ng, C.H.; Mansir, N.; Ma, T.; Choong, S.T.; Taufiq-Yap, Y.H. Methoxy-functionalized mesostructured stable carbon catalysts for effective biodiesel production from non-edible feedstock. Chem. Eng. J. 2018, 334, 1851–1868. [Google Scholar] [CrossRef]
- Amani, H.; Ahmad, Z.; Hameed, B. Highly active alumina-supported Cs–Zr mixed oxide catalysts for low-temperature transesterification of waste cooking oil. Appl. Catal. A Gen. 2014, 487, 16–25. [Google Scholar] [CrossRef]
- Xie, W.; Peng, H.; Chen, L. Calcined Mg–Al hydrotalcites as solid base catalysts for methanolysis of soybean oil. J. Mol. Catal. A Chem. 2006, 246, 24–32. [Google Scholar] [CrossRef]
- Marinković, D.M.; Stanković, M.V.; Veličković, A.V.; Avramović, J.M.; Miladinović, M.R.; Stamenković, O.O.; Veljković, V.B.; Jovanović, D.M. Calcium oxide as a promising heterogeneous catalyst for biodiesel production: Current state and perspectives. Renew. Sustain. Energy Rev. 2016, 56, 1387–1408. [Google Scholar] [CrossRef]
- Saba, T.; Estephane, J.; El Khoury, B.; El Khoury, M.; Khazma, M.; El Zakhem, H.; Aouad, S. Biodiesel production from refined sunflower vegetable oil over KOH/ZSM5 catalysts. Renew. Energy 2016, 90, 301–306. [Google Scholar] [CrossRef]
- Kaur, N.; Ali, A. Preparation and application of Ce/ZrO2− TiO2/SO42− as solid catalyst for the esterification of fatty acids. Renew. Energy 2015, 81, 421–431. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaik, M.R.; Khan, M.; Kumar, J.V.S.; Ashraf, M.; Khan, M.; Kuniyil, M.; Assal, M.E.; Al-Warthan, A.; Siddiqui, M.R.H.; Khan, A.; et al. Nano Nickel-Zirconia: An Effective Catalyst for the Production of Biodiesel from Waste Cooking Oil. Crystals 2023, 13, 592. https://doi.org/10.3390/cryst13040592
Shaik MR, Khan M, Kumar JVS, Ashraf M, Khan M, Kuniyil M, Assal ME, Al-Warthan A, Siddiqui MRH, Khan A, et al. Nano Nickel-Zirconia: An Effective Catalyst for the Production of Biodiesel from Waste Cooking Oil. Crystals. 2023; 13(4):592. https://doi.org/10.3390/cryst13040592
Chicago/Turabian StyleShaik, Mohammed Rafi, Mujeeb Khan, J. V. Shanmukha Kumar, Muhammad Ashraf, Majad Khan, Mufsir Kuniyil, Mohamed E. Assal, Abdulrahman Al-Warthan, Mohammed Rafiq H. Siddiqui, Aslam Khan, and et al. 2023. "Nano Nickel-Zirconia: An Effective Catalyst for the Production of Biodiesel from Waste Cooking Oil" Crystals 13, no. 4: 592. https://doi.org/10.3390/cryst13040592








