Novel Mononuclear Tetrabromonitrosylrhenate(II) Complexes Containing Azole-Type Ligands: Magnetostructural Characterization through Hirshfeld Surfaces Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Physical Methods
2.3. Synthesis of the Complexes
2.4. X-ray Data Collection and Structure Refinement
2.5. Hirshfeld Surface Analysis
3. Results
3.1. Details on the Preparation and Characterization of the Complexes
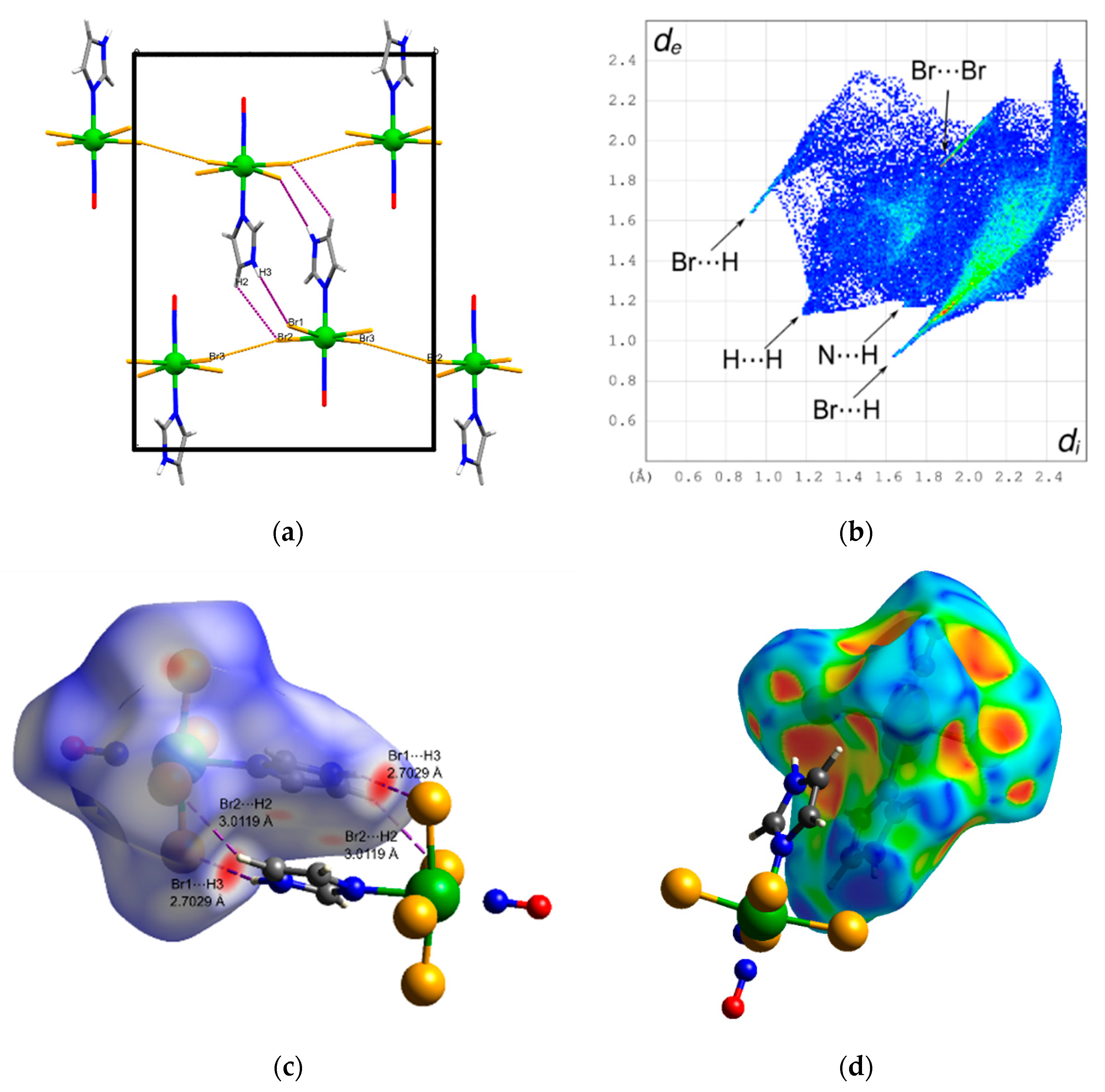
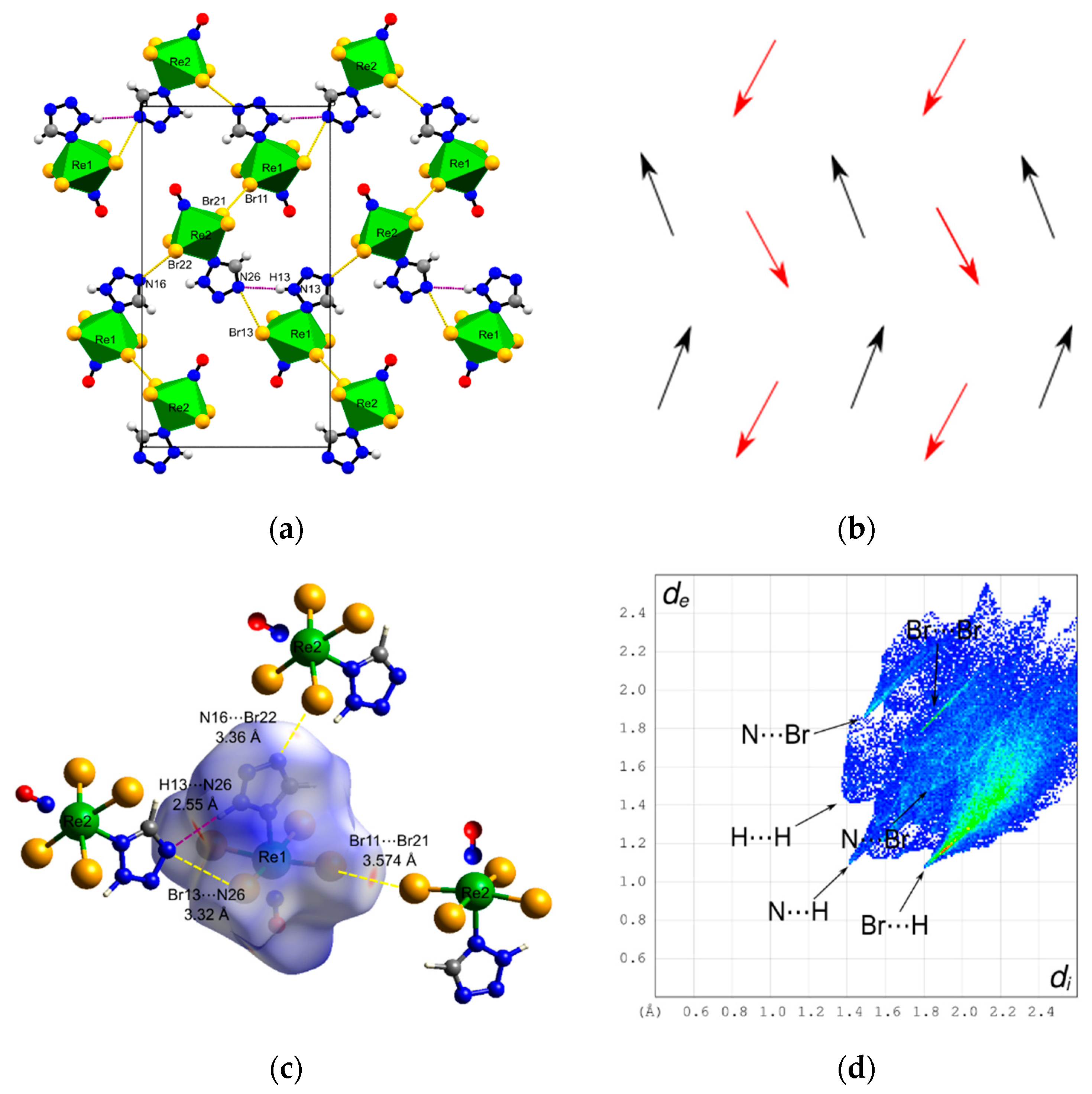
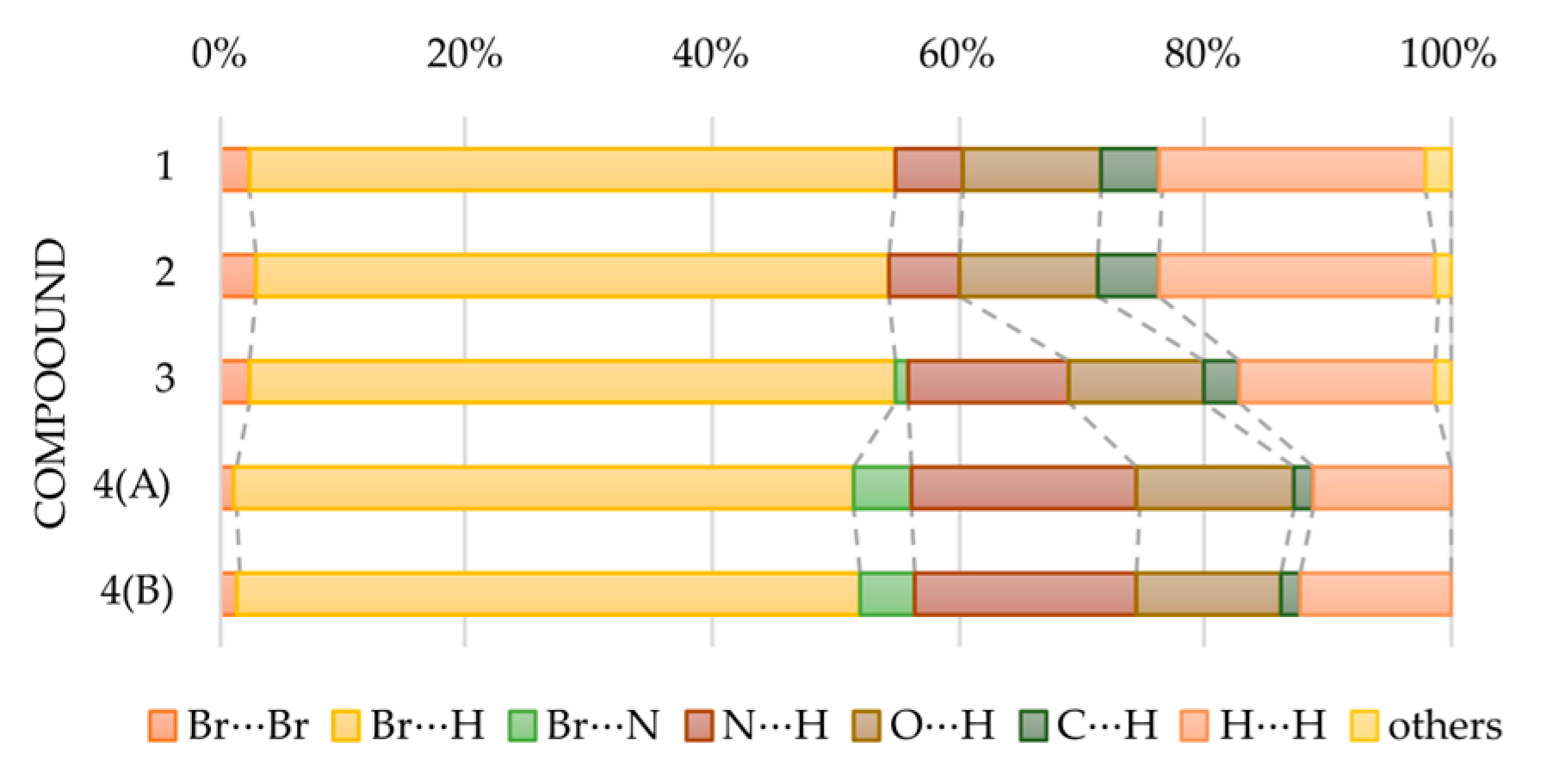
3.2. Crystal Structure and Hirshfeld Analysis
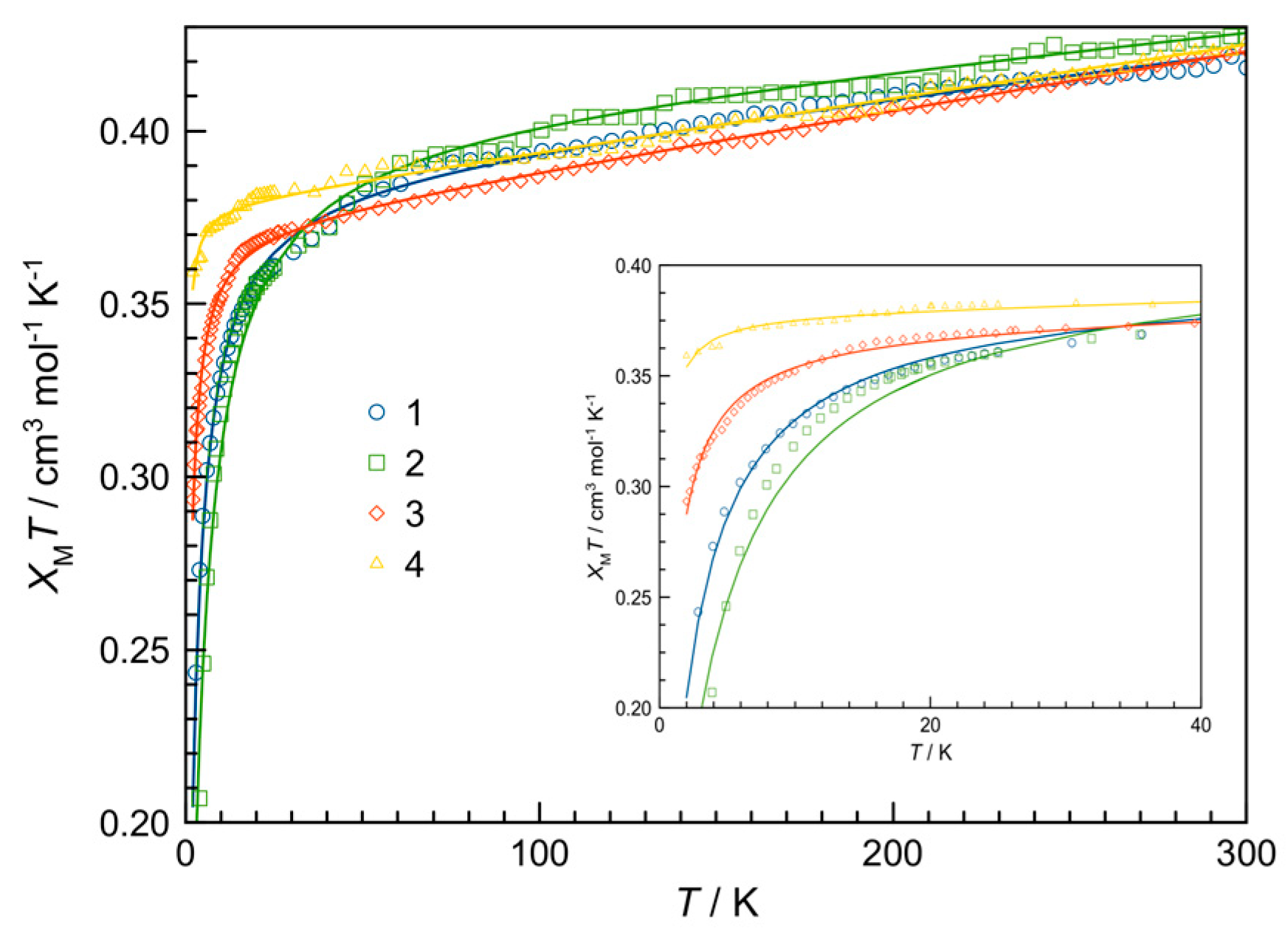
3.3. Magnetic Properties
 (1800 to 1899 cm−1) are indicative of a significant covalence degree of the rhenium to ligand interaction in 1–4. The value of Δ (axial distortion) is positive in sign, indicating that the orbital singlet (2B2) is lower in energy than the doublet (2E) (Figure 6), in agreement with the electronic configuration expected for a tetragonal Re(II)-nitrosyl complex [51]. A simulation of the dependence of the gu value with the distortion parameter ν by using the equations in Table 3, shown in Figure 7a, agrees with the experimental g∥ < g⊥ pattern determined experimentally. Additionally, there is a good agreement between the experimental values calculated for the g∥ < g⊥ pattern and those inferred from Figure 7b. (g⊥ = 2.2 − 2.7 and g∥ = 0.9 − 1.4), considering λ = –2000 cm−1 and values of κ in the range 0.5–0.75.
(1800 to 1899 cm−1) are indicative of a significant covalence degree of the rhenium to ligand interaction in 1–4. The value of Δ (axial distortion) is positive in sign, indicating that the orbital singlet (2B2) is lower in energy than the doublet (2E) (Figure 6), in agreement with the electronic configuration expected for a tetragonal Re(II)-nitrosyl complex [51]. A simulation of the dependence of the gu value with the distortion parameter ν by using the equations in Table 3, shown in Figure 7a, agrees with the experimental g∥ < g⊥ pattern determined experimentally. Additionally, there is a good agreement between the experimental values calculated for the g∥ < g⊥ pattern and those inferred from Figure 7b. (g⊥ = 2.2 − 2.7 and g∥ = 0.9 − 1.4), considering λ = –2000 cm−1 and values of κ in the range 0.5–0.75.4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dudle, B.; Rajesh, K.; Blacque, O.; Berke, H. Rhenium in Homogeneous Catalysis: [ReBrH(NO)(Labile Ligand)(Large-Bite-Angle Diphosphine)] Complexes as Highly Active Catalysts in Olefin Hydrogenations. J. Am. Chem. Soc. 2011, 133, 8168–8178. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Blacque, O.; Berke, H. Probing the Catalytic Potential of Chloro Nitrosyl Rhenium(I) Complexes. Dalton Trans. 2011, 40, 2578–2587. [Google Scholar] [CrossRef]
- Zobi, F.; Blacque, O. Reactivity of 17 E − Complex [ReIIBr4(CO)2]2− with Bridging Aromatic Ligands. Characterization and CO-Releasing Properties. Dalton Trans. 2011, 40, 4994–5001. [Google Scholar] [CrossRef]
- Adams, J.J.; Arulsamy, N.; Sullivan, B.P.; Roddick, D.M.; Neuberger, A.; Schmehl, R.H. Homoleptic Tris-Diphosphine Re(I) and Re(II) Complexes and Re(II) Photophysics and Photochemistry. Inorg. Chem. 2015, 54, 11136–11149. [Google Scholar] [CrossRef]
- Bar, A.K.; Pichon, C.; Sutter, J.-P. Magnetic Anisotropy in Two- to Eight-Coordinated Transition–Metal Complexes: Recent Developments in Molecular Magnetism. Coord. Chem. Rev. 2016, 308, 346–380. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Avendaño, C.; Dunbar, K.R. Molecular Magnetic Materials Based on 4d and 5d Transition Metals. Chem. Soc. Rev. 2011, 40, 3213–3238. [Google Scholar] [CrossRef] [PubMed]
- Schelter, E.J.; Prosvirin, A.V.; Dunbar, K.R. Molecular Cube of ReII and MnII That Exhibits Single-Molecule Magnetism. J. Am. Chem. Soc. 2004, 126, 15004–15005. [Google Scholar] [CrossRef] [PubMed]
- Schelter, E.J.; Prosvirin, A.V.; Reiff, W.M.; Dunbar, K.R. Unusual Magnetic Metal–Cyanide Cubes of ReII with Alternating Octahedral and Tetrahedral Corners. Angew. Chem. Int. Ed. 2004, 43, 4912–4915. [Google Scholar] [CrossRef]
- Pacheco, M.; Cuevas, A.; González-Platas, J.; Faccio, R.; Lloret, F.; Julve, M.; Kremer, C. Synthesis, Crystal Structure and Magnetic Properties of the Re(Ii) Complexes NBu4[Re(NO)Br4(L)] (L = Pyridine and Diazine Type Ligands). Dalton Trans. 2013, 42, 15361–15371. [Google Scholar] [CrossRef]
- Pacheco, M.; Cuevas, A.; González-Platas, J.; Lloret, F.; Julve, M.; Kremer, C. The Crystal Structure and Magnetic Properties of 3-Pyridinecarboxylate-Bridged Re(ii)M(ii) Complexes (M = Cu, Ni, Co and Mn). Dalton Trans. 2015, 44, 11636–11648. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.; González-Platas, J.; Julve, M.; Lloret, F.; Kremer, C.; Cuevas, A. Crystal Structure and Magnetic Properties of 3,5-Pyridinedicarboxylate-Bridged Re(II)M(II) Heterodinuclear Complexes (M = Cu, Ni and Co). Polyhedron 2021, 208, 115414. [Google Scholar] [CrossRef]
- Rosas, K.S.; Torres, J.; Pacheco, M.; Ramos, J.; Gancheff, J.S. Proton-Transfer Reactions of Re(II)-Nitrosyl Complexes: Potentiometric Studies, DFT and TD-DFT Calculations. Results Chem. 2022, 4, 100455. [Google Scholar] [CrossRef]
- Pacheco, M.; Cuevas, A.; González-Platas, J.; Gancheff, J.S.; Kremer, C. Complex Salts of [ReII(NO)Br4(Pyz)]−: Synthesis, Crystal Structures, and DFT Studies. J. Coord. Chem. 2014, 67, 4028–4038. [Google Scholar] [CrossRef]
- Pacheco, M.; Alvarez, N.; Cuevas, A.; Romerosa, A.; Lloret, F.; Kremer, C. Crystal Structure and Magnetic Study of the Complex Salt [RuCp(PTA)2–μ-CN-1κC:2κN–RuCp(PTA)2][Re(NO)Br4(EtOH)0.5(MeOH)0.5]. Acta Crystallogr. Sect. E Crystallogr. Commun. 2021, 77, 749–754. [Google Scholar] [CrossRef]
- Pacheco, M.; Cuevas, A.; González-Platas, J.; Kremer, C. Synthesis, Spectroscopic Characterization and Crystal Structure of [ReV(O)2(Pyz)4][ReII(NO)Br4(Pyz)] (Pyz = Pyrazine). Commun. Inorg. Synth. 2015, 2, 20–24. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; Wiley-VCH: New York, NY, USA, 2001; ISBN 978-0-471-18838-4. [Google Scholar]
- Barbour, L.J. Single-Crystal X-ray Diffraction. In Comprehensive Supramolecular Chemistry II; Elsevier: Amsterdam, The Netherlands, 2017; pp. 23–43. ISBN 978-0-12-803199-5. [Google Scholar]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards Quantitative Analysis of Intermolecular Interactions with Hirshfeld Surfaces. Chem. Commun. 2007, 7, 3814–3816. [Google Scholar] [CrossRef]
- Aromí, G.; Barrios, L.A.; Roubeau, O.; Gamez, P. Triazoles and Tetrazoles: Prime Ligands to Generate Remarkable Coordination Materials. Coord. Chem. Rev. 2011, 255, 485–546. [Google Scholar] [CrossRef]
- Chen, S.-S. The Roles of Imidazole Ligands in Coordination Supramolecular Systems. Crystengcomm 2016, 18, 6543–6565. [Google Scholar] [CrossRef]
- Haasnoot, J.G. Mononuclear, Oligonuclear and Polynuclear Metal Coordination Compounds with 1,2,4-Triazole Derivatives as Ligands. Coord. Chem. Rev. 2000, 200–202, 131–185. [Google Scholar] [CrossRef]
- Li, H.; Zhang, S.-G.; Xie, L.-M.; Yu, L.; Shi, J.-M. π–π Stacking, Hydrogen Bonding and Anti-Ferromagnetic Coupling Mechanism on a Mononuclear Cu(II) Complex. J. Coord. Chem. 2011, 64, 1456–1468. [Google Scholar] [CrossRef]
- Earnshaw, A. Introduction to Magnetochemistry; Elsevier Science: Saint Louis, MI, USA, 2013; ISBN 978-1-4832-7069-2. [Google Scholar]
- AIST: Spectral Database for Organic Compounds, SDBS. Available online: http://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_index.cgi (accessed on 15 October 2016).
- Nakamoto, K. Complexes of Alcoxides, Alcohols, Ethers, Ketones, Aldehydes, Esteres, and Carboxylic Acids. En: Infrared and Raman Spectra of Inorganic and Coordination Compounds. In Part II: Applications in Coordination, Organometallic, and Bioinorganic; John Wiley & Sons: Hoboken, NJ, USA, 1997. [Google Scholar]
- Billes, F.; Endrédi, H.; Keresztury, G. Vibrational Spectroscopy of Triazoles and Tetrazole. J. Mol. Struct. THEOCHEM 2000, 530, 183–200. [Google Scholar] [CrossRef]
- Agilent Technologies. CrysAlisPro (v42). Agilent Technologies, Oxfordshire, UK. 2021. Available online: https://www.rigaku.com/products/crystallography/crysalis (accessed on 6 April 2023).
- BRUKER APEX2. Available online: https://www.cif.iastate.edu/x-ray/apexii (accessed on 3 April 2023).
- Palatinus, L.; Chapuis, G. Superflip—A Computer Program for the Solution of Crystal Structures by Charge Flipping in Arbitrary Dimensions. J. Appl. Crystallogr. 2007, 40, 786–790. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Speck, A. Platon: A Multipurpose Crystalographic Tool; Utrecht University: Utrecht, The Netherlands, 2002. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld Surface Analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17; University of Western Australia: Perth, Australia, 2017. [Google Scholar]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting Intermolecular Interactions in Molecular Crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Spackman, M.A.; Mitchell, A.S. Novel Tools for Visualizing and Exploring Intermolecular Interactions in Molecular Crystals. Acta Crystallogr. Sect. B Struct. Sci. 2004, 60, 627–668. [Google Scholar] [CrossRef]
- Ciani, G.; Giusto, D.; Manassero, M.; Sansoni, M. Reactions of Pentachloro- and Pentabromonitrosylrhenate(II) Anions with N-Donor Ligands. Gazz. Chim. Ital. 1977, 107, 429–430. [Google Scholar]
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry, 2nd ed.; Wiley: Chichester, UK, 2009; ISBN 978-0-470-51233-3. [Google Scholar]
- Alkorta, I.; Elguero, J.; Frontera, A. Not Only Hydrogen Bonds: Other Noncovalent Interactions. Crystals 2020, 10, 180. [Google Scholar] [CrossRef] [Green Version]
- Cuevas, A.; Chiozzone, R.; Kremer, C.; Suescun, L.; Mombrú, A.; Armentano, D.; De Munno, G.; Lloret, F.; Cano, J.; Faus, J. Rhenium (IV)-Copper (II) Heterobimetallic Complexes with a Bridge Malonato Ligand. Synthesis, Crystal Structure, and Magnetic Properties. Inorg. Chem. 2004, 43, 7823–7831. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, A.; Kremer, C.; Hummert, M.; Schumann, H.; Lloret, F.; Julve, M.; Faus, J. Magnetic Properties and Molecular Structures of Binuclear (2-Pyrazinecarboxylate)-Bridged Complexes Containing Re (IV) and M (II)(M = Co, Ni). Dalton Trans. 2007, 3, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lillo, J.; Armentano, D.; Munno, G.D.; Julve, M.; Lloret, F.; Faus, J. Ferromagnetic Coupling and Spin Canting Behaviour in Heterobimetallic ReIVMII/III (M = CoII/III, NiII) Species. Dalton Trans. 2013, 42, 1687–1695. [Google Scholar] [CrossRef]
- Schiemenz, G.P. The Sum of van der Waals Radii—A Pitfall in the Search for Bonding. Z. Für Nat. B 2007, 62, 235–243. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.L.; Jotani, M.M.; Tiekink, E.R.T. Utilizing Hirshfeld Surface Calculations, Non-Covalent Interaction (NCI) Plots and the Calculation of Interaction Energies in the Analysis of Molecular Packing. Acta Crystallogr. Sect. E Cryst. Commun. 2019, 75, 308–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lloret, F.; Julve, M.; Cano, J.; Ruiz-García, R.; Pardo, E. Magnetic Properties of Six-Coordinated High-Spin Cobalt(II) Complexes: Theoretical Background and Its Application. Inorg. Chim. Acta 2008, 361, 3432–3445. [Google Scholar] [CrossRef]
- Machura, B. Structural and Spectroscopic Properties of Rhenium Nitrosyl Complexes. Coord. Chem. Rev. 2005, 249, 2277–2307. [Google Scholar] [CrossRef]
- Figgis, B.N. The Magnetic Properties of Transition Metal Ions in Asymmetric Ligand Fields. Part 3.—The Behaviour of Some T52g Complexes. Trans. Faraday Soc. 1961, 57, 204–209. [Google Scholar] [CrossRef]
- Mabbs, F.E.; Machin, D.J. Magnetism and Transition Metal Complexes; Springer: Berlin/Heidelberg, Germany, 1973; ISBN 978-1-5041-2035-7. [Google Scholar]
- Cano, J. VPMAG; University of Valencia: Valencia, Spain, 2003. [Google Scholar]
- Schelter, E.J.; Bera, J.K.; Bacsa, J.; Galán-Mascarós, J.R.; Dunbar, K.R. New Paramagnetic Re(II) Compounds with Nitrile and Cyanide Ligands Prepared by Homolytic Scission of Dirhenium Complexes. Inorg. Chem. 2003, 42, 4256–4258. [Google Scholar] [CrossRef]
- Silva, C.P.; Junior, H.C.S.; Santos, I.F.; Bernardino, A.M.R.; Cassaro, R.A.A.; Novak, M.A.; Vaz, M.G.F.; Guedes, G.P. Synthesis, Crystal Structure, Magnetic Properties and DFT Calculations of a Mononuclear Copper(II) Complex: Relevance of Halogen Bonding for Magnetic Interaction. Inorg. Chim. Acta 2018, 482, 395–401. [Google Scholar] [CrossRef]
- Martinez.Lillo, J.; Faus, J.; Lloret., F.; Julve, M. Towards multifunctional magnetic systems through molecular-programmed self assembly of Re(IV) metalloligands. Coord. Chem. Rev. 2015, 289–290, 215–237. [Google Scholar] [CrossRef]



| Bond Lengths (Å) | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4A/4B | |
| Re1−N2L | 2.173(4) | 2.191(3) | 2.194(4) | 2.171(12)/2.183(12) |
| Re1−N1NO | 1.736(4) | 1.732(4) | 1.743(4) | 1.735(13)/1.759(14) |
| Re1−Br1 | 2.5628(5) | 2.5335(5) | 2.5558(5) | 2.5013(16)/2.5020(18) |
| Re1−Br2 | 2.5347(5) | 2.5346(5) | 2.5290(4) | 2.5197(17)/2.5286(18) |
| Re1−Br3 | 2.5103(5) | 2.5250(5) | 2.5041(4) | 2.5172(15)/2.5017(13) |
| Re1−Br4 | 2.5274(5) | 2.5130(5) | 2.5228(5) | 2.5145(16)/2.5070(16) |
| N1NO−O1 | 1.185(5) | 1.176(5) | 1.179(5) | 1.139(13)/1.100(14) |
| Bond angles (°) | ||||
| N1NO−Re1−N2L | 178.69(14) | 177.6(1) | 177.9(1) | 178.8(5)/179.2(6) |
| Re1−NNO−O1 | 177.8(4) | 177.5(3) | 176.8(3) | 177.3(13)/177.6(16) |
| Br1−Re1−Br3 | 172.141(19) | 173.98(2) | 171.41(2) | 171.11(6)/171.99(6) |
| Br2−Re1−Br4 | 175.197(18) | 171.72(2) | 173.71(2) | 173.20(6)/172.77(7) |
| N1NO−Re1−Br2 | 90.93(12) | 96.5(1) | 94.1(1) | 91.7(4)/93.0(4) |
| N2L−Re1−Br2 | 88.13(9) | 85.87(9) | 87.03(9) | 87.3(3)/87.0(3) |
| Direction (u) | ||
|---|---|---|
| Parallel (z) | ||
| Perpendicular (x and y) |
| Compound | Δ | λ | κ | ν | TIP b | θ/K | g|| | g⊥ | gav | R c × 106 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2225 | –1847 | 0.62 | –1.94 | 125 | –1.78 | 1.25 | 2.33 | 2.03 | 0.5 |
| 2 | 2589 | –1800 | 0.63 | –2.28 | 98 | –3.12 | 1.43 | 2.34 | 2.08 | 1.8 |
| 3 | 1850 | –1899 | 0.63 | –1.55 | 169 | –0.59 | 1.01 | 2.32 | 1.98 | 0.2 |
| 4 d | 2016 | –1849 | 0.64 | –1.70 | 155 | –0.14 | 1.07 | 2.34 | 2.01 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco, M.; González-Platas, J.; Kremer, C.; Julve, M.; Lloret, F.; Cuevas, A. Novel Mononuclear Tetrabromonitrosylrhenate(II) Complexes Containing Azole-Type Ligands: Magnetostructural Characterization through Hirshfeld Surfaces Analysis. Crystals 2023, 13, 658. https://doi.org/10.3390/cryst13040658
Pacheco M, González-Platas J, Kremer C, Julve M, Lloret F, Cuevas A. Novel Mononuclear Tetrabromonitrosylrhenate(II) Complexes Containing Azole-Type Ligands: Magnetostructural Characterization through Hirshfeld Surfaces Analysis. Crystals. 2023; 13(4):658. https://doi.org/10.3390/cryst13040658
Chicago/Turabian StylePacheco, Mario, Javier González-Platas, Carlos Kremer, Miguel Julve, Francesc Lloret, and Alicia Cuevas. 2023. "Novel Mononuclear Tetrabromonitrosylrhenate(II) Complexes Containing Azole-Type Ligands: Magnetostructural Characterization through Hirshfeld Surfaces Analysis" Crystals 13, no. 4: 658. https://doi.org/10.3390/cryst13040658