Spectroscopic and Crystallographic Characterization of Two Hydrochloride Cathinones: 1-(4-fluoro-3-methylphenyl)-2-(pyrrolidin-1-yl)pentan-1-one (4-F-3-Me-α-PVP) and N-ethyl-2-amino-1-phenylheptan-1-one (N-ethylheptedrone)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
2.4. Direct Infusion Electrospray Ionization Mass Spectrometry (ESI-MS)
2.5. High-Resolution Mass Spectrometry (HR-MS)
2.6. NMR Spectroscopy
2.7. Fourier Transform Infrared (FTIR) Spectroscopy
2.8. Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC)
2.9. X-ray Single Crystal Diffraction Studies
3. Results and Discussion
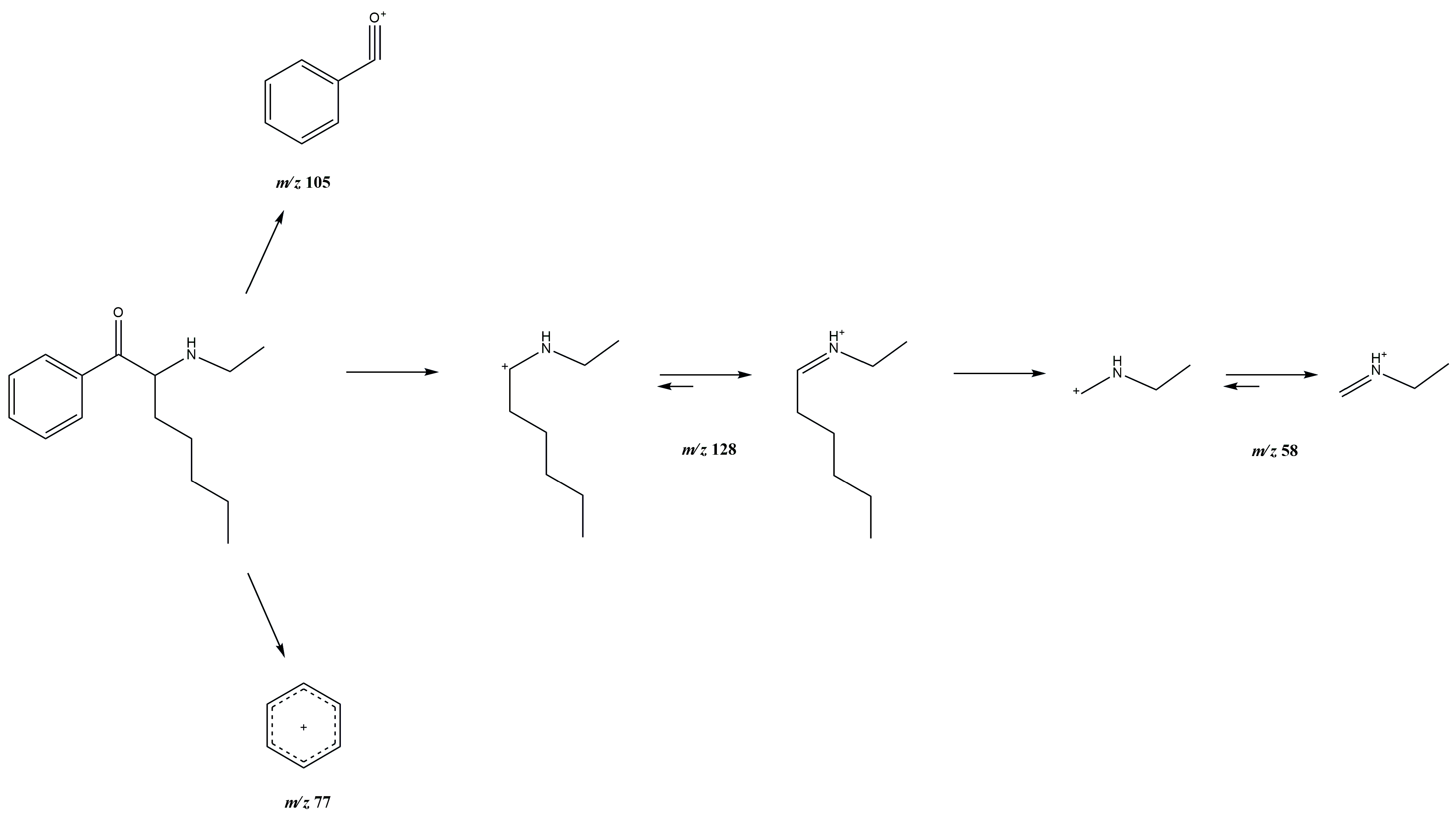
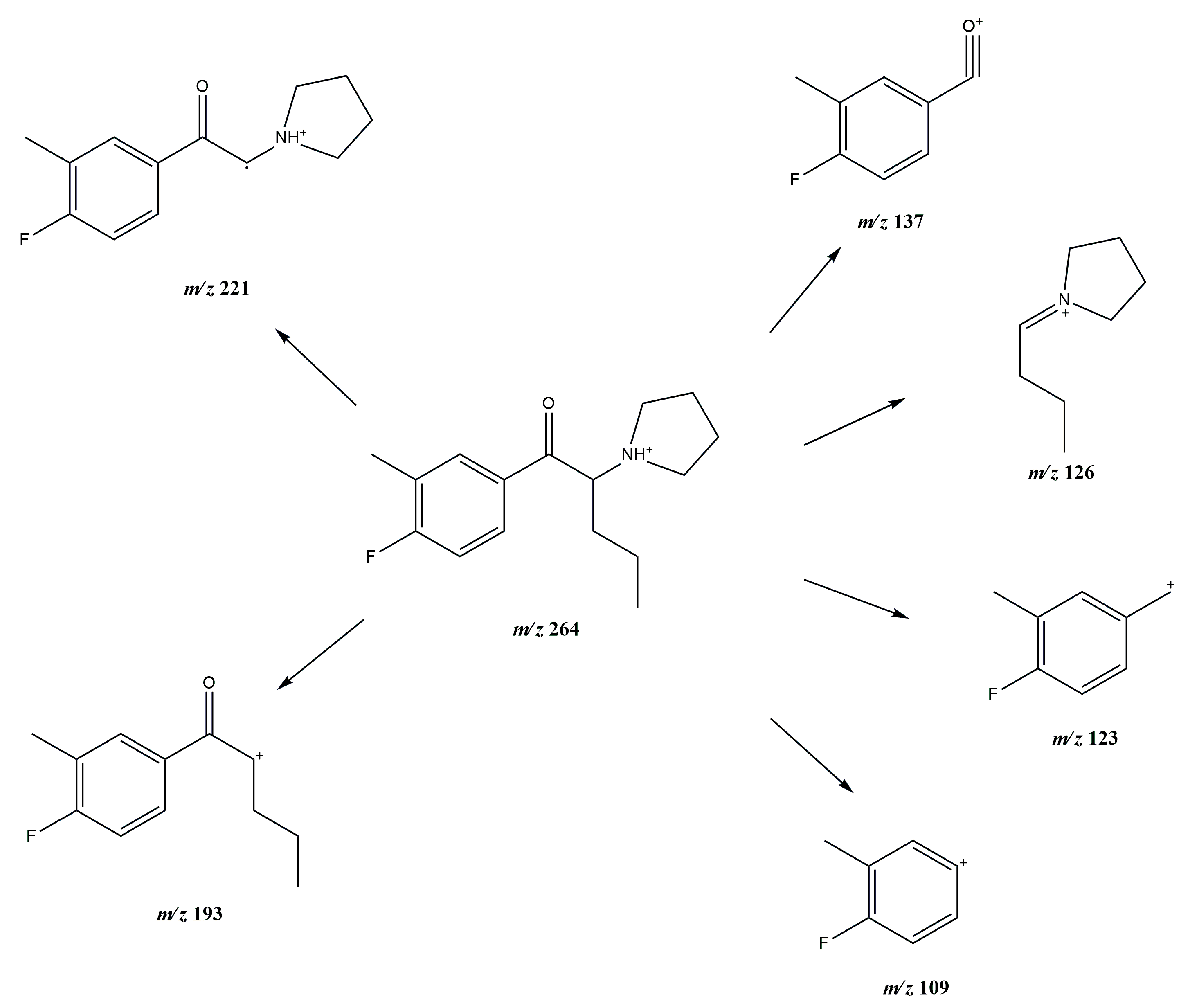
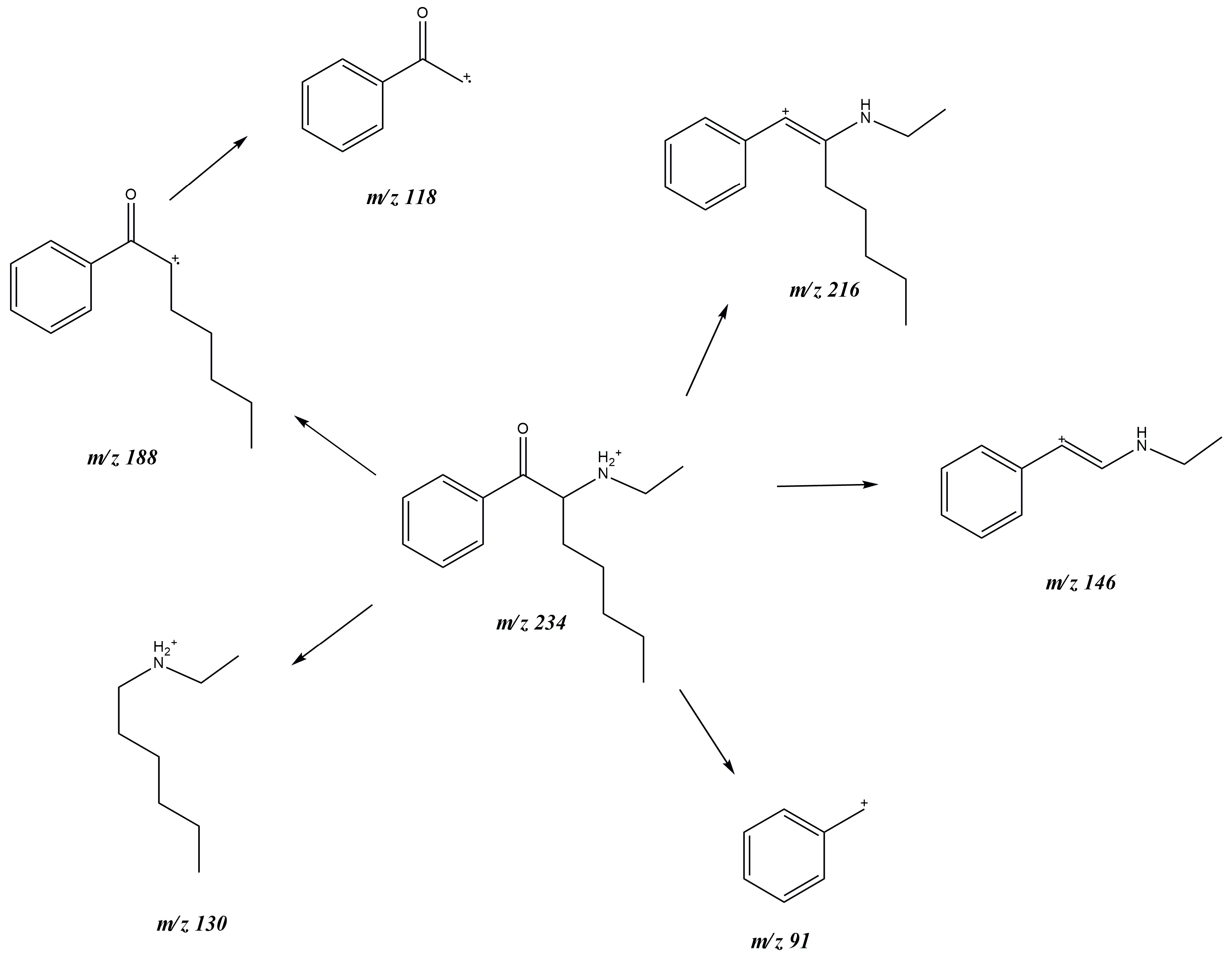
3.1. GC-MS and ESI-MS
3.2. 1H and 13C Nuclear Magnetic Resonance Spectroscopy
3.3. IR Spectra, TGA and DSC
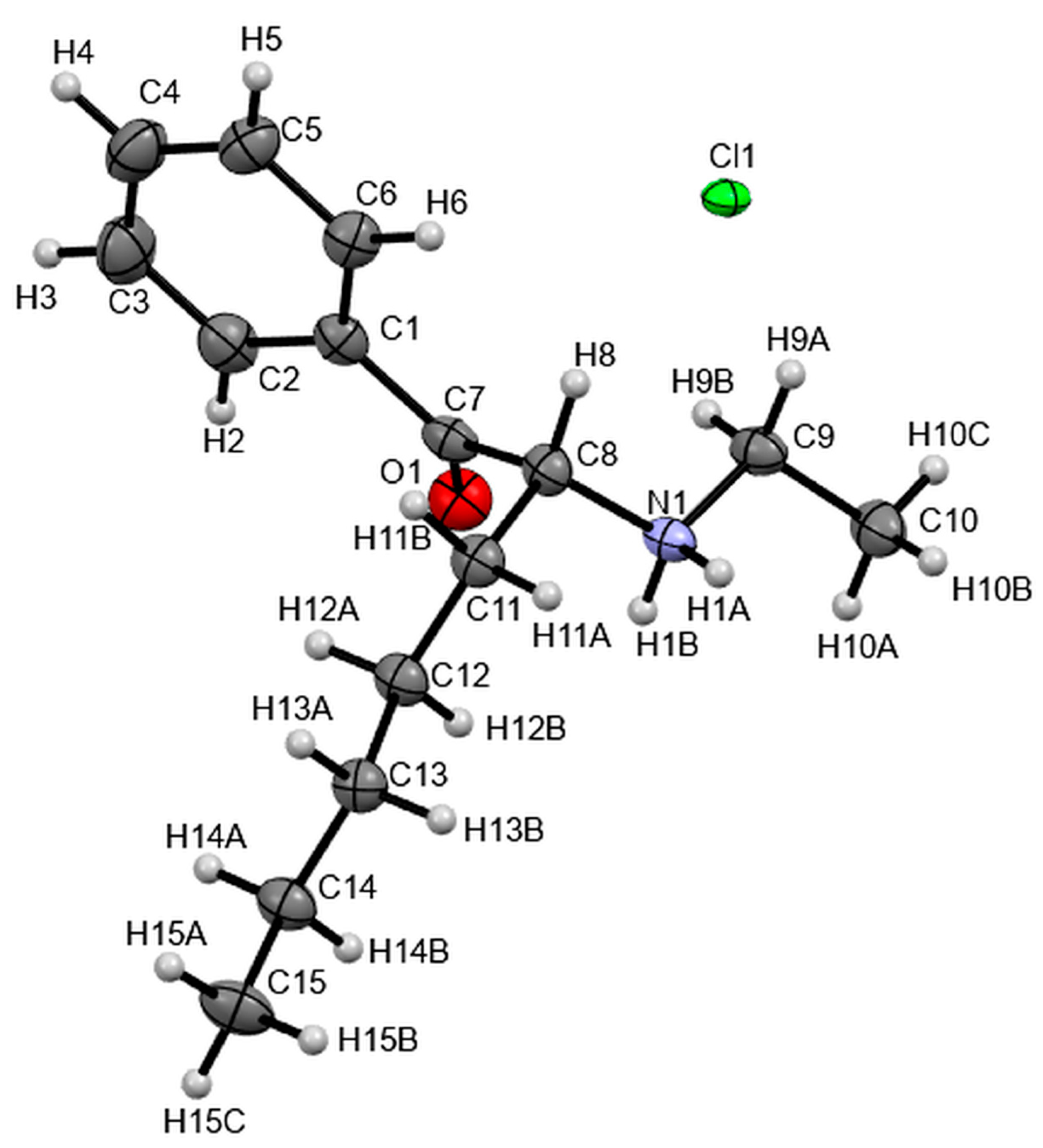
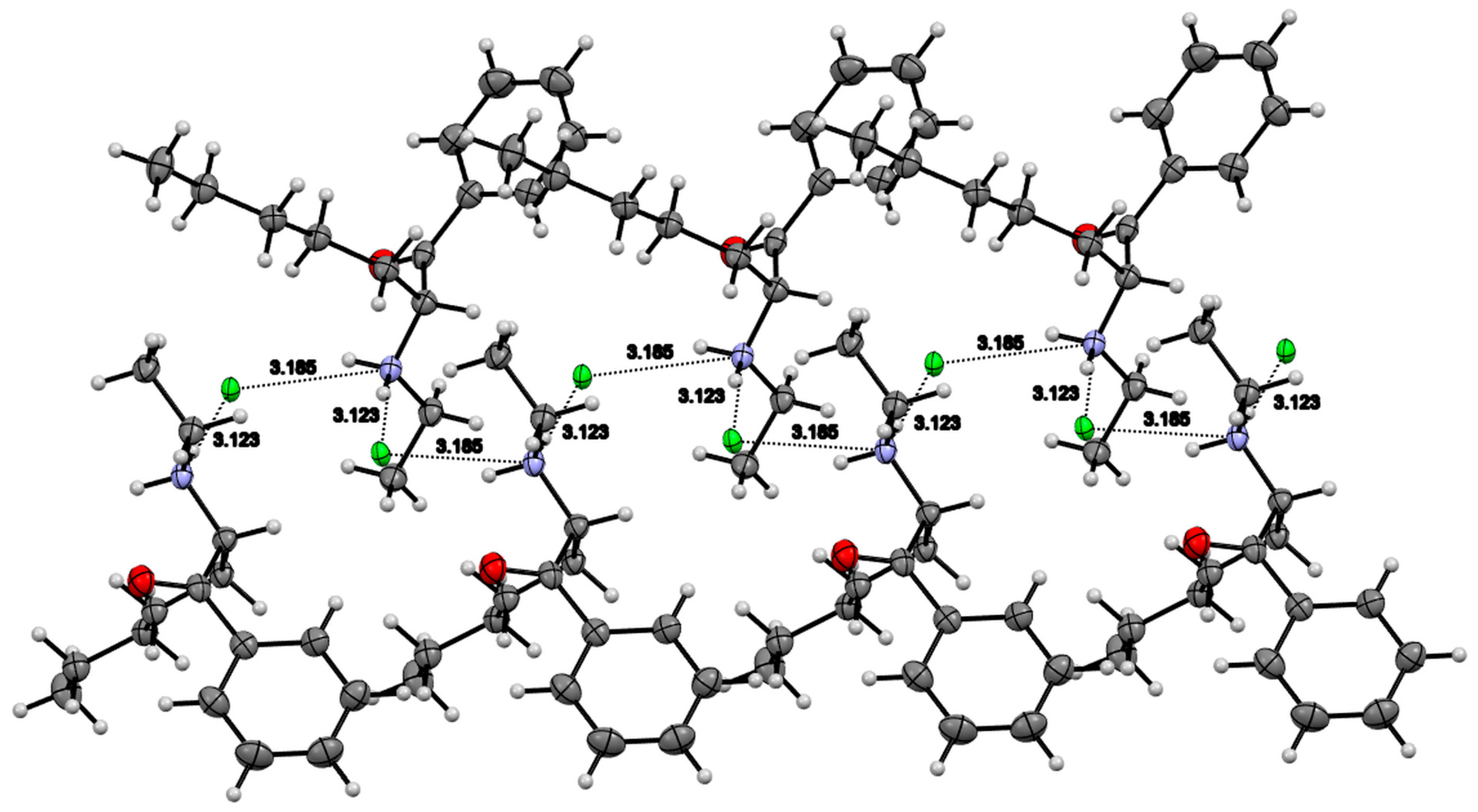
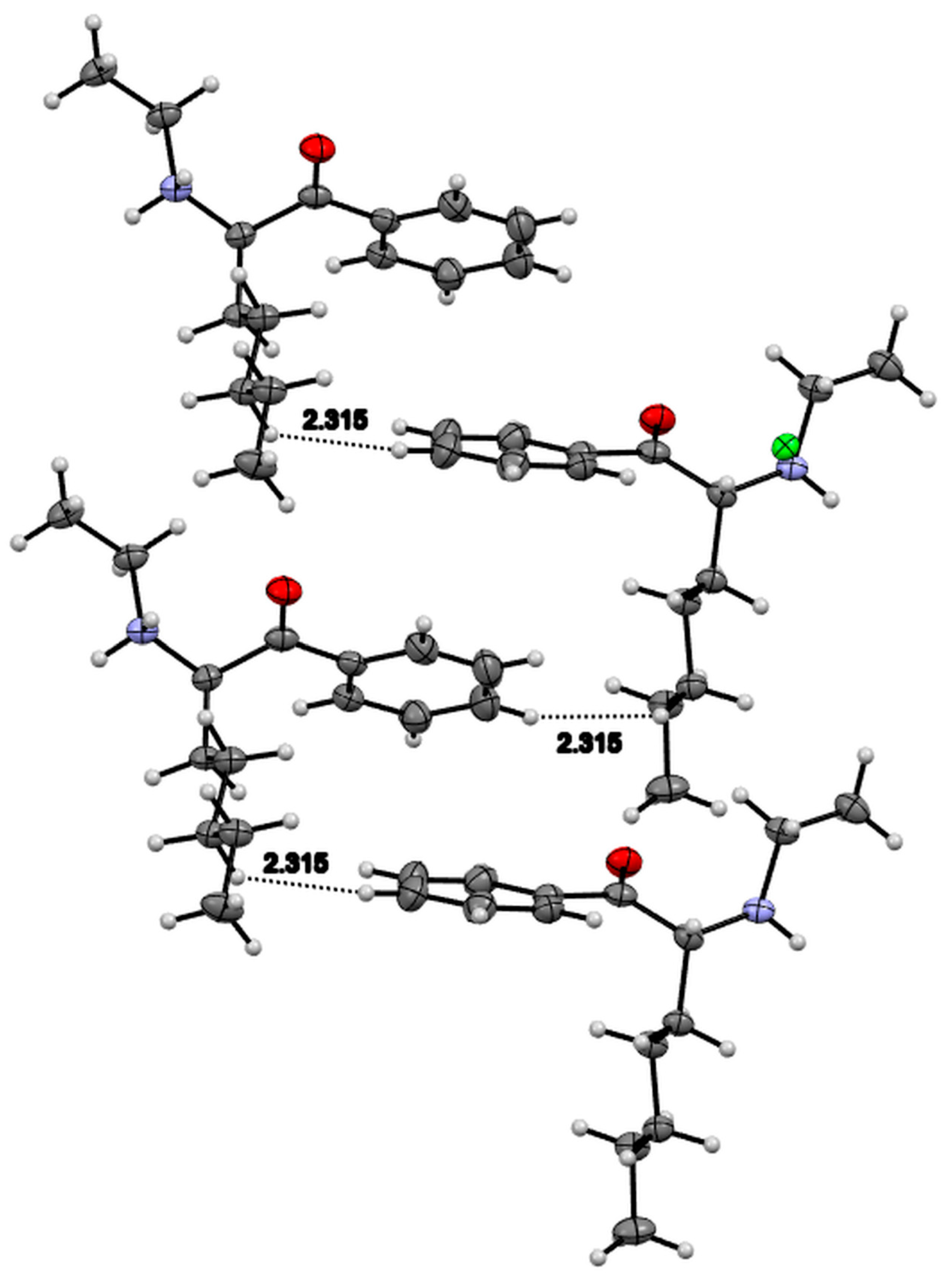
3.4. X-ray Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- EMCDDA European Drug Report 2022: Trends and Developments. 2022. Available online: https://www.emcdda.europa.eu/system/files/publications/13236/TDAT20001ENN_web.pdf (accessed on 22 December 2022).
- Valente, M.J.; Guedes de Pinho, P.; de Lourdes Bastos, M.; Carvalho, F.; Carvalho, M. Khat and synthetic cathinones: A review. Arch. Toxicol. 2014, 88, 15–45. [Google Scholar] [CrossRef] [PubMed]
- Szendrei, K. The chemistry of khat. Bull. Narc. 1980, 32, 5–35. [Google Scholar] [PubMed]
- Patel, N.B. “Natural Amphetamine” Khat: A Cultural Tradition or a Drug of Abuse? Int. Rev. Neurobiol. 2015, 120, 235–255. [Google Scholar] [CrossRef] [PubMed]
- Brenneisen, R.; Fisch, H.U.; Koelbing, U.; Geisshüsler, S.; Kalix, P. Amphetamine-like effects in humans of the khat alkaloid cathinone. Br. J. Clin. Pharmacol. 1990, 30, 825–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaizaki, A.; Tanaka, S.; Numazawa, S. New recreational drug 1-phenyl-2-(1-pyrrolidinyl)-1-pentanone (alpha-PVP) activates central nervous system via dopaminergic neuron. J. Toxicol. Sci. 2014, 39, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uralets, V.; Rana, S.; Morgan, S.; Ross, W. Testing for designer stimulants: Metabolic profiles of 16 synthetic cathinones excreted free in human urine. J. Anal. Toxicol. 2014, 38, 233–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Forensic Laboratory. Slovenia. Analytical Report: 4F-3Me-α-PVP. 2020. Available online: https://www.policija.si/apps/nfl_response_web/0_Analytical_Reports_final/4F-3Me-%CE%B1-PVP-ID-2187-20_report.pdf (accessed on 20 December 2022).
- Hungarian Institute for Forensic Science. Hungary. Analytical Data for Ethyl-Heptedrone. 2019. Available online: https://www.policija.si/apps/nfl_response_web/0_Analytical_Reports_final/ETHYLHEPTEDRON-ID-HIFS-012.pdf (accessed on 20 December 2022).
- Rigaku Oxford Diffraction. CrysAlisPro Software System, Version 1.171.39.46; Rigaku Corporation: Wroclaw, Poland, 2018. [Google Scholar]
- Rigaku Oxford Diffraction. CrysAlisPro Software System, Version 1.171.38.41q; Rigaku Corporation: Wroclaw, Poland, 2015. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuta, S.; Katagi, M.; Nishioka, H.; Kamata, H.; Sasaki, K.; Shima, N.; Kamata, T.; Miki, A.; Tatsuno, M.; Zaitsu, K.; et al. Structural characterization of cathinone-type designer drugs by EI mass spectrometry. Jpn. J. Forensic Sci. Technol. 2014, 19, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Fornal, E. Study of collision-induced dissociation of electrospray-generated protonated cathinones. Drug Test. Anal. 2014, 6, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Fornal, E. Formation of odd-electron product ions in collision-induced fragmentation of electrospray-generated protonated cathinone derivatives: Aryl α-primary amino ketones. Rapid Commun. Mass Spectrom. 2013, 27, 1858–1866. [Google Scholar] [CrossRef] [PubMed]
- Kuś, P.; Rojkiewicz, M.; Kusz, J.; Książek, M.; Sochanik, A. Spectroscopic characterization and crystal structures of four hydrochloride cathinones: N-ethyl-2-amino-1-phenylhexan-1-one (hexen, NEH), N-methyl-2-amino-1-(4-methylphenyl)-3-methoxypropan-1-one (mexedrone), N-ethyl-2-amino-1-(3,4-methylenedioxyphenyl)pentan-1-one (ephylone) and N-butyl-2-amino-1-(4-chlorophenyl)propan-1-one (4-chlorobutylcathinone). Forensic Toxicol. 2019, 37, 456–464. [Google Scholar] [CrossRef] [Green Version]
- UNODC Data from the UNODC Early Warning Advisory on the New Psychoactive Substances. 2022. Available online: https://www.unodc.org/unodc/en/scientists/ewa/data.html (accessed on 22 December 2022).
- Hobbs, J.M.; DeRienz, R.T.; Baker, D.D.; Shuttleworth, M.R.; Pandey, M. Fatal intoxication by the novel cathinone 4-fuoro-3-methyl-α-PVP. J. Anal. Toxicol. 2022, 46, e101–e104. [Google Scholar] [CrossRef] [PubMed]
- Kuropka, P.; Zawadzki, M.; Szpot, P. A review of synthetic cathinones emerging in recent years (2019–2022). Forensic Toxicol. 2023, 41, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Kolaczynska, K.E.; Thomann, J.; Hoener, M.C.; Liechti, M.E. The pharmacological profile of second generation pyrovalerone cathinones and related cathinone derivative. Int. J. Mol. Sci. 2021, 22, 8277. [Google Scholar] [CrossRef] [PubMed]
- Gatch, M.B.; Forster, M.J. Methylenedioxymethamphetamine-like discriminative stimulus effects of pyrrolidinyl cathinones in rats. J. Psychopharmacol. 2020, 34, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Gatch, M.B.; Shetty, R.A.; Sumien, N.; Forster, M.J. Behavioral effects of four novel synthetic cathinone analogs in rodents. Addict. Biol. 2021, 26, e12987. [Google Scholar] [CrossRef] [PubMed]
- Mikołajczyk, A.; Adamowicz, P.; Tokarczyk, B.; Sekuła, K.; Gieroń, J.; Wrzesień, W.; Stanaszek, R. Determination of N-ethylhexedrone, a new cathinone derivative, in blood collected from drivers—Analysis of three cases. Prob. Forensic Sci. 2017, 109, 53–63. [Google Scholar]

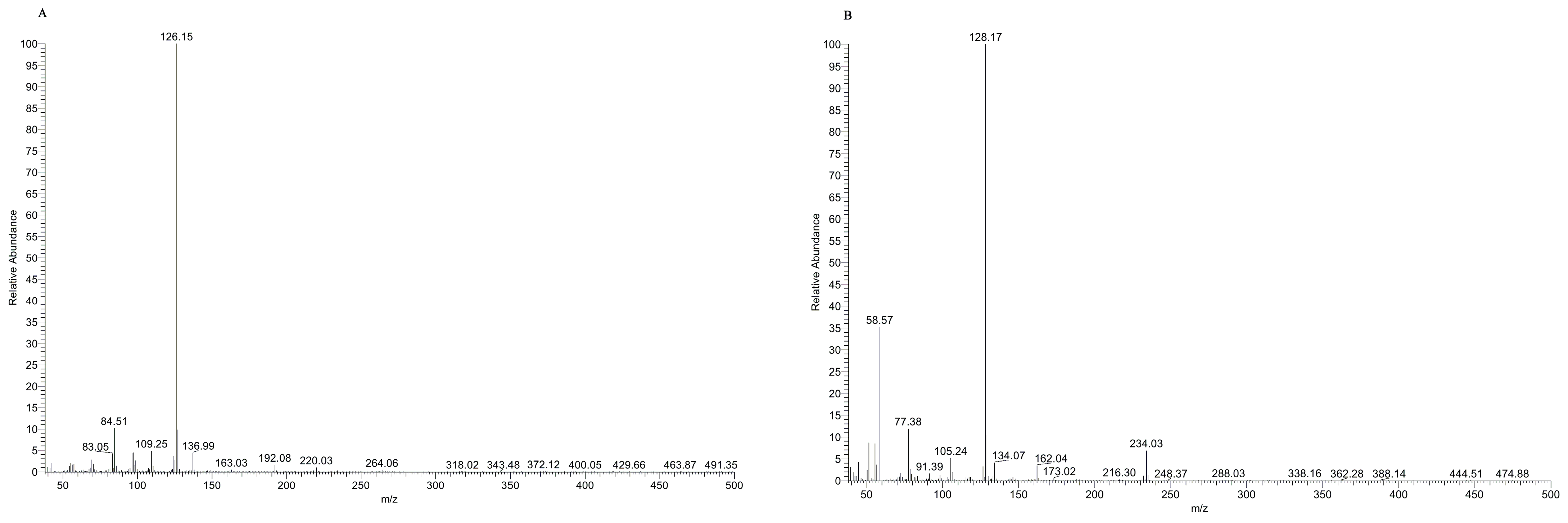



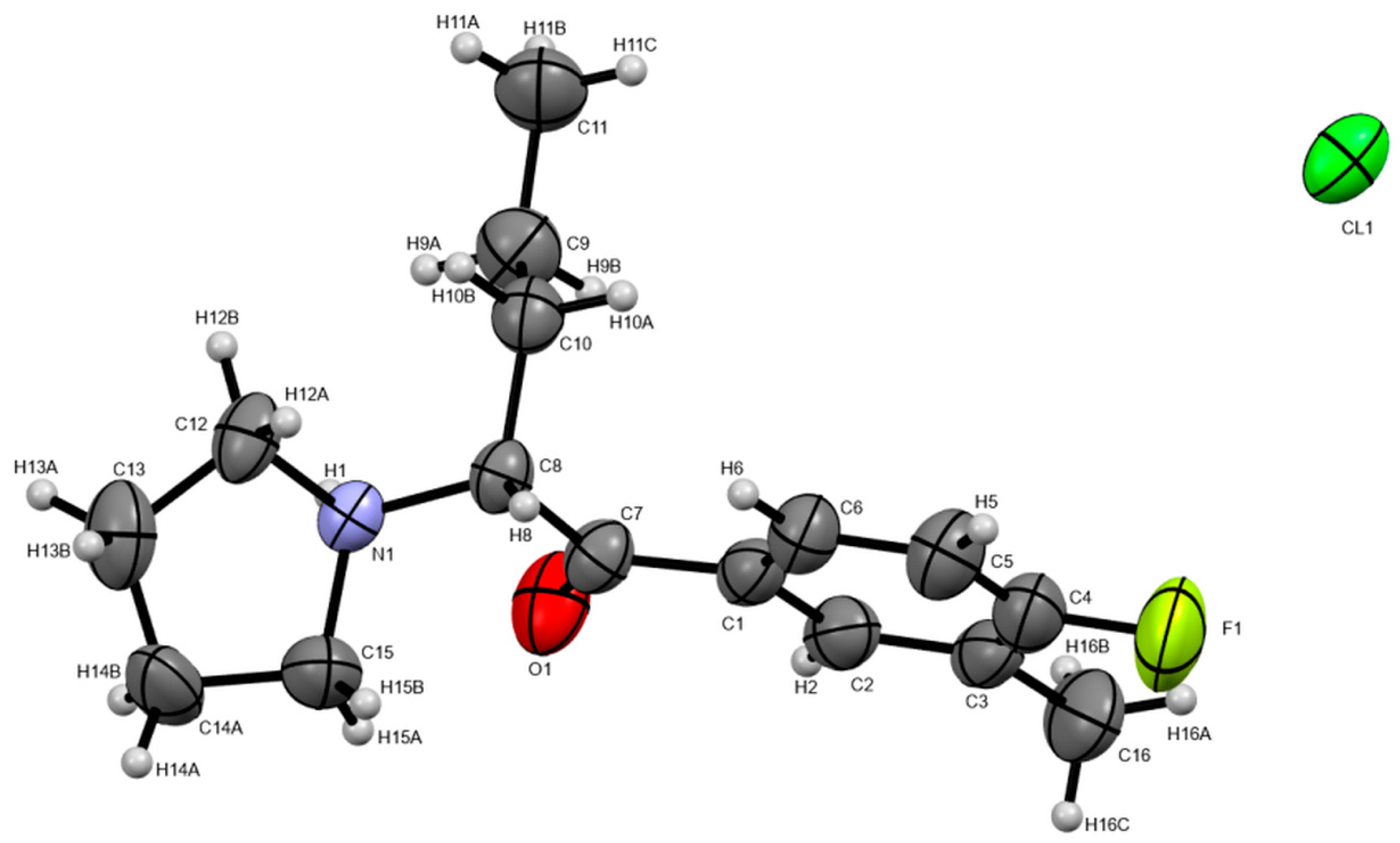
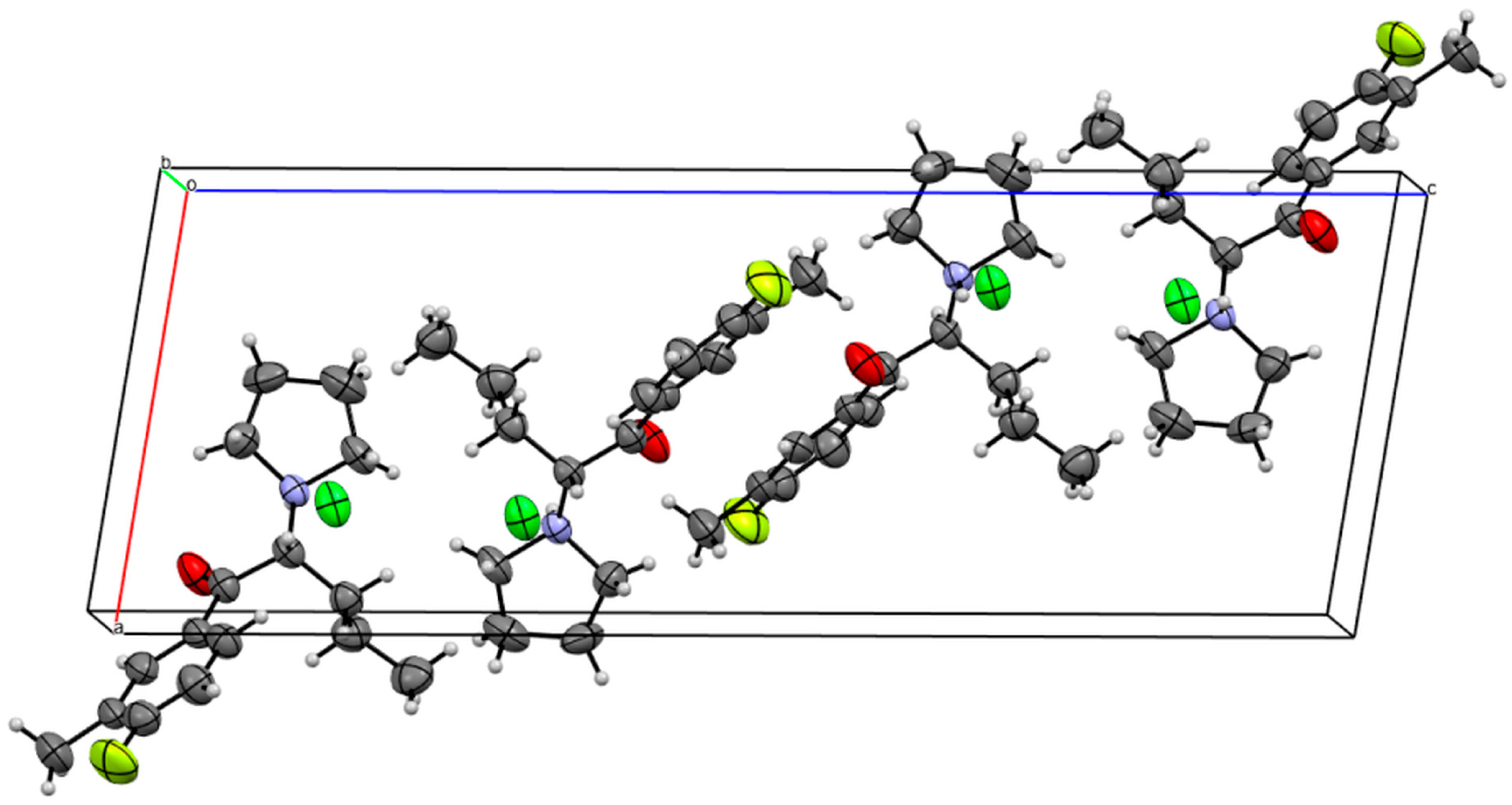
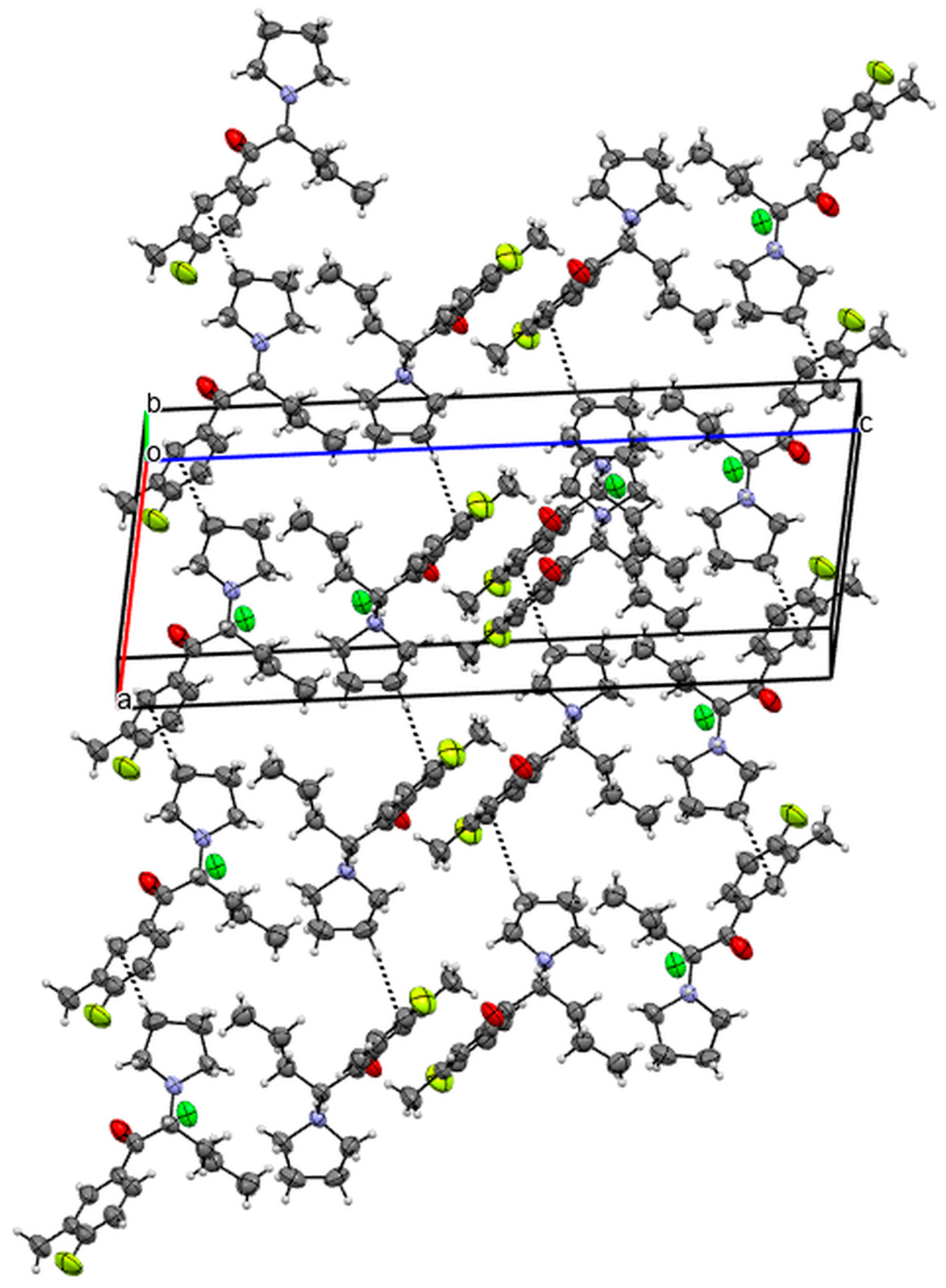
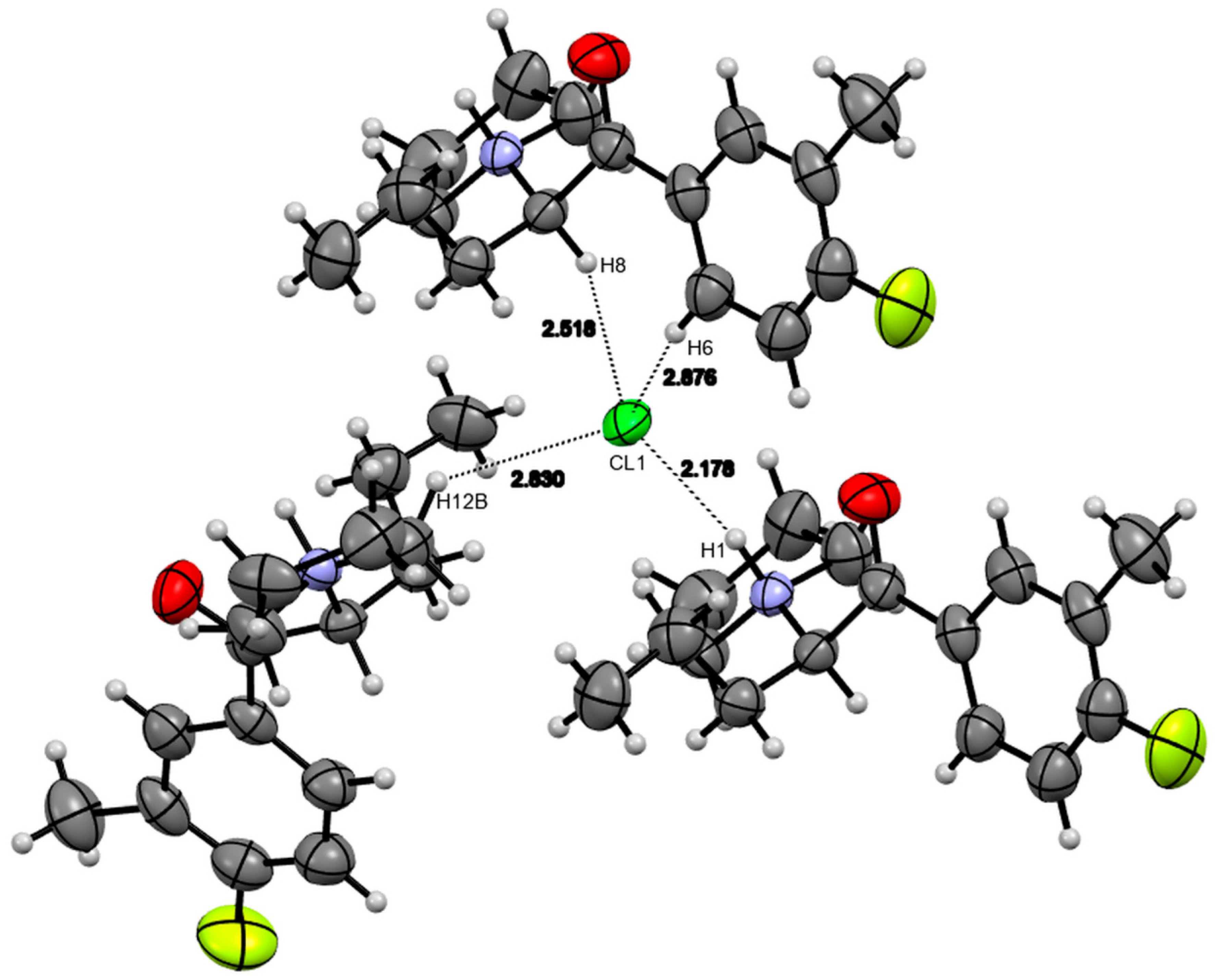

| Compound 1 | Compound 2 | |
|---|---|---|
| Chemical formula | C16H23FClNO | C15H24ClNO |
| Mr | 299.80 | 269.80 |
| Crystal system, space group | Monoclinic, P21/n | Orthorhombic, P212121 |
| Temperature (K) | 293 | 100 |
| a, b, c (Å) | 9.1188 (12), 7.0997 (8), 25.262 (4) | 7.0850 (5), 7.8818 (6), 27.695 (2) |
| β (°) | 98.951 (14) | 90 |
| V (Å3) | 1615.6 (4) | 1546.6 (2) |
| Z | 4 | 4 |
| Radiation type | MoKα | CuKα |
| µ (mm−1) | 0.24 | 2.09 |
| Crystal size (mm) | 0.18 × 0.07 × 0.03 | 0.600 × 0.060 × 0.020 |
| Tmin, Tmax | 0.869; 1.000 | 0.605; 1.000 |
| No. of measured, independent andobserved [I > 2σ(I)] reflections | 18582, 6413, 2936 | 20690, 3057, 2911 |
| Rint | 0.048 | 0.054 |
| (sin θ/λ)max (Å−1) | 0.800 | 0.622 |
| R[F2 > 2σ(F2)], wR(F2), S | 0.080, 0.269, 1.03 | 0.040, 0.113, 1.13 |
| No. of reflections | 6413 | 3057 |
| No. of parameters | 193 | 166 |
| Δρmax, Δρmin (e Å−3) | 0.69, −0.37 | 0.51, −0.32 |
| CCDC | 2249500 | 2249501 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojkiewicz, M.; Kuś, P.; Kusz, J.; Książek, M.; Staszek, D. Spectroscopic and Crystallographic Characterization of Two Hydrochloride Cathinones: 1-(4-fluoro-3-methylphenyl)-2-(pyrrolidin-1-yl)pentan-1-one (4-F-3-Me-α-PVP) and N-ethyl-2-amino-1-phenylheptan-1-one (N-ethylheptedrone). Crystals 2023, 13, 934. https://doi.org/10.3390/cryst13060934
Rojkiewicz M, Kuś P, Kusz J, Książek M, Staszek D. Spectroscopic and Crystallographic Characterization of Two Hydrochloride Cathinones: 1-(4-fluoro-3-methylphenyl)-2-(pyrrolidin-1-yl)pentan-1-one (4-F-3-Me-α-PVP) and N-ethyl-2-amino-1-phenylheptan-1-one (N-ethylheptedrone). Crystals. 2023; 13(6):934. https://doi.org/10.3390/cryst13060934
Chicago/Turabian StyleRojkiewicz, Marcin, Piotr Kuś, Joachim Kusz, Maria Książek, and Dorota Staszek. 2023. "Spectroscopic and Crystallographic Characterization of Two Hydrochloride Cathinones: 1-(4-fluoro-3-methylphenyl)-2-(pyrrolidin-1-yl)pentan-1-one (4-F-3-Me-α-PVP) and N-ethyl-2-amino-1-phenylheptan-1-one (N-ethylheptedrone)" Crystals 13, no. 6: 934. https://doi.org/10.3390/cryst13060934





