In Situ Study on Cu-to-Cu Thermal Compression Bonding
Abstract
:1. Introduction
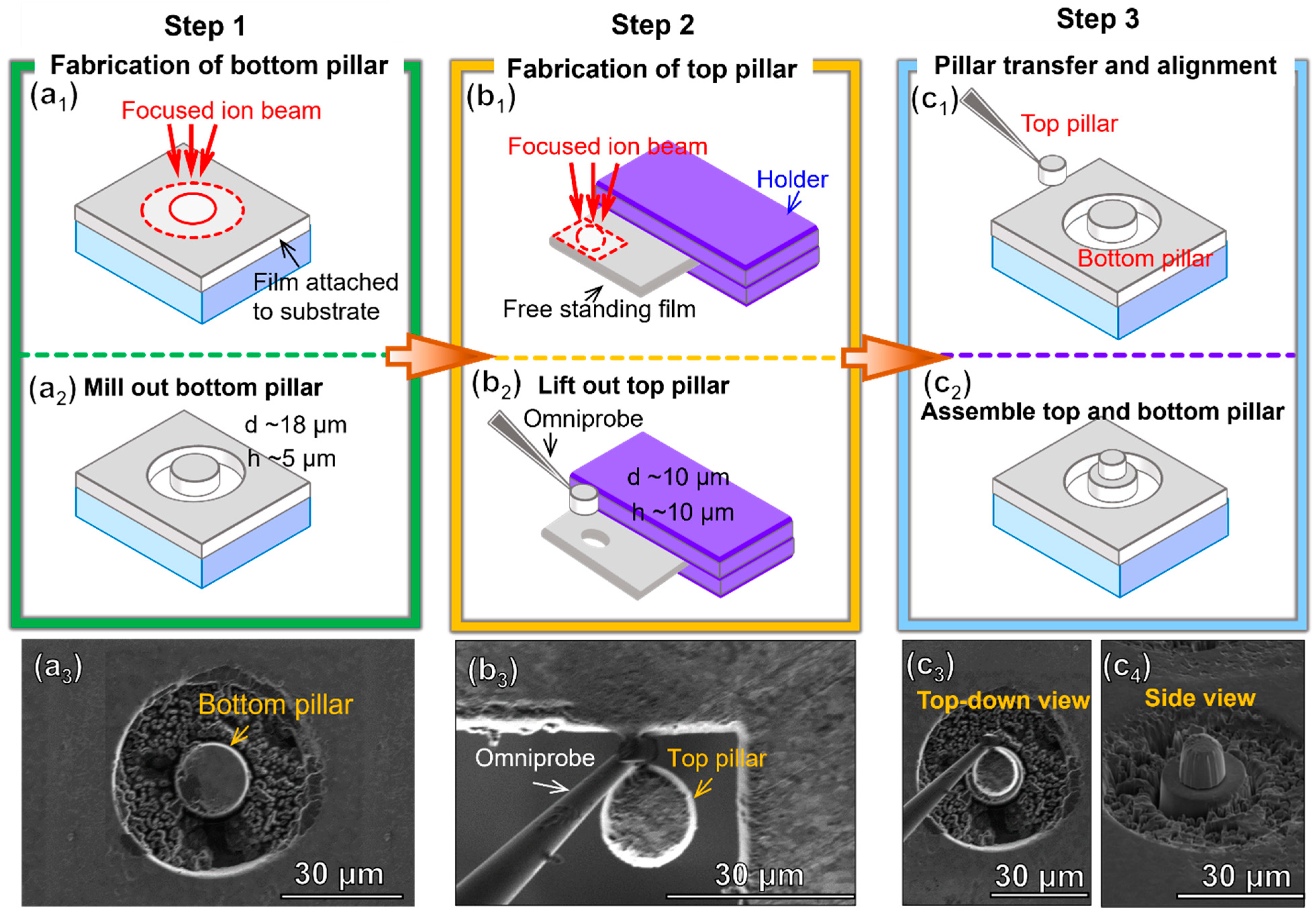
2. Materials and Methods
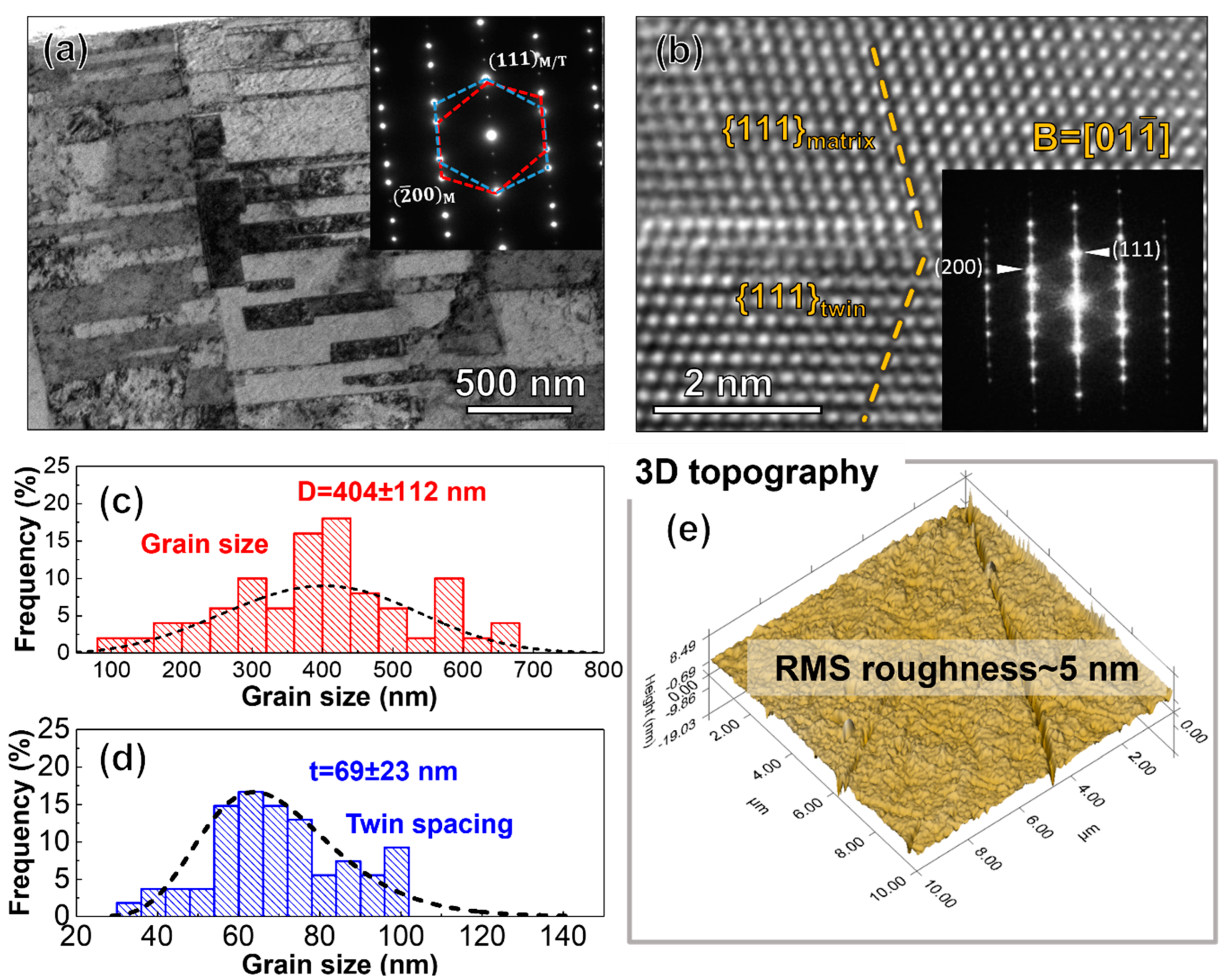
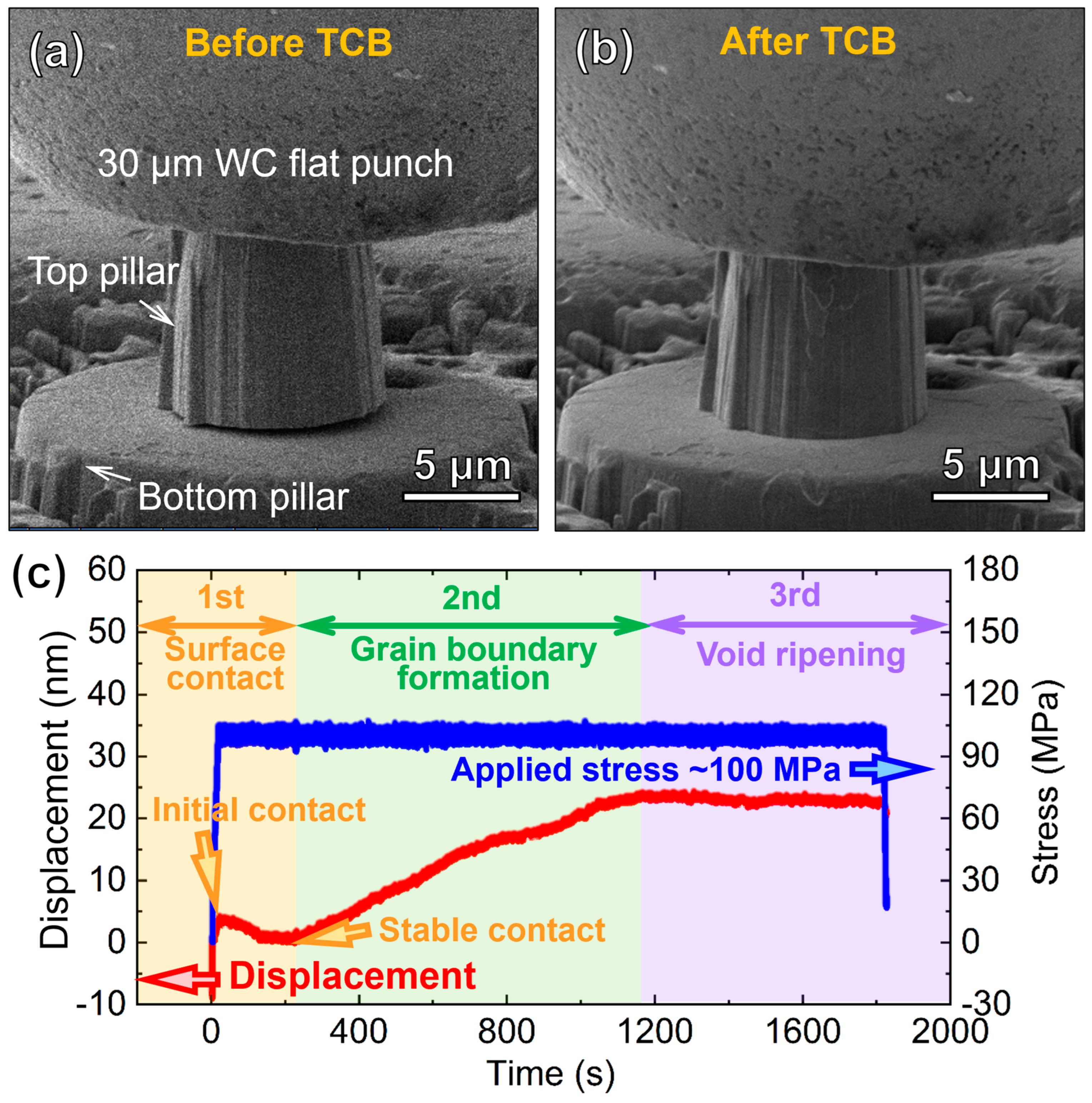
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, K.-N.; Lee, S.H.; Andry, P.S.; Tsang, C.K.; Topol, A.W.; Lin, Y.-M.; Lu, J.-Q.; Young, A.M.; Ieong, M.; Haensch, W. Structure, design and process control for Cu bonded interconnects in 3D integrated circuits. In Proceedings of the 2006 International Electron Devices Meeting, San Francisco, CA, USA, 11–13 December 2006; pp. 1–4. [Google Scholar]
- Topol, A.W.; La Tulipe, D.C.; Shi, L.; Frank, D.J.; Bernstein, K.; Steen, S.E.; Kumar, A.; Singco, G.U.; Young, A.M.; Guarini, K.W. Three-dimensional integrated circuits. IBM J. Res. Dev. 2006, 50, 491–506. [Google Scholar] [CrossRef]
- Di Cioccio, L.; Radu, I.; Gueguen, P.; Sadaka, M. Direct bonding for wafer level 3D integration. In Proceedings of the 2010 IEEE International Conference on Integrated Circuit Design and Technology, Grenoble, France, 2–4 June 2010; pp. 110–113. [Google Scholar]
- Enquist, P.; Fountain, G.; Petteway, C.; Hollingsworth, A.; Grady, H. Low cost of ownership scalable copper direct bond interconnect 3D IC technology for three dimensional integrated circuit applications. In Proceedings of the 2009 IEEE International Conference on 3D System Integration, San Francisco, CA, USA, 28–30 September 2009; pp. 1–6. [Google Scholar]
- Lu, J.-Q.; Jindal, A.; Kwon, Y.; McMahon, J.J.; Rasco, M.; Augur, R.; Cale, T.S.; Gutmann, R.J. Evaluation procedures for wafer bonding and thinning of interconnect test structures for 3D ICs. In Proceedings of the IEEE 2003 International Interconnect Technology Conference (Cat. No. 03TH8695), Burlingame, CA, USA, 4 June 2003; pp. 74–76. [Google Scholar]
- Niklaus, F.; Kumar, R.J.; McMahon, J.J.; Yu, J.; Lu, J.-Q.; Cale, T.S.; Gutmann, R.J. Adhesive wafer bonding using partially cured benzocyclobutene for three-dimensional integration. J. Electrochem. Soc. 2006, 153, G291. [Google Scholar] [CrossRef]
- Made, R.I.; Gan, C.L.; Yan, L.; Kor, K.H.B.; Chia, H.L.; Pey, K.L.; Thompson, C.V. Experimental characterization and modeling of the mechanical properties of Cu–Cu thermocompression bonds for three-dimensional integrated circuits. Acta Mater. 2012, 60, 578–587. [Google Scholar] [CrossRef]
- Gao, G.; Mirkarimi, L.; Workman, T.; Fountain, G.; Theil, J.; Guevara, G.; Liu, P.; Lee, B.; Mrozek, P.; Huynh, M. Low temperature Cu interconnect with chip to wafer hybrid bonding. In Proceedings of the 2019 IEEE 69th Electronic Components and Technology Conference (ECTC), Las Vegas, NV, USA, 28–31 May 2019; pp. 628–635. [Google Scholar]
- Save, D.; Braud, F.; Torres, J.; Binder, F.; Müller, C.; Weidner, J.O.; Hasse, W. Electromigration resistance of copper interconnects. Microelectron. Eng. 1997, 33, 75–84. [Google Scholar] [CrossRef]
- Liu, C.-M.; Lin, H.-W.; Huang, Y.-S.; Chu, Y.-C.; Chen, C.; Lyu, D.-R.; Chen, K.-N.; Tu, K.-N. Low-temperature direct copper-to-copper bonding enabled by creep on (111) surfaces of nanotwinned Cu. Sci. Rep. 2015, 5, 9734. [Google Scholar] [CrossRef] [Green Version]
- Panigrahy, A.K.; Chen, K.-N. Low temperature Cu–Cu bonding technology in three-dimensional integration: An extensive review. J. Electron. Packag. 2018, 140, 010801. [Google Scholar] [CrossRef] [Green Version]
- Juang, J.-Y.; Lu, C.-L.; Chen, K.-J.; Chen, C.-C.A.; Hsu, P.-N.; Chen, C.; Tu, K.-N. Copper-to-copper direct bonding on highly (111)-oriented nanotwinned copper in no-vacuum ambient. Sci. Rep. 2018, 8, 13910. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.S.; Reif, R. Silicon multilayer stacking based on copper wafer bonding. Electrochem. Solid-State Lett. 2005, 8, G147. [Google Scholar] [CrossRef]
- Park, H.; Kim, S.E. Two-step plasma treatment on copper surface for low-temperature Cu thermo-compression bonding. IEEE Trans. Compon. Packag. Manuf Technol. 2019, 10, 332–338. [Google Scholar] [CrossRef]
- Tseng, C.-H.; Tu, K.-N.; Chen, C. Comparison of oxidation in uni-directionally and randomly oriented Cu films for low temperature Cu-to-Cu direct bonding. Sci. Rep. 2018, 8, 10671. [Google Scholar] [CrossRef] [Green Version]
- Moriceau, H.; Rieutord, F.; Fournel, F.; Di Cioccio, L.; Moulet, C.; Libralesso, L.; Gueguen, P.; Taibi, R.; Deguet, C. Low temperature direct bonding: An attractive technique for heterostructures build-up. Microelectron. Reliab. 2012, 52, 331–341. [Google Scholar] [CrossRef]
- Park, M.; Baek, S.; Kim, S.; Kim, S.E. Argon plasma treatment on Cu surface for Cu bonding in 3D integration and their characteristics. Appl. Surf. Sci. 2015, 324, 168–173. [Google Scholar] [CrossRef]
- Takagi, H.; Kikuchi, K.; Maeda, R.; Chung, T.R.; Suga, T. Surface activated bonding of silicon wafers at room temperature. Appl. Phys. Lett. 1996, 68, 2222–2224. [Google Scholar] [CrossRef]
- Tan, C.S.; Lim, D.F.; Singh, S.G.; Goulet, S.K.; Bergkvist, M. Cu–Cu diffusion bonding enhancement at low temperature by surface passivation using self-assembled monolayer of alkane-thiol. Appl. Phys. Lett. 2009, 95, 192108. [Google Scholar] [CrossRef]
- Ghosh, T.; Krushnamurthy, K.; Panigrahi, A.K.; Dutta, A.; Subrahmanyam, C.; Vanjari, S.R.K.; Singh, S.G. Facile non thermal plasma based desorption of self assembled monolayers for achieving low temperature and low pressure Cu–Cu thermo-compression bonding. RSC Adv. 2015, 5, 103643–103648. [Google Scholar] [CrossRef]
- Tan, C.S.; Lim, D.F.; Ang, X.F.; Wei, J.; Leong, K.C. Low temperature CuCu thermo-compression bonding with temporary passivation of self-assembled monolayer and its bond strength enhancement. Microelectron. Reliab. 2012, 52, 321–324. [Google Scholar] [CrossRef]
- Huang, Y.-P.; Chien, Y.-S.; Tzeng, R.-N.; Shy, M.-S.; Lin, T.-H.; Chen, K.-H.; Chiu, C.-T.; Chiou, J.-C.; Chuang, C.-T.; Hwang, W. Novel Cu-to-Cu Bonding With Ti Passivation at 180 °C in 3-D Integration. IEEE Electron Device Lett. 2013, 34, 1551–1553. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Hsu, S.-Y.; Chen, K.-N. Electrical performance and reliability investigation of cosputtered Cu/Ti bonded interconnects. IEEE Trans. Electron Devices 2013, 60, 3521–3526. [Google Scholar] [CrossRef]
- Panigrahi, A.K.; Bonam, S.; Ghosh, T.; Singh, S.G.; Vanjari, S.R.K. Ultra-thin Ti passivation mediated breakthrough in high quality Cu-Cu bonding at low temperature and pressure. Mater. Lett. 2016, 169, 269–272. [Google Scholar] [CrossRef]
- Bonam, S.; Panigrahi, A.K.; Kumar, C.H.; Vanjari, S.R.K.; Singh, S.G. Interface and reliability analysis of Au-passivated Cu–Cu fine-pitch thermocompression bonding for 3-D IC applications. IEEE Trans. Compon. Packag. Manuf. Technol. 2019, 9, 1227–1234. [Google Scholar] [CrossRef]
- Lin, T.; Li, C.; Si, X.; Li, X.; Yang, B.; Feng, J.; Cao, J. An investigation on diffusion bonding of Cu/Cu using various grain size of Ni interlayers at low temperature. Materialia 2020, 14, 100882. [Google Scholar] [CrossRef]
- Panigrahi, A.K.; Kumar, C.H.; Bonam, S.; Brince, P.K.; Ghosh, T.; Paul, N.; Vanjari, S.R.K.; Singh, S.G. Metal-alloy Cu surface passivation leads to high quality fine-pitch bump-less Cu-Cu bonding for 3D IC and heterogeneous integration applications. In Proceedings of the 2018 IEEE 68th Electronic Components and Technology Conference (ECTC), San Diego, CA, USA, 29 May–1 June 2018; pp. 1561–1566. [Google Scholar]
- Al Farisi, M.S.; Hirano, H.; Tanaka, S. Low-temperature hermetic thermo-compression bonding using electroplated copper sealing frame planarized by fly-cutting for wafer-level MEMS packaging. Sens. Actuators A Phys. 2018, 279, 671–679. [Google Scholar] [CrossRef]
- Shie, K.-C.; Gusak, A.M.; Tu, K.-N.; Chen, C. A kinetic model of copper-to-copper direct bonding under thermal compression. J. Mater. Res. Technol. 2021, 15, 2332–2344. [Google Scholar] [CrossRef]
- Agrawal, P.M.; Rice, B.M.; Thompson, D.L. Predicting trends in rate parameters for self-diffusion on FCC metal surfaces. Surf. Sci. 2002, 515, 21–35. [Google Scholar] [CrossRef]
- Juang, J.-Y.; Lu, C.-L.; Li, Y.-J.; Tu, K.-N.; Chen, C. Correlation between the microstructures of bonding interfaces and the shear strength of Cu-to-Cu joints using (111)-oriented and nanotwinned Cu. Materials 2018, 11, 2368. [Google Scholar] [CrossRef] [Green Version]
- Rebhan, B.; Hingerl, K. Physical mechanisms of copper-copper wafer bonding. J. Appl. Phys. 2015, 118, 135301. [Google Scholar] [CrossRef]
- Fan, A.; Rahman, A.; Reif, R. Copper wafer bonding. Electrochem. Solid-State Lett. 1999, 2, 534. [Google Scholar] [CrossRef]
- Di Cioccio, L.; Gueguen, P.; Taibi, R.; Landru, D.; Gaudin, G.; Chappaz, C.; Rieutord, F.; de Crecy, F.; Radu, I.; Chapelon, L.L. An overview of patterned metal/dielectric surface bonding: Mechanism, alignment and characterization. J. Electrochem. Soc. 2011, 158, P81. [Google Scholar] [CrossRef] [Green Version]
- Rebhan, B.; Svoboda, J.; Panholzer, M. A thermodynamic study of voiding phenomena in Cu–Cu thermo-compression wafer bonding. Microsyst. Technol. 2018, 24, 815–822. [Google Scholar] [CrossRef]
- Sun, T.; Cho, J.; Shang, Z.; Niu, T.; Ding, J.; Wang, J.; Wang, H.; Zhang, X. Deformation mechanism in nanolaminate FeCrAl alloys by in situ micromechanical strain rate jump tests at elevated temperatures. Scr. Mater. 2022, 215, 114698. [Google Scholar] [CrossRef]
- Sun, T.; Shang, Z.; Cho, J.; Ding, J.; Niu, T.; Zhang, Y.; Yang, B.; Xie, D.; Wang, J.; Wang, H. Ultra-fine-grained and gradient FeCrAl alloys with outstanding work hardening capability. Acta Mater. 2021, 215, 117049. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Li, Q.; Xue, S.C.; Ding, J.; Xie, D.Y.; Li, J.; Niu, T.; Wang, H.; Wang, H.; Wang, J. Ultra-strong nanotwinned Al–Ni solid solution alloys with significant plasticity. Nanoscale 2018, 10, 22025–22034. [Google Scholar] [CrossRef] [PubMed]
- Niu, T.; Li, J.; Zhang, Y.; Cho, J.; Ding, J.; Su, R.; Xue, S.; Fan, C.; Shang, Z.; Chen, D.; et al. In-situ studies on the mechanical properties of He ion irradiated nanotwinned Ag. J. Nucl. Mater. 2020, 540, 152392. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Li, Q.; Gong, M.; Xue, S.; Ding, J.; Li, J.; Cho, J.; Niu, T.; Su, R.; Richter, N.A.; et al. Deformation behavior and phase transformation of nanotwinned Al/Ti multilayers. Appl. Surf. Sci. 2020, 527, 146776. [Google Scholar] [CrossRef]
- Chen, K.-N.; Fan, A.; Reif, R. Interfacial morphologies and possible mechanisms of copper wafer bonding. J. Mater. Sci. 2002, 37, 3441–3446. [Google Scholar] [CrossRef]
- Derby, B.; Wallach, E.R. Theoretical model for diffusion bonding. Met. Sci. 1982, 16, 49–56. [Google Scholar] [CrossRef]
- Shie, K.C.; Juang, J.-Y.; Chen, C. Instant Cu-to-Cu direct bonding enabled by <111>-oriented nanotwinned Cu bumps. Jpn. J. Appl. Phys. 2019, 59, SBBA03. [Google Scholar]
- Chang, L.-P.; Wang, J.-J.; Hung, T.-H.; Chen, K.-N.; Ouyang, F.-Y. Direct metal bonding using nanotwinned Ag films with (111) surface orientation under air atmosphere for heterogeneous integration. Appl. Surf. Sci. 2022, 576, 151845. [Google Scholar] [CrossRef]
- Wu, Y.S.; Lai, T.-Y.; Li, M.; Lu, T.-F.; Wang, Y.H.; Tseng, T.Y. Bonding mechanisms of roughened nanotwinned-Cu surface at temperature as low as 120 °C. ECS J. Solid State Sci. Technol. 2020, 9, 124005. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, T.; Xu, K.; Shen, C.; Sun, T.; Oberst, J.; Handwerker, C.A.; Subbarayan, G.; Wang, H.; Zhang, X. In Situ Study on Cu-to-Cu Thermal Compression Bonding. Crystals 2023, 13, 989. https://doi.org/10.3390/cryst13070989
Niu T, Xu K, Shen C, Sun T, Oberst J, Handwerker CA, Subbarayan G, Wang H, Zhang X. In Situ Study on Cu-to-Cu Thermal Compression Bonding. Crystals. 2023; 13(7):989. https://doi.org/10.3390/cryst13070989
Chicago/Turabian StyleNiu, Tongjun, Ke Xu, Chao Shen, Tianyi Sun, Justin Oberst, Carol A. Handwerker, Ganesh Subbarayan, Haiyan Wang, and Xinghang Zhang. 2023. "In Situ Study on Cu-to-Cu Thermal Compression Bonding" Crystals 13, no. 7: 989. https://doi.org/10.3390/cryst13070989




