Room Temperature Corrosion Behavior of Selective Laser Melting (SLM)-Processed Ni-Fe Superalloy (Inconel 718) in 3.5% NaCl Solution at Different pH Conditions: Role of Microstructures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Heat Treatment
2.3. Microstructural Characterization
2.4. Electrochemical Tests
3. Results
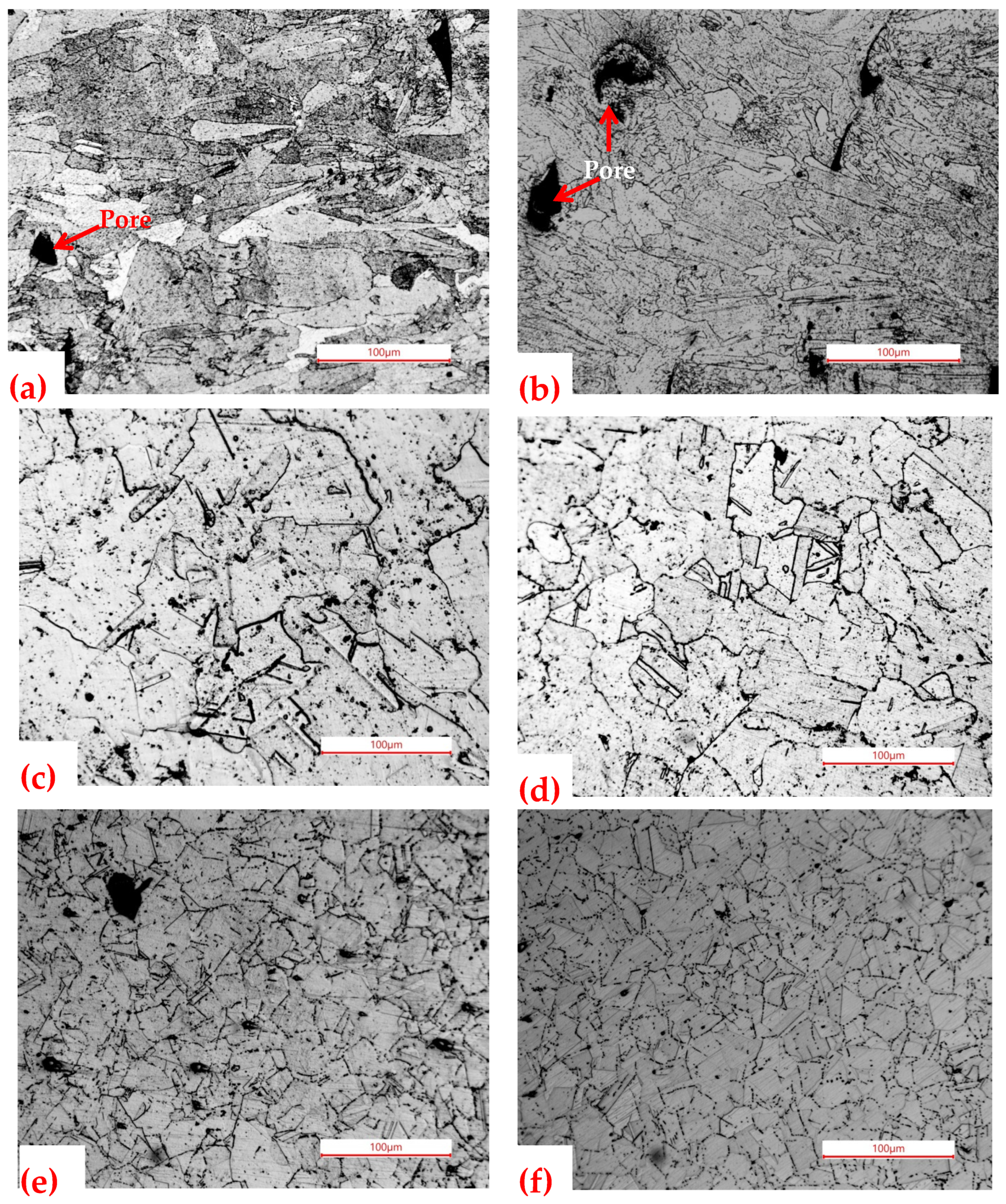
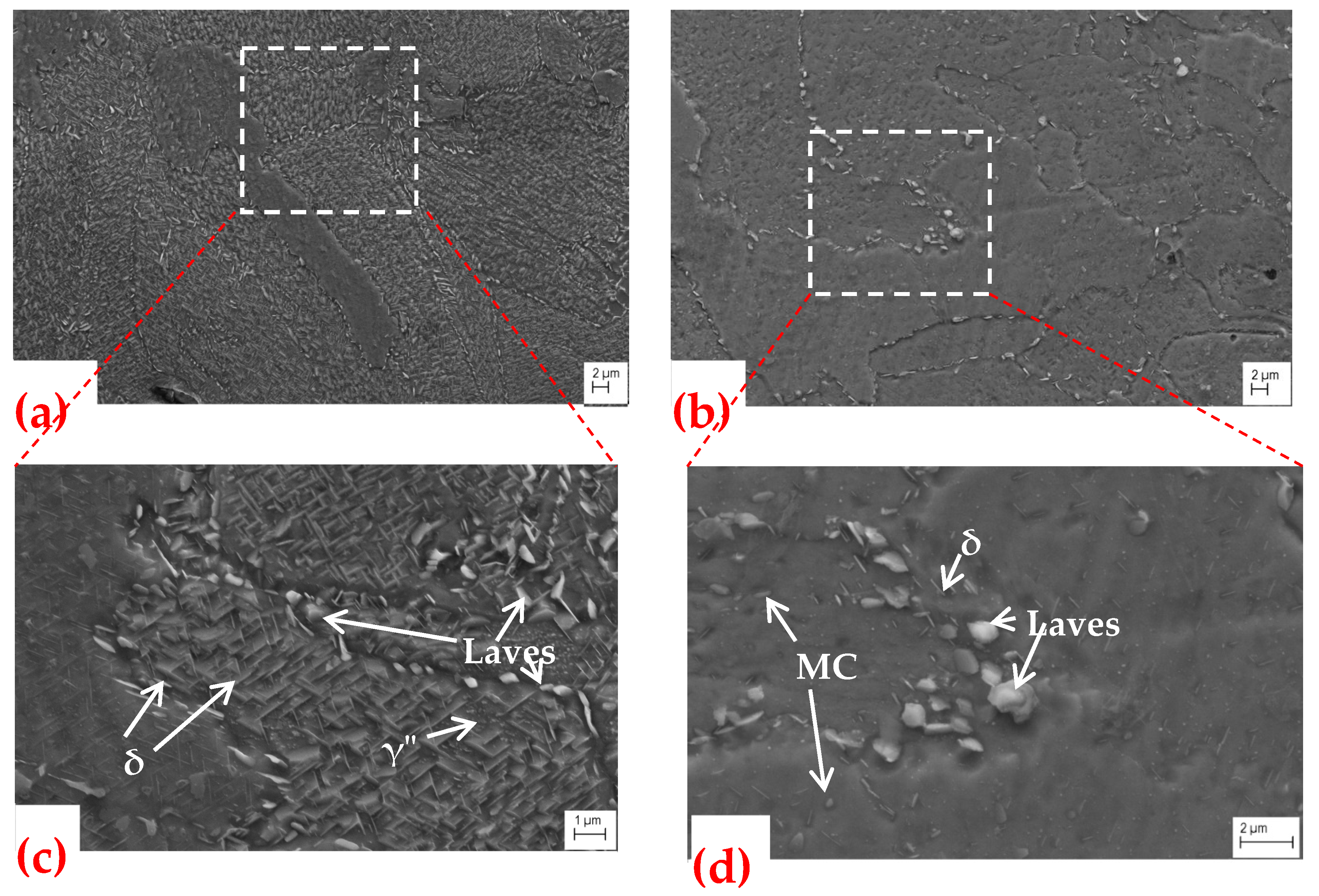
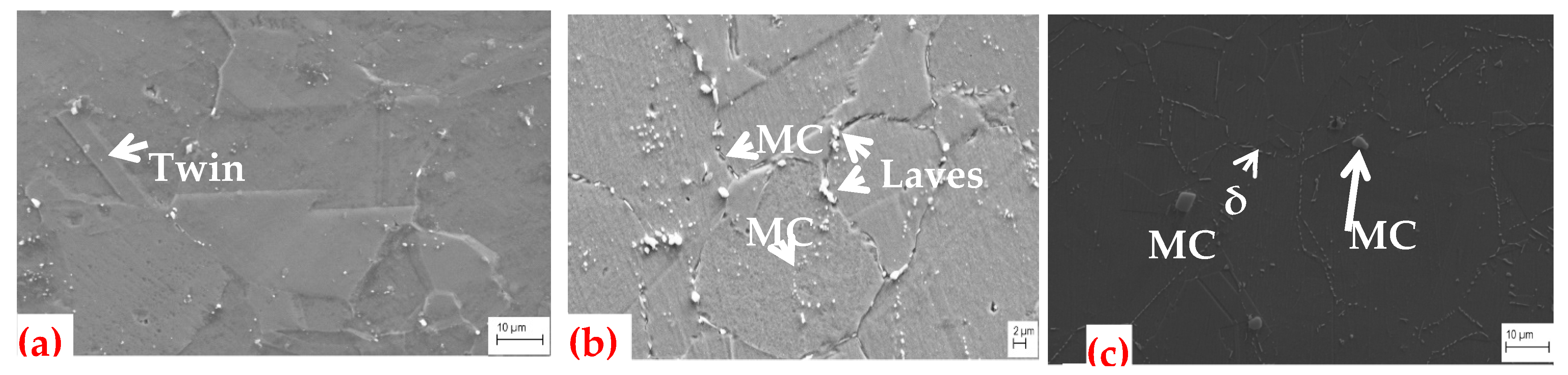
3.1. Microstructure
3.1.1. XRD Results
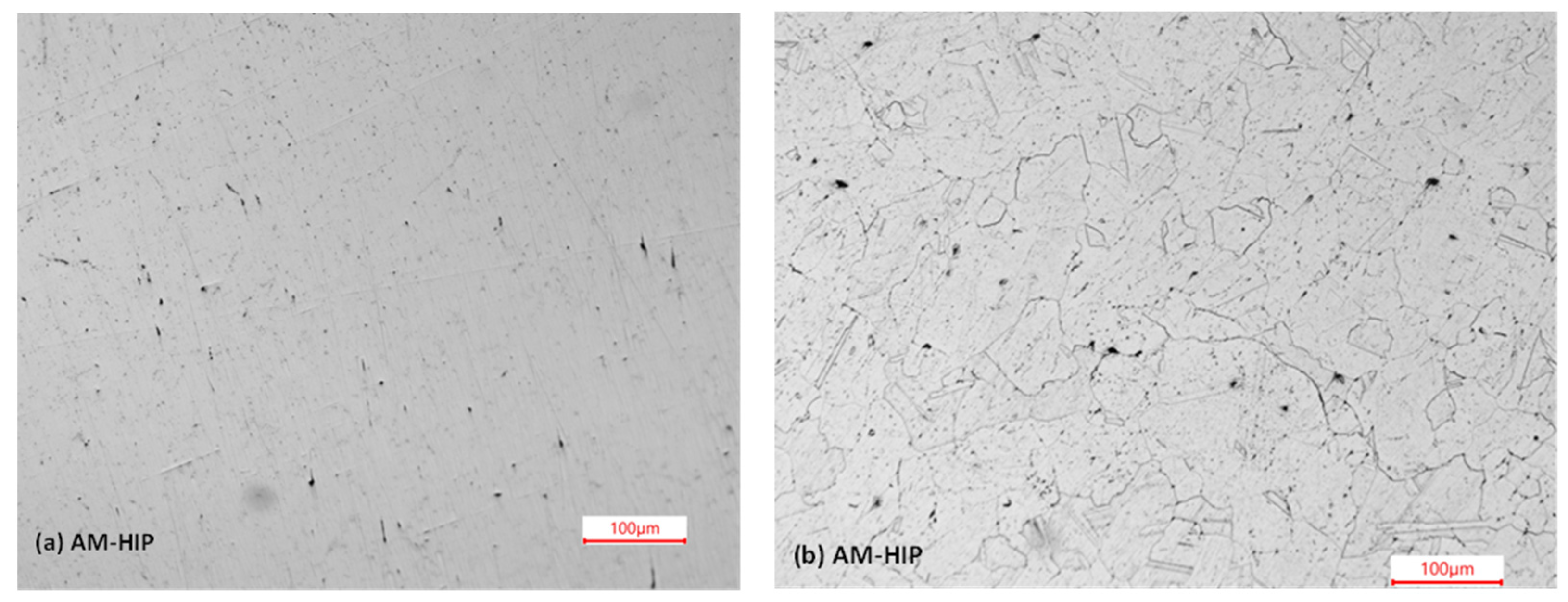
3.1.2. Optical Microstructure
3.1.3. SEM Microstructures
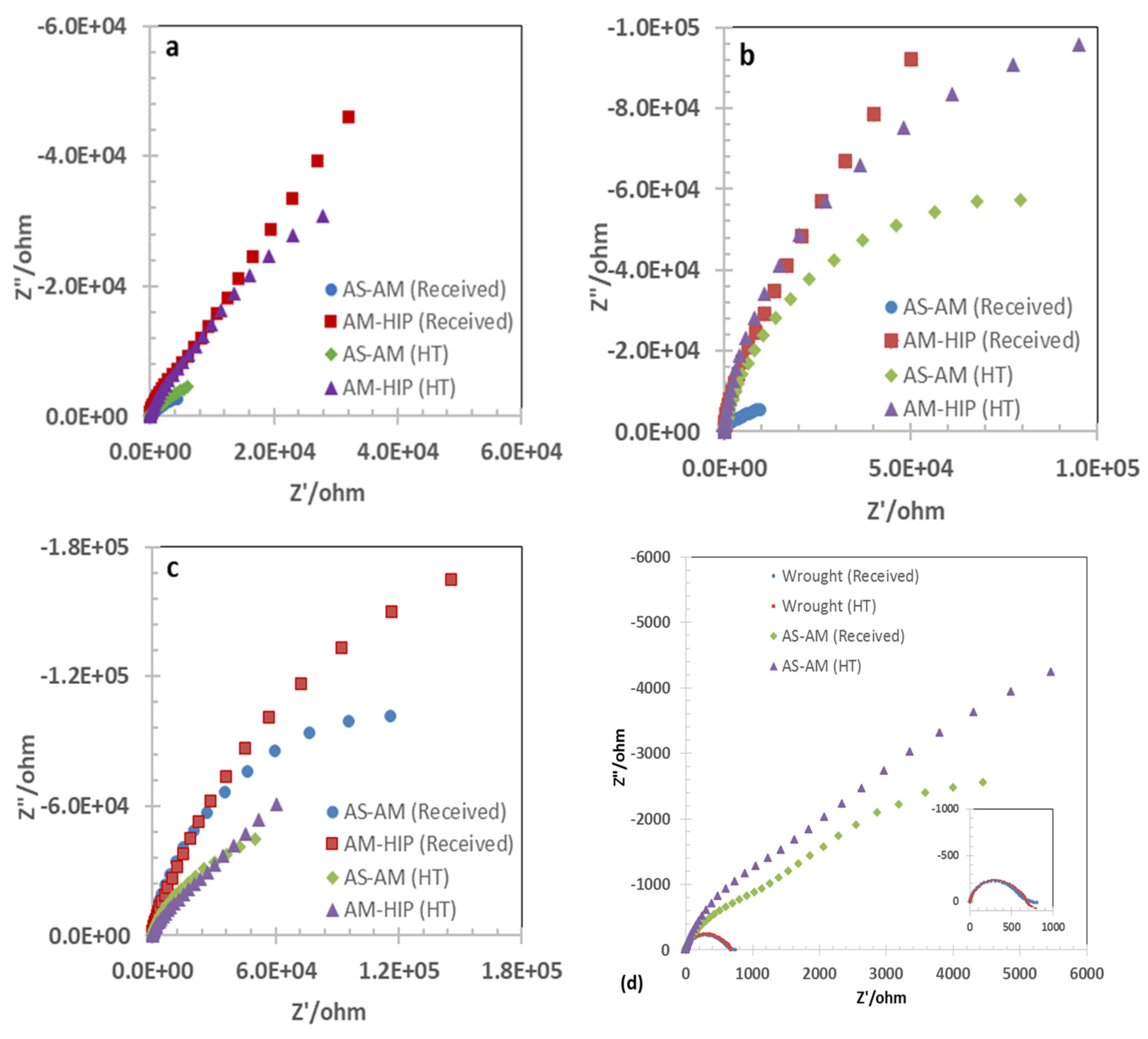
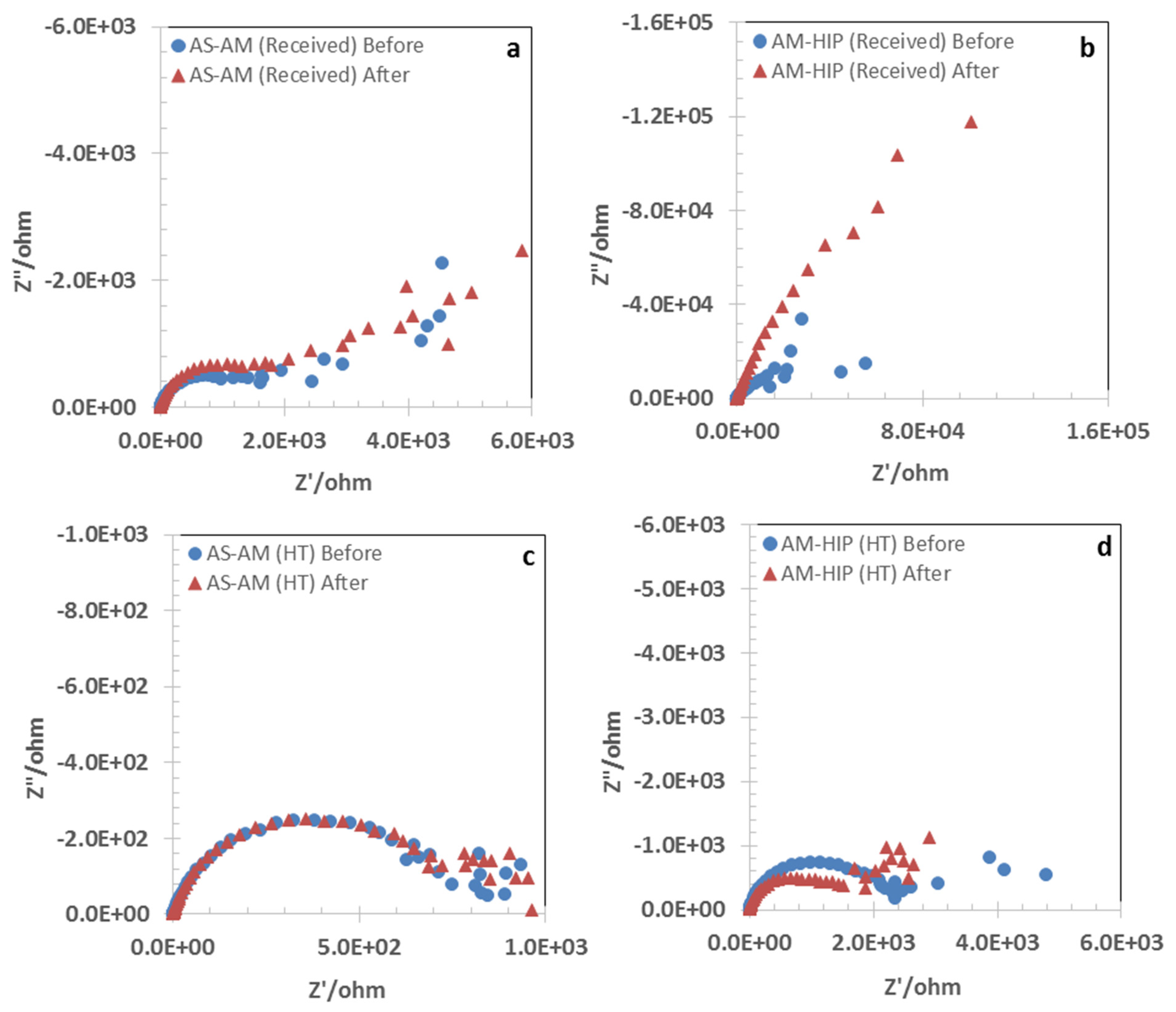
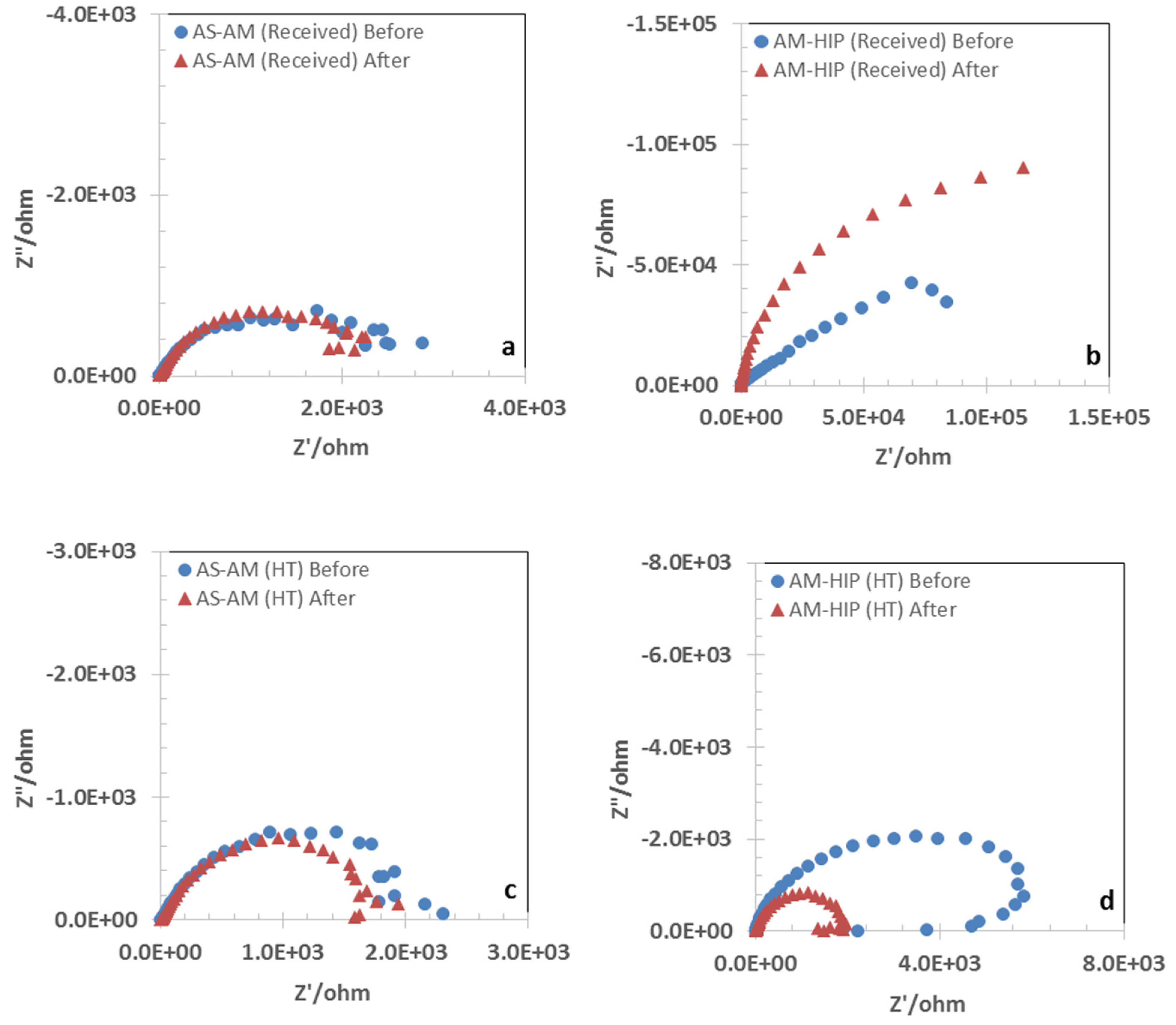
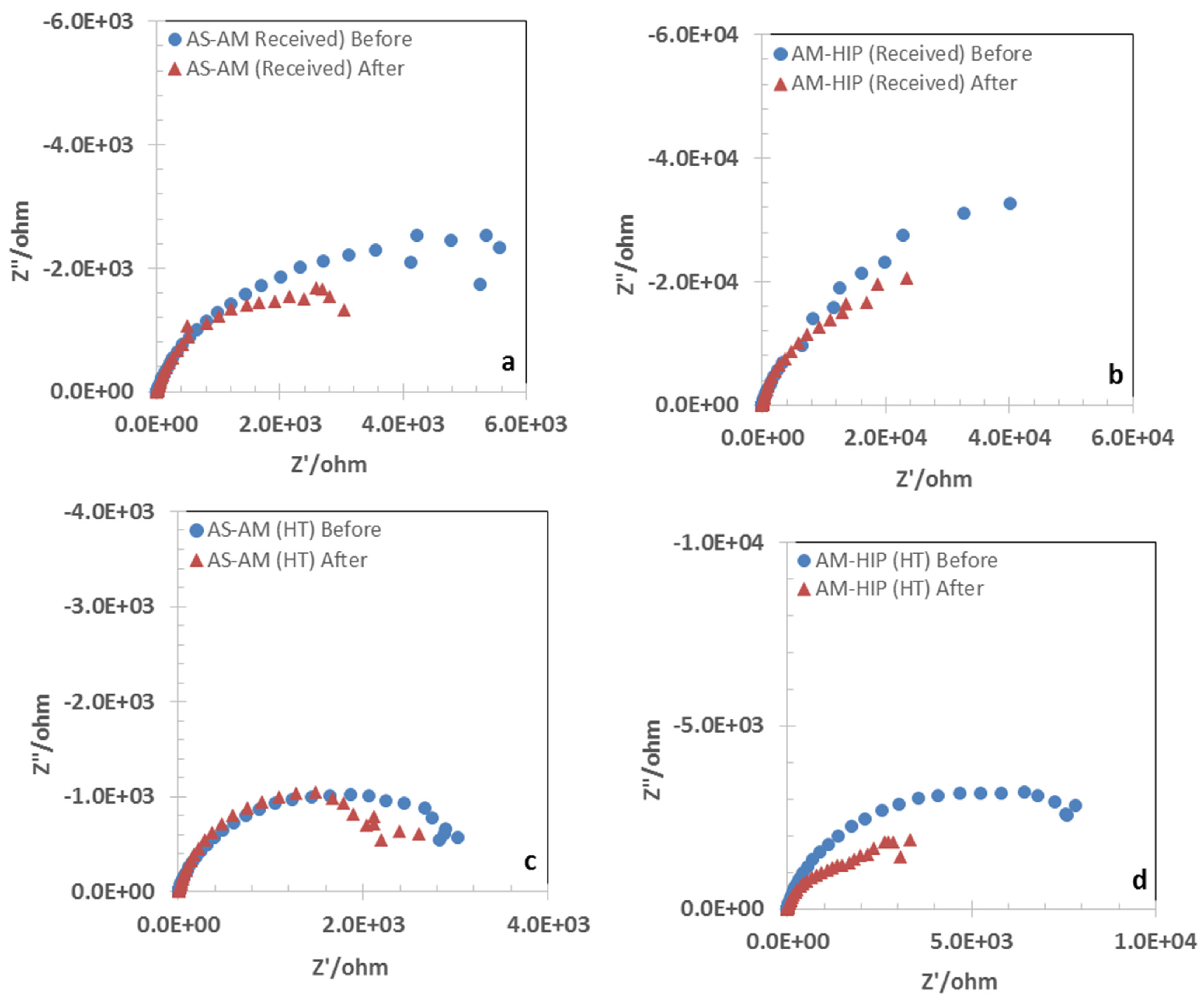
3.2. Open Circuit Potential and Impedance
3.3. Cyclic Polarization
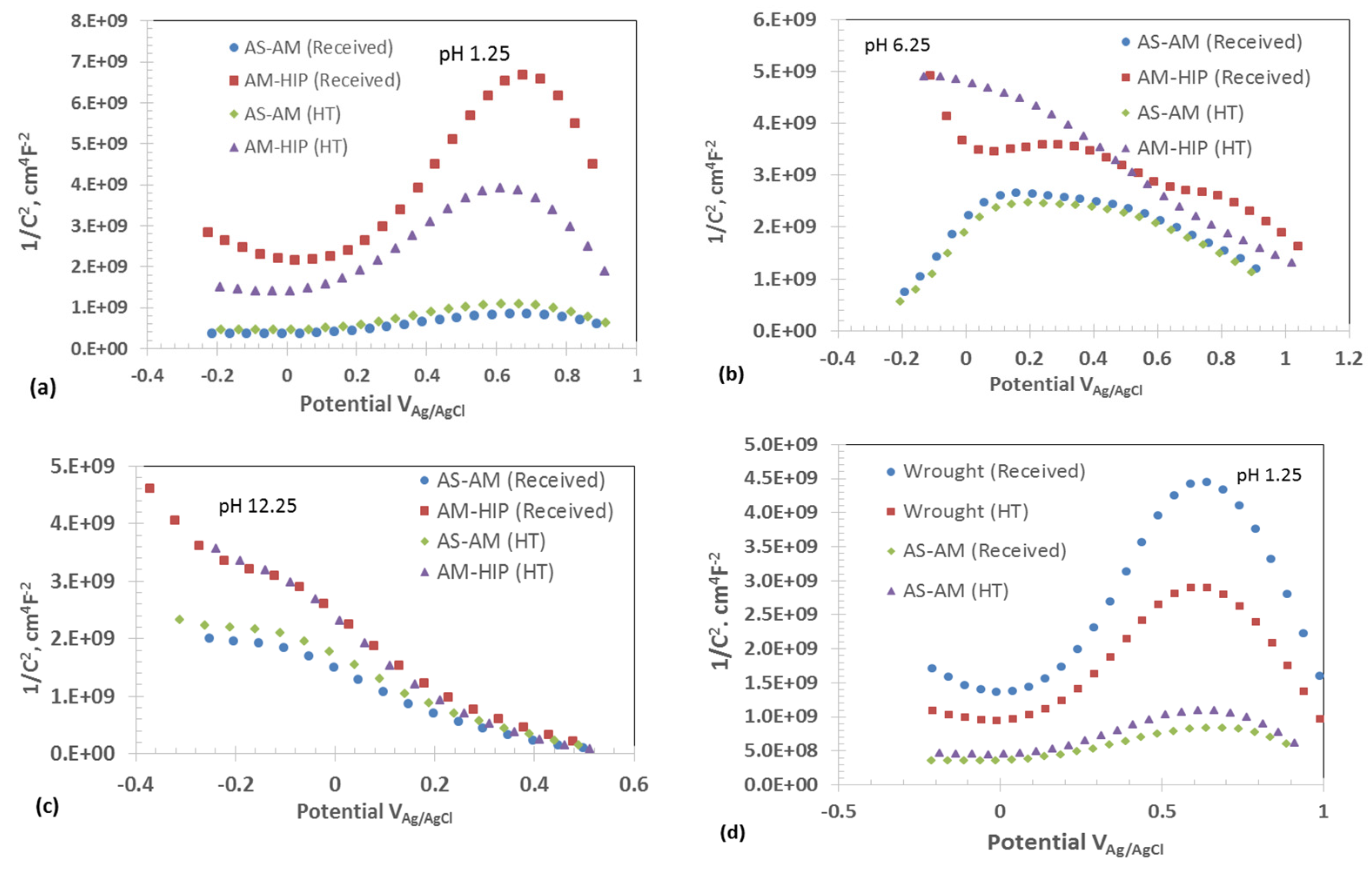
3.4. Potentiostatic Conditioning
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sims, C.T.; Hagel, W.C. The Superalloys; John Wiley & Sons: Hoboken, NJ, USA, 1972; pp. 113–143. [Google Scholar]
- Luo, S.; Huang, W.; Yang, H.; Yang, J.; Wang, Z.; Zeng, X. Microstructural evolution and corrosion behaviors of Inconel 718 alloy produced by selective laser melting following different heat treatments. Addit. Manuf. 2019, 30, 100875. [Google Scholar]
- Sahraeian, R.; Omidvar, H.; Hadavi, S.M.M.; Shakerin, S.; Maleki, V. An Investigation on high-temperature oxidation and hot corrosion resistance behavior of coated TLP (Transient Liquid Phase)-bonded IN738-LC. Trans. Indian Inst. Met. 2018, 71, 2903–2918. [Google Scholar] [CrossRef]
- Cozar, R.; Pineau, A. Morphology of γ′ and γ″ precipitates and thermal stability of inconel 718 type alloys. Metall. Trans. 1973, 4, 47–59. [Google Scholar] [CrossRef]
- Thomas, A.; El-Wahabi, M.; Cabrera, J.M.; Prado, J.M. High temperature deformation of Inconel 718. J. Mater. Process. Technol. 2006, 177, 469–472. [Google Scholar]
- Janaki Ram, G.D.; Venugopal Reddy, A.; Prasad Rao, K.; Madhusudhan Reddy, G. Microstructure and mechanical properties of Inconel 718 electron beam welds. Mater. Sci. Technol. 2005, 21, 1132–1138. [Google Scholar]
- Gao, M.; Wei, R.P. Grain boundary niobium carbides in Inconel 718. Scr. Mater. 2006, 37, 1843–1849. [Google Scholar]
- INCONEL®alloy 718 (UNS N07718/W.Nr. 2.4668), Technical Brochure, Special Metals. Available online: www.specialmetals.com (accessed on 14 November 2020).
- API SPEC 6A718-2004; Specification of Nickel Base Alloy 718 (UNS N07718) for Oil and Gas Drilling and Production Equipment. American Petroleum Institute: Washington, DC, USA, 2004.
- Valle, L.C.M.; Santana, A.I.C.; Rezende, M.C.; Dille, J.; Mattos, O.R.; de Almeida, L.H. The influence of heat treatments on the corrosion behavior of nickel-based alloy 718. J. Alloys Compd. 2019, 809, 151781. [Google Scholar] [CrossRef]
- Chen, T.; John, H.; Xu, J.; Lu, Q.; Hawk, J.; Liu, X. Influence of surface modifications on pitting corrosion behavior of nickel-base alloy 718. Part 1: Effect of machine hammer peening. Corros. Sci. 2013, 77, 230–245. [Google Scholar] [CrossRef]
- Wei, X.; Zheng, W.J. Effect of carbides on sensitivity of pitting corrosion in Inconel 718 alloy. Anti-Corros. Methods Mater. 2020, 67, 158–165. [Google Scholar] [CrossRef]
- Schirra, J.J.; Caless, R.H.; Hatala, R.W. The effect of Laves phase on the mechanical properties of wrought and cast + HIP Inconel 718. In Superalloys 718,625 and Various Derivatives; Edward, J.L., Ed.; The Minerals, Metals & Materials Society: Warrendale, PA, USA, 1991; pp. 375–388. [Google Scholar]
- Radhakrishna, C.; Rao, K.P. The formation and control of Laves phase in superalloy 718 welds. J. Mater. Sci. 1997, 32, 1977–1984. [Google Scholar] [CrossRef]
- Kruk, A.; Cempura, G. Decomposition of the Laves phase in the fusion zone of the Inconel 718/ATI 718Plus® welded joint during isothermal holding at a temperature of 649 °C. Mater. Charact. 2023, 196, 112560. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Lin, X.; Yang, H.; Huang, W. The formation and dissolution mechanisms of Laves phase in Inconel 718 fabricated by selective laser melting compared to directed energy deposition and cast. Compos. Part B 2022, 239, 109994. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, X.; Wang, L.; Yu, X.; Huc, Y.; Yang, H.; Lei, L.; Huang, W. Strengthening mechanisms in selective laser-melted Inconel718 superalloy. Mater. Sci. Eng. A 2021, 812, 141145. [Google Scholar] [CrossRef]
- Ghaemifar, S.; Mirzadeh, H. Dissolution kinetics of Laves phase during homogenization heat treatment of additively manufactured Inconel 718 superalloy. J. Mater. Res. Technol. 2023, 24, 3491–3501. [Google Scholar]
- Sander, G.; Tan, J.; Balan, P.; Gharbi, O.; Feenstra, D.R.; Singer, L.; Thomas, S.; Kelly, R.G.; Scully, J.R.; Birbilis, N. Corrosion of Additively Manufactured Alloys: A Review. Corrosion 2018, 74, 1318–1350. [Google Scholar] [CrossRef] [PubMed]
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive manufacturing of metals. Acta Mater. 2016, 117, 371–392. [Google Scholar] [CrossRef]
- Guo, P.; Lin, X.; Xu, J.; Li, J.; Liu, J.; Huang, W. Electrochemical Removal of Different Phases from Laser Solid Formed Inconel 718. J. Electrochem. Soc. 2017, 164, E151–E157. [Google Scholar] [CrossRef]
- Guo, P.; Lin, X.; Li, J.; Zhang, Y.; Song, M.; Huang, W. Electrochemical behavior of Inconel 718 fabricated by laser solid forming on different sections. Corros. Sci. 2018, 132, 79–89. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, H.; Yang, Y. Effects of Solution Heat Treatment on Laves Phase and Corrosion Behaviors in Selective Laser Melted Inconel 718 Alloy. IOP Conf. Ser. Mater. Sci. Eng. 2019, 677, 022081. [Google Scholar] [CrossRef]
- Du, D.; Dong, A.; Shu, D.; Zhu, G.G.; Sun, B.; Li, X.; Lavernia, E. Influence of build orientation on microstructure, mechanical and corrosion behavior of Inconel 718 processed by selective laser melting. Mater. Sci. Eng. A 2019, 760, 469–480. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Z.Y.; Bai, P.K.; Qu, H.Q.; Liu, B.; Li, L.; Wu, L.Y.; Guan, R.G.; Liu, H.; Guo, Z.H. Microstructural evolution and mechanical properties of IN718 alloy fabricated by selective laser melting following different heat treatments. J. Alloys Compd. 2019, 772, 861–870. [Google Scholar]
- Siddaiah, A.; Kasar, A.; Kumar, P.; Akram, J.; Misra, M.; Menezes, P.L. Tribocorrosion Behavior of Inconel 718 Fabricated by Laser Powder Bed Fusion-Based Additive Manufacturing. Coatings 2021, 11, 195. [Google Scholar]
- Diljith, P.K.; Jinoop, A.N.; Paul, C.P.; Krishna, P.; Bontha, S.; Bindra, K.S. Understanding the corrosion behaviour of laser directed energy deposition-based additive manufacturing built Inconel 718 under acidic environments. Prog. Addit. Manuf. 2021, 6, 395–406. [Google Scholar]
- Tang, Y.; Shen, X.; Liu, Z.; Xiao, Y.; Yang, L.; Lu, D.; Zhou, J.; Xu, J. Corrosion Behaviors of Selective Laser Melted Inconel 718 Alloy in NaOH Solution. Acta Metall. Sin. 2022, 58, 324–333. [Google Scholar]
- Zhang, Y.; Yua, J.; Lin, X.; Guo, P.; Yan, Q.; Liu, J.; Huang, W. Synergistic effect of transpassive film and gamma double prime phase on the electrochemical dissolution behavior of Inconel 718 manufactured by laser directed energy deposition. Mater. Charact. 2023, 204, 113207. [Google Scholar] [CrossRef]
- Zhang, Y.; Yua, J.; Lin, X.; Guo, P.; Yan, Q.; Liu, J.; Huang, W. Passive behavior of laser directed energy deposited Inconel 718 after homogenization and aging heat treatment. Corros. Sci. 2022, 205, 110439. [Google Scholar] [CrossRef]
- Koutiri, I.; Pessard, E.; Peyre, P.; Amlou, O.; De Terris, T. Influence of SLM process parameters on the surface finish, porosity rate and fatigue behavior of as-built Inconel 625 parts. J. Mater. Process. Technol. 2018, 255, 536–546. [Google Scholar] [CrossRef]
- Dilip, J.J.S.; Zhang, S.; Teng, C.; Zeng, K.; Robinson, C.; Pal, D.; Stucker, B. Influence of processing parameters on the evolution of melt pool, porosity, and microstructures in Ti-6Al-4V alloy parts fabricated by selective laser melting. Prog. Addit. Manuf. 2017, 2, 157–167. [Google Scholar]
- Kumar, P.; Farah, J.; Akram, J.; Teng, C.; Ginn, J.; Misra, M. Influence of laser processing parameters on porosity in Inconel 718 during additive manufacturing. Int. J. Adv. Manuf. Technol. 2019, 103, 1497–1507. [Google Scholar]
- Sander, G.; Thomas, S.; Cruz, V.; Jurg, M.; Birbilis, N.; Gao, X.; Brameld, M.; Hutchinson, C.R. On The Corrosion and Metastable Pitting Characteristics of 316L Stainless Steel Produced by Selective Laser Melting. J. Electrochem. Soc. 2017, 164, C250–C257. [Google Scholar] [CrossRef]
- Koizumi, M. Hot Isostatic Pressing Theory and Applications, International Conference on Hot Isostatic Pressing; Elsevier Applied Science: London, UK; New York, NY, USA, 1992; pp. 11–187. [Google Scholar]
- Lee, S.C.; Chang, S.H.; Tang, T.P.; Ho, H.H.; Chen, J.K. Improvement in the Microstructure and Tensile Properties of Inconel 718 Superalloy by HIP Treatment. Mater. Trans. 2006, 47, 2877–2881. [Google Scholar] [CrossRef]
- Vikram, R.J.; Singh, A.; Suwas, S. Effect of heat treatment on the modification of microstructure of selective laser melted (SLM) IN718 and its consequences on mechanical behavior. J. Mater. Res. 2020, 35, 1949–1962. [Google Scholar]
- Popovich, V.A.; Borisov, E.V.; Popovich, A.A.; Sufiiarov, V.S.; Masaylo, D.V.; Alzina, L. Impact of heat treatment on mechanical behaviour of Inconel 718 processed with tailored microstructure by selective laser melting. Mater. Des. 2017, 131, 12–22. [Google Scholar] [CrossRef]
- Zhang, L.N.; Ojo, O.A. Corrosion behavior of wire arc additive manufactured Inconel 718 superalloy. J. Alloys Compd. 2020, 829, 154455. [Google Scholar] [CrossRef]
- Luo, K.; Roberts, M.R.; Guerrini, N.; Tapia-Ruiz, N.; Hao, R.; Massel, F.; Pickup, D.M.; Ramos, S.; Liu, Y.S.; Guo, J.; et al. Anion redox chemistry in the cobalt free 3d transition metal oxide intercalation electrode Li[Li0.2Ni0.2Mn0.6]O2. J. Am. Chem. Soc. 2016, 138, 11211–11218. [Google Scholar] [PubMed]
- Raja, K.S.; Jones, D.A. Effects of dissolved oxygen on passive behavior of stainless alloys. Corros. Sci. 2006, 48, 1623–1638. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Wang, F. Influence of micro-structure on corrosion behavior of a Ni-based superalloy in 3.5% NaCl. Electrochim. Acta 2007, 52, 7193–7202. [Google Scholar] [CrossRef]
- Raja, K.S.; Shoji, T. Semiconducting properties of surface oxide films of pure iron, nickel and chromium metals in pure water at 288 °C. J. Mater. Sci. Lett. 2003, 22, 1347–1349. [Google Scholar] [CrossRef]
- Zhang, B.; Xiu, M.; Tan, Y.T.; Wei, J.; Wang, P. Pitting corrosion of SLM Inconel 718 sample under surface and heat treatments. Appl. Surf. Sci. 2019, 490, 556–567. [Google Scholar]





| Specimen ID | Processing Condition |
|---|---|
| AS-AM or AS-AM (Received) | SLM 3D-printed without further conditioning |
| AS-AM (HT) | 3D-printed and subjected to the solution annealing at 980 °C followed by two-step aging treatment |
| AM-HIP or AM-HIP (Received) | 3D-printed and hot isostatically pressed |
| AM-HIP (HR) | 3D-printed and hot isostatically pressed + heat-treated (solution annealing at 980 °C followed by two-step aging treatment). |
| Wrought (Received) | Traditionally manufactured wrought material in mill annealed condition—as-received |
| Wrought (HT) | Wrought material heat-treated (solution annealing at 980 °C followed by two-step aging treatment) |
| Specimen ID | Vickers Hardness—Range (Average), kgf/mm2 | ASTM Grain Size Number (Average Diameter, μm) | Porosity, vol% |
|---|---|---|---|
| AS-AM (Received) | 360–510 (420) | 9.5 (13.3) | 2.92 ± 0.2 |
| AS-AM (HT) | 419–535 (474) | 9.5 (13.2) | 2.77 ± 0.15 |
| AM-HIP (Received) | 344–454 (408) | 8 (22.5) | 0.67 ± 0.06 |
| AM-HIP (HT) | 483–546 (516) | 7.5 (25.8) | 0.69 ± 0.05 |
| Wrought—Received | 339–409 (370) | 7.5 (26.7) | N.A |
| Wrought (HT) | 518–600 (562) | 7.0 (32.7) | N.A |
| pH | Specimen ID | Linear Polarization Resistance (ohm·cm2) | OCP (VAg/AgCl) | Passivation Current Density, ip (A/cm2) | Transpassive Potential, ETP (VAg/AgCl) | Pitting Protection Potential, EPP (VAg/AgCl) |
|---|---|---|---|---|---|---|
| 1.25 | AS-AM Received | 5427.3 | −0.090 | 2.90 × 10−5 | 0.937 | −0.029 |
| AS-AM (HT) | 7580.3 | −0.159 | 6.67 × 10−5 | 0.962 | 0.082 | |
| AM-HIP Received | 63,682 | −0.093 | 4.52 × 10−6 | 0.925 | 0.047 | |
| AM-HIP (HT) | 47,586 | −0.086 | 5.01 × 10−5 | 0.960 | −0.129 | |
| Wrought Received | 658 | −0.216 | 1.76 × 10−3 | 1.04 | - | |
| Wrought (HT) | 467 | −0.220 | 1.83 × 10−3 | 1.04 | - | |
| 6.25 | AS-AM Received | 281,180 | −0.333 | 3.28 × 10−5 | 0.959 | 0.072 |
| AS-AM (HT) | 91,058 | −0.196 | 5.16 × 10−5 | 0.944 | −0.115 | |
| AM-HIP Received | 222,641 | −0.143 | 3.88 × 10−6 | 1.090 | 1.050 | |
| AM-HIP (HT) | 157,219 | −0.155 | 8.03 × 10−5 | 1.070 | 0.880 | |
| 12.25 | AS-AM Received | 162,270 | −0.329 | 2.02 × 10−5 | 0.547 | - |
| AS-AM (HT) | 55,027 | −0.291 | 8.21 × 10−6 | 0.539 | - | |
| AM-HIP Received | 211,407 | −0.277 | 4.33 × 10−6 | 0.528 | - | |
| AM-HIP (HT) | 93,244 | −0.306 | 6.32 × 10−6 | 0.560 | - |
| pH = 1.25 | n-Type | ||
|---|---|---|---|
| Specimen | Slope of Log(i)–Log(t) Plot | Charge Carrier Density (cm−3) | Flat Band Potential (VAg/AgCl) |
| AS-AM Received | −0.39 | 1.03 × 1022 | −0.23 |
| AS-AM (HT) | −0.16 | 7.35 × 1021 | −0.19 |
| AM-HIP Received | −0.62 | 9.79 × 1020 | 0.02 |
| AM-HIP (HT) | −0.30 | 1.68 × 1021 | −0.07 |
| Wrought (Received) | −0.42 | 1.32 × 1021 | 0.09 |
| Wrought (HT) | −0.94 | 2.21 × 1021 | −0.05 |
| pH = 6.25 | p-Type | n-Type | ||||
|---|---|---|---|---|---|---|
| Specimen | Slope | Charge Carrier Density (cm−3) | Flat Band Potential, (VAg/AgCl) | Slope | Charge Carrier Density (cm−3) | Flat Band Potential (VAg/AgCl) |
| AS-AM Received | −3.01 × 109 | 3.60 × 1021 | 0.32 | 8.18 × 109 | 1.33 × 1021 | −0.27 |
| AS-AM (HT) | −3.10 × 109 | 3.50 × 1021 | 1.28 | 7.87 × 109 | 1.38 × 1021 | −0.25 |
| AM-HIP Received | −3.13 × 109 | 3.47 × 1021 | 1.49 | - | - | - |
| AM-HIP (HT) | −4.52 × 109 | 2.40 × 1021 | 1.19 | - | - | - |
| pH = 12.25 | p-Type | ||
|---|---|---|---|
| Specimen | Slope | Charge Carrier Density (cm−3) | Flat Band Potential |
| AS-AM Received | −4.09 × 109 | 2.65 × 1021 | 0.36 |
| AS-AM (HT) | −4.79 × 109 | 2.27 × 1021 | 0.36 |
| AM-HIP Received | −7.24 × 109 | 1.50 × 1021 | 0.34 |
| AM-HIP (HT) | −1.16 × 109 | 9.40 × 1021 | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thuneman, T.; Raja, K.S.; Charit, I. Room Temperature Corrosion Behavior of Selective Laser Melting (SLM)-Processed Ni-Fe Superalloy (Inconel 718) in 3.5% NaCl Solution at Different pH Conditions: Role of Microstructures. Crystals 2024, 14, 89. https://doi.org/10.3390/cryst14010089
Thuneman T, Raja KS, Charit I. Room Temperature Corrosion Behavior of Selective Laser Melting (SLM)-Processed Ni-Fe Superalloy (Inconel 718) in 3.5% NaCl Solution at Different pH Conditions: Role of Microstructures. Crystals. 2024; 14(1):89. https://doi.org/10.3390/cryst14010089
Chicago/Turabian StyleThuneman, Thomas, Krishnan S. Raja, and Indrajit Charit. 2024. "Room Temperature Corrosion Behavior of Selective Laser Melting (SLM)-Processed Ni-Fe Superalloy (Inconel 718) in 3.5% NaCl Solution at Different pH Conditions: Role of Microstructures" Crystals 14, no. 1: 89. https://doi.org/10.3390/cryst14010089





