Insights into the Electronic, Optical, and Anti-Corrosion Properties of Two-Dimensional ZnO: First-Principles Study
Abstract
:1. Introduction
2. Computational Model
3. Results and Discussions
3.1. Structure Stability
3.2. Electronic Properties
3.3. Anticorrosion Performance
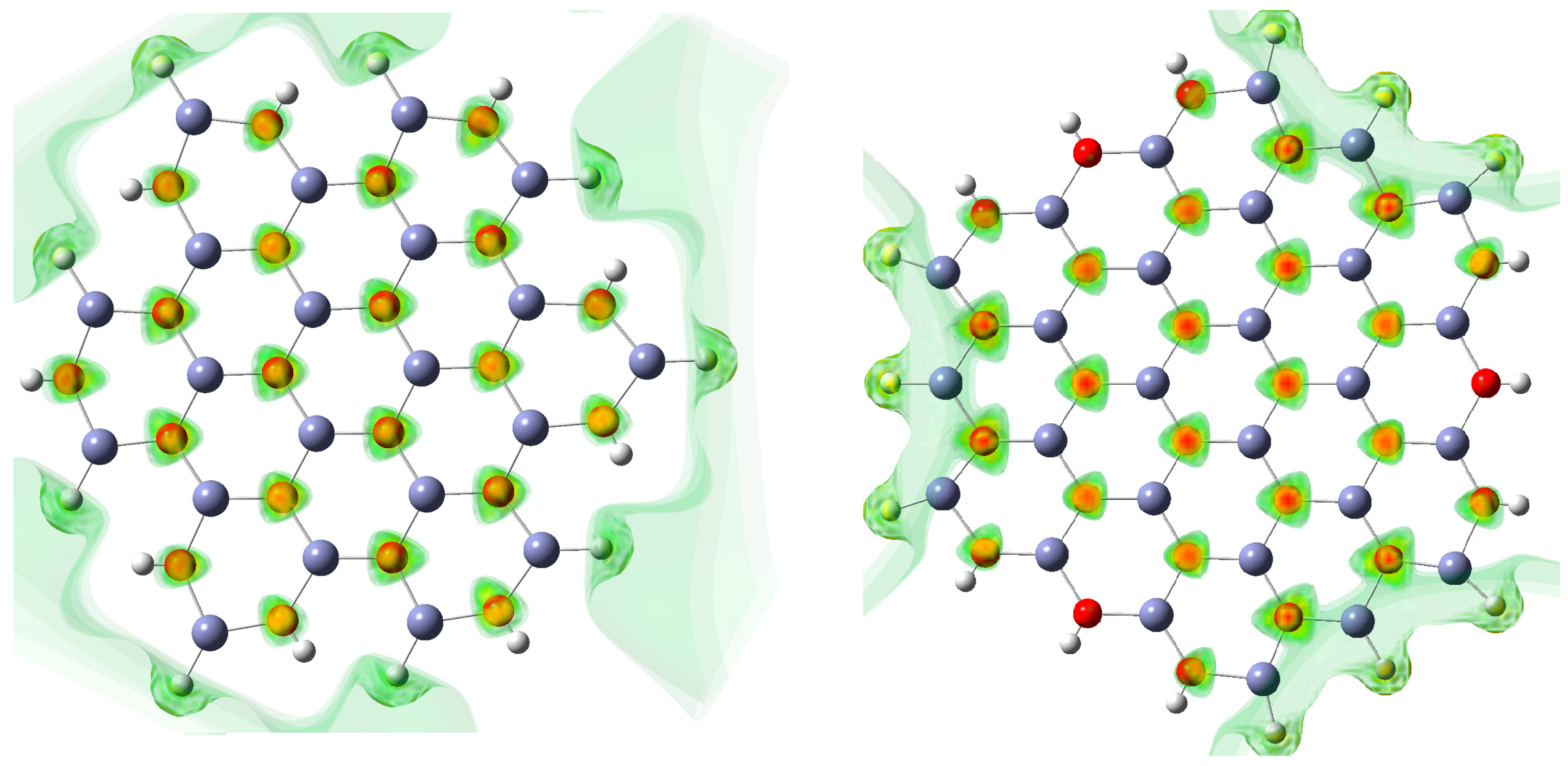
3.4. Molecular Electrostatic Potentials (MEP)
3.5. UV-Vis Absorption Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Morab, S.; Sundaram, M.M.; Pivrikas, A. Review on charge carrier transport in inorganic and organic semiconductors. Coatings 2023, 13, 1657. [Google Scholar] [CrossRef]
- Brütting, W. Introduction to the physics of organic semiconductors. In Physics of Organic Semiconductors; Wiley: Hoboken, NJ, USA, 2005; pp. 1–14. [Google Scholar]
- Yadav, S.; Jena, S.R.; MB, B.; Altaee, A.; Saxena, M.; Samal, A.K. Heterostructures of 2D materials-quantum dots (QDs) for optoelectronic devices: Challenges and opportunities. Emergent Mater. 2021, 4, 901–922. [Google Scholar] [CrossRef]
- Sakthivel, R.; Keerthi, M.; Chung, R.-J.; He, J.-H. Heterostructures of 2D materials and their applications in biosensing. Prog. Mater. Sci. 2023, 132, 101024. [Google Scholar] [CrossRef]
- Mas-Balleste, R.; Gomez-Navarro, C.; Gomez-Herrero, J.; Zamora, F. 2D materials: To graphene and beyond. Nanoscale 2011, 3, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Velický, M.; Toth, P.S. From two-dimensional materials to their heterostructures: An electrochemist’s perspective. Appl. Mater. Today 2017, 8, 68–103. [Google Scholar] [CrossRef]
- Duong, D.L.; Yun, S.J.; Lee, Y.H. van der Waals layered materials: Opportunities and challenges. ACS Nano 2017, 11, 11803–11830. [Google Scholar] [CrossRef]
- Medina, H.; Li, J.-G.; Su, T.-Y.; Lan, Y.-W.; Lee, S.-H.; Chen, C.-W.; Chen, Y.-Z.; Manikandan, A.; Tsai, S.-H.; Navabi, A. Wafer-scale growth of wse2 monolayers toward phase-engineered hybrid wo x/wse2 films with sub-ppb no x gas sensing by a low-temperature plasma-assisted selenization process. Chem. Mater. 2017, 29, 1587–1598. [Google Scholar] [CrossRef]
- Qu, Y.; Medina, H.; Wang, S.W.; Wang, Y.C.; Chen, C.W.; Su, T.Y.; Manikandan, A.; Wang, K.; Shih, Y.C.; Chang, J.W. Wafer scale phase-engineered 1T-and 2H-MoSe2/Mo core–shell 3D-hierarchical nanostructures toward efficient electrocatalytic hydrogen evolution reaction. Adv. Mater. 2016, 28, 9831–9838. [Google Scholar] [CrossRef]
- Waleed, A.; Tavakoli, M.M.; Gu, L.; Wang, Z.; Zhang, D.; Manikandan, A.; Zhang, Q.; Zhang, R.; Chueh, Y.-L.; Fan, Z. Lead-free perovskite nanowire array photodetectors with drastically improved stability in nanoengineering templates. Nano Lett. 2017, 17, 523–530. [Google Scholar] [CrossRef]
- Bollella, P.; Fusco, G.; Tortolini, C.; Sanzò, G.; Favero, G.; Gorton, L.; Antiochia, R. Beyond graphene: Electrochemical sensors and biosensors for biomarkers detection. Biosens. Bioelectron. 2017, 89, 152–166. [Google Scholar] [CrossRef]
- Manikandan, A.; Chen, Y.-Z.; Shen, C.-C.; Sher, C.-W.; Kuo, H.-C.; Chueh, Y.-L. A critical review on two-dimensional quantum dots (2D QDs): From synthesis toward applications in energy and optoelectronics. Prog. Quantum Electron. 2019, 68, 100226. [Google Scholar] [CrossRef]
- Xu, M.; Liang, T.; Shi, M.; Chen, H. Graphene-like two-dimensional materials. Chem. Rev. 2013, 113, 3766–3798. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Neal, A.T.; Zhu, Z.; Tomanek, D.; Ye, P.D. Phosphorene: A new 2D material with high carrier mobility. arXiv 2014, arXiv:1401.4133. [Google Scholar]
- Naguib, M.; Come, J.; Dyatkin, B.; Presser, V.; Taberna, P.-L.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. MXene: A promising transition metal carbide anode for lithium-ion batteries. Electrochem. Commun. 2012, 16, 61–64. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, H. Two-dimensional transition metal dichalcogenide nanosheet-based composites. Chem. Soc. Rev. 2015, 44, 2713–2731. [Google Scholar] [CrossRef]
- Lv, L.; Yang, Z.; Chen, K.; Wang, C.; Xiong, Y. 2D layered double hydroxides for oxygen evolution reaction: From fundamental design to application. Adv. Energy Mater. 2019, 9, 1803358. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Anasori, B. The rise of MXenes. ACS Nano 2019, 13, 8491–8494. [Google Scholar] [CrossRef]
- dos Santos, R.B.; Rivelino, R.; Gueorguiev, G.K.; Kakanakova-Georgieva, A. Exploring 2D structures of indium oxide of different stoichiometry. CrystEngComm 2021, 23, 6661–6667. [Google Scholar] [CrossRef]
- Alves Machado Filho, M.; Hsiao, C.-L.; Dos Santos, R.B.; Hultman, L.; Birch, J.; Gueorguiev, G.K. Self-Induced Core–Shell InAlN Nanorods: Formation and Stability Unraveled by Ab Initio Simulations. ACS Nanosci. Au 2022, 3, 84–93. [Google Scholar] [CrossRef]
- Huang, M.; Wang, M.; Chen, C.; Ma, Z.; Li, X.; Han, J.; Wu, Y. Broadband black-phosphorus photodetectors with high responsivity. Adv. Mater. 2016, 28, 3481–3485. [Google Scholar] [CrossRef]
- Pavliček, N.; Mistry, A.; Majzik, Z.; Moll, N.; Meyer, G.; Fox, D.J.; Gross, L. Synthesis and characterization of triangulene. Nat. Nanotechnol. 2017, 12, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Kabel, J.; Sharma, S.; Acharya, A.; Zhang, D.; Yap, Y.K. Molybdenum disulfide quantum dots: Properties, synthesis, and applications. C 2021, 7, 45. [Google Scholar] [CrossRef]
- Prasannachandran, R.; Vineesh, T.V.; Anil, A.; Krishna, B.M.; Shaijumon, M.M. Functionalized phosphorene quantum dots as efficient electrocatalyst for oxygen evolution reaction. ACS Nano 2018, 12, 11511–11519. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, H.; Zhang, Q. Spintronic properties of 2D heterostructures from laterally connected graphene and hBN quantum dots. Chem. Phys. Lett. 2023, 825, 140591. [Google Scholar] [CrossRef]
- Jin, S.H.; Kim, D.H.; Jun, G.H.; Hong, S.H.; Jeon, S. Tuning the photoluminescence of graphene quantum dots through the charge transfer effect of functional groups. ACS Nano 2013, 7, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Golovynskyi, S.; Datsenko, O.I.; Dong, D.; Lin, Y.; Golovynska, I.; Jin, Z.; Li, B.; Wu, H. MoS2 monolayer quantum dots on a flake: Efficient sensitization of exciton and trion photoluminescence via resonant nonradiative energy and charge transfers. Appl. Surf. Sci. 2022, 601, 154209. [Google Scholar] [CrossRef]
- Angizi, S.; Alem, S.A.A.; Azar, M.H.; Shayeganfar, F.; Manning, M.I.; Hatamie, A.; Pakdel, A.; Simchi, A. A comprehensive review on planar boron nitride nanomaterials: From 2D nanosheets towards 0D quantum dots. Prog. Mater. Sci. 2022, 124, 100884. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, A.; Xu, Y.; Shan, F.; Li, A.; Wang, J.; Yang, W.; Barrow, C.; Liu, J. Graphene quantum dots directly generated from graphite via magnetron sputtering and the application in thin-film transistors. Carbon 2015, 88, 225–232. [Google Scholar] [CrossRef]
- Long, M.; Wang, P.; Fang, H.; Hu, W. Progress, challenges, and opportunities for 2D material based photodetectors. Adv. Funct. Mater. 2019, 29, 1803807. [Google Scholar] [CrossRef]
- Abdelsalam, H.; Atta, M.M.; Saroka, V.A.; Zhang, Q. Anomalous magnetic and transport properties of laterally connected graphene quantum dots. J. Mater. Sci. 2022, 57, 14356–14370. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef] [PubMed]
- Sakr, M.A.; Saad, M.A.; Abdelsalam, H.; Abd-Elkader, O.H.; Aleya, L.; Zhang, Q. Two-Dimensional ZnS Quantum Dots for Gas Sensors: Electronic and Adsorption Properties. J. Electron. Mater. 2023, 52, 5227–5238. [Google Scholar] [CrossRef]
- Abdelsalam, H.; Atta, M.M.; Osman, W.; Zhang, Q. Two-dimensional quantum dots for highly efficient heterojunction solar cells. J. Colloid Interface Sci. 2021, 603, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, H.; Li, J. Graphene and graphene-like layered transition metal dichalcogenides in energy conversion and storage. Small 2014, 10, 2165–2181. [Google Scholar] [CrossRef] [PubMed]
- Marcus, P. Corrosion Mechanisms in Theory and Practice; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- de Rincon, O.T.; Perez, O.; Paredes, E.; Caldera, Y.; Urdaneta, C.; Sandoval, I. Long-term performance of ZnO as a rebar corrosion inhibitor. Cem. Concr. Compos. 2002, 24, 79–87. [Google Scholar] [CrossRef]
- Al-Dahiri, R.H.; Turkustani, A.M.; Salam, M.A. The application of zinc oxide nanoparticles as an eco-friendly inhibitor for steel in acidic solution. Int. J. Electrochem. Sci. 2020, 15, 442–457. [Google Scholar] [CrossRef]
- Kamburova, K.; Boshkova, N.; Boshkov, N.; Radeva, T. Composite coatings with polymeric modified ZnO nanoparticles and nanocontainers with inhibitor for corrosion protection of low carbon steel. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125741. [Google Scholar] [CrossRef]
- Quadri, T.W.; Olasunkanmi, L.O.; Fayemi, O.E.; Ebenso, E.E. Utilization of ZnO-based materials as anticorrosive agents: A review. Inorg. Anticorros. Mater. 2022, 161–182. [Google Scholar] [CrossRef]
- Nazari, M.H.; Zhang, Y.; Mahmoodi, A.; Xu, G.; Yu, J.; Wu, J.; Shi, X. Nanocomposite organic coatings for corrosion protection of metals: A review of recent advances. Prog. Org. Coat. 2022, 162, 106573. [Google Scholar] [CrossRef]
- Ammar, S.; Ramesh, K.; Vengadaesvaran, B.; Ramesh, S.; Arof, A.K. Formulation and characterization of hybrid polymeric/ZnO nanocomposite coatings with remarkable anti-corrosion and hydrophobic characteristics. J. Coat. Technol. Res. 2016, 13, 921–930. [Google Scholar] [CrossRef]
- Shi, X.; Nguyen, T.A.; Suo, Z.; Liu, Y.; Avci, R. Effect of nanoparticles on the anticorrosion and mechanical properties of epoxy coating. Surf. Coat. Technol. 2009, 204, 237–245. [Google Scholar] [CrossRef]
- Puspasari, V.; Ridhova, A.; Hermawan, A.; Amal, M.I.; Khan, M.M. ZnO-based antimicrobial coatings for biomedical applications. Bioprocess Biosyst. Eng. 2022, 45, 1421–1445. [Google Scholar] [CrossRef]
- Sakellis, I. Determining the activation volumes in ZnO. J. Appl. Phys. 2012, 112, 013504. [Google Scholar] [CrossRef]
- Frisch, M.E.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H. Gaussian 16, Revision C. 01. 2016. Available online: https://gaussian.com/citation/ (accessed on 10 January 2024).
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self—Consistent molecular orbital methods. XII. Further extensions of Gaussian—Type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Yang, Y.; Weaver, M.N.; Merz, K.M., Jr. Assessment of the “6-31 + G** + LANL2DZ” mixed basis set coupled with density functional theory methods and the effective core potential: Prediction of heats of formation and ionization potentials for first-row-transition-metal complexes. J. Phys. Chem. A 2009, 113, 9843–9851. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. I. The effect of the exchange-only gradient correction. J. Chem. Phys. 1992, 96, 2155–2160. [Google Scholar] [CrossRef]
- Povie, G.; Segawa, Y.; Nishihara, T.; Miyauchi, Y.; Itami, K. Synthesis and size-dependent properties of [12], [16], and [24] carbon nanobelts. J. Am. Chem. Soc. 2018, 140, 10054–10059. [Google Scholar] [CrossRef]
- Hegazy, M.A.; Ezzat, H.A.; Yahia, I.S.; Zahran, H.Y.; Elhaes, H.; Matar, H.; Ibrahim, M. Effect of Metal Oxides and Graphene Upon The Electronic Properties of Polyvinyl Alcohol. 2021; preprint. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Kushwaha, A.K.; Sahoo, M.R.; Ray, M.; Das, D.; Nayak, S.; Maity, A.; Sarkar, K.; Bhagat, A.N.; Pal, A.R.; Rout, T.K. Functional Pyromellitic Diimide as a Corrosion Inhibitor for Galvanized Steel: An Atomic-Scale Engineering. ACS Omega 2022, 7, 27116–27125. [Google Scholar] [CrossRef]
- Radhi, A.H.; Du, E.A.; Khazaal, F.A.; Abbas, Z.M.; Aljelawi, O.H.; Hamadan, S.D.; Almashhadani, H.A.; Kadhim, M.M. HOMO-LUMO energies and geometrical structures effecton corrosion inhibition for organic compounds predict by DFT and PM3 methods. NeuroQuantology 2020, 18, 37. [Google Scholar] [CrossRef]
- Zarrouk, A.; El Ouali, I.; Bouachrine, M.; Hammouti, B.; Ramli, Y.; Essassi, E.; Warad, I.; Aouniti, A.; Salghi, R. Theoretical approach to the corrosion inhibition efficiency of some quinoxaline derivatives of steel in acid media using the DFT method. Res. Chem. Intermed. 2013, 39, 1125–1133. [Google Scholar] [CrossRef]
- Gece, G. The use of quantum chemical methods in corrosion inhibitor studies. Corros. Sci. 2008, 50, 2981–2992. [Google Scholar] [CrossRef]
- Kokalj, A. Is the analysis of molecular electronic structure of corrosion inhibitors sufficient to predict the trend of their inhibition performance. Electrochim. Acta 2010, 56, 745–755. [Google Scholar] [CrossRef]
- Kokalj, A. On the HSAB based estimate of charge transfer between adsorbates and metal surfaces. Chem. Phys. 2012, 393, 1–12. [Google Scholar] [CrossRef]
- Lee, J.; Sorescu, D.C.; Deng, X. Tunable lattice constant and band gap of single-and few-layer ZnO. J. Phys. Chem. Lett. 2016, 7, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, N.; Zhou, S.; Zhao, J. Two-dimensional ZnO for the selective photoreduction of CO2. J. Mater. Chem. A 2019, 7, 16294–16303. [Google Scholar] [CrossRef]

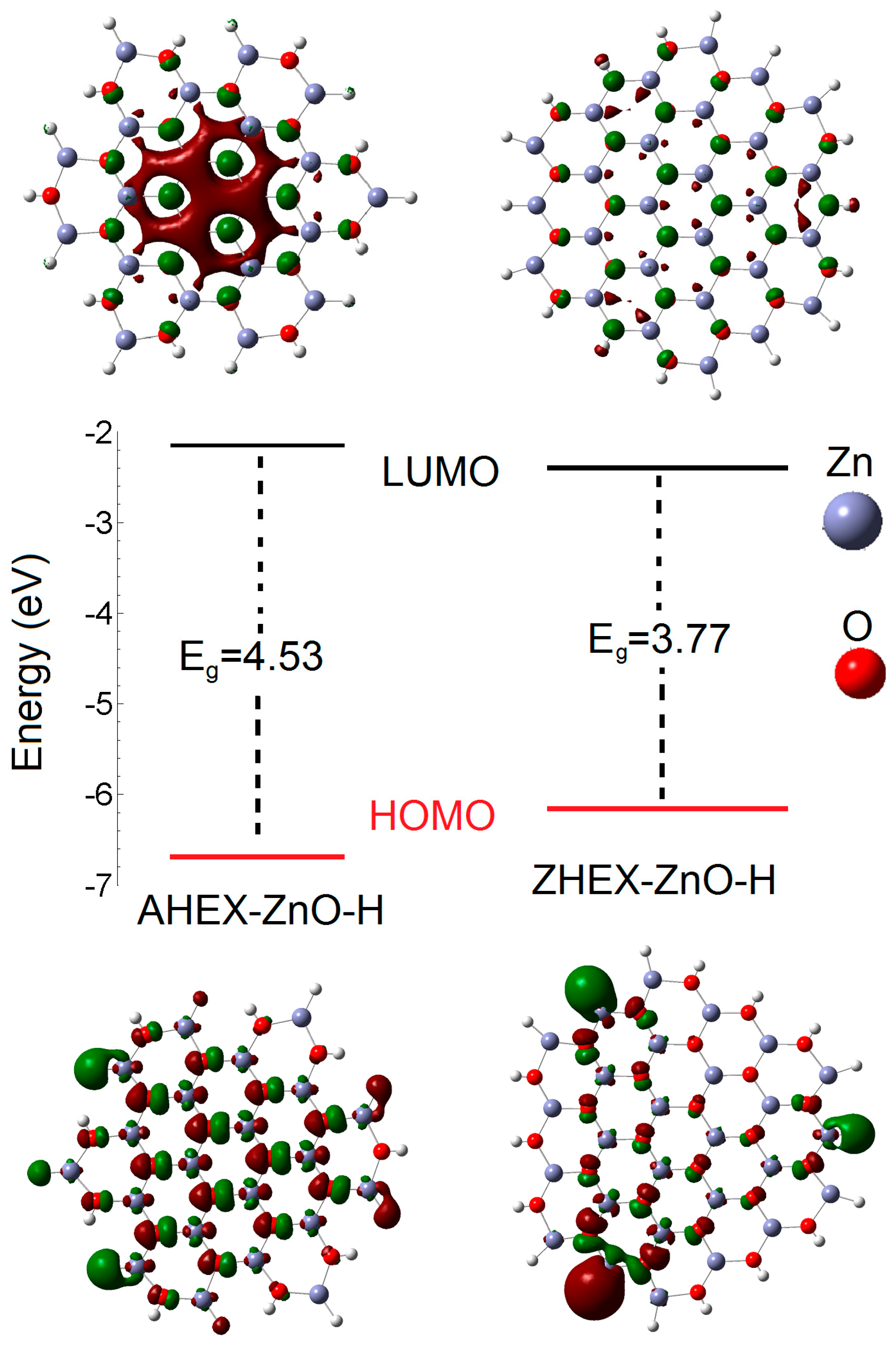

| Structure | Bond Lengths (Å) | Binding Energy (EB) (eV) | ||
|---|---|---|---|---|
| H–O | H–Zn | Zn–O | ||
| AHEX–ZnO | 0.971–0.974 | 1.615–1.640 | 1.897–1.977 | −0.16 |
| ZHEX–ZnO | 0.969–0.970 | 1.622–1.631 | 1.877–2.056 | −0.15 |
| Structure | ELUMO (eV) | EHOMO (eV) | ∆E (eV) | η (eV) | σ (eV−1) | χ (eV) | ΔN |
|---|---|---|---|---|---|---|---|
| AHEX–ZnO | −2.17 | −6.70 | 4.53 | 2.27 | 0.44 | 4.44 | 0.56 |
| ZHEX–ZnO | −2.39 | −6.17 | 3.77 | 1.89 | 0.53 | 4.28 | 0.72 |
| 4ZHEX–ZnO | −2.71 | −5.83 | 3.12 | 1.56 | 0.64 | 4.27 | 0.88 |
| 5ZHEX–ZnO | −3.06 | −5.38 | 2.32 | 1.16 | 0.86 | 4.22 | 1.19 |
| ZnO-Crystal | 0.78 | −2.65 | 3.43 | 1.72 | 0.58 | 0.94 | 1.76 |
| Absorption Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| ES | ET | ΔE (eV) | λ (nm) | f | ES | ET | ΔE (eV) | λ (nm) | f |
| AHEX–ZnO | ZHEX–ZnO-H | ||||||||
| H-11 → L | |||||||||
| H-7 → L | |||||||||
| 1 | H → L | 3.93 | 316 | 0.46 | 8 | H-4 → L | 3.80 | 326 | 0.34 |
| H-2 → L | |||||||||
| H-10 → L | |||||||||
| H-6 → L | |||||||||
| 2 | H-2 → L | 3.93 | 315 | 0.46 | 9 | H-5 → L | 3.80 | 326 | 0.34 |
| H-2 → L | |||||||||
| H-4 → L | |||||||||
| 4 | H-3 → L | 4.28 | 290 | 0.002 | 1 | H → L | 3.38 | 367 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elwahab, F.A.; Teleb, N.H.; Abdelsalam, H.; Abd-Elkader, O.H.; Zhang, Q. Insights into the Electronic, Optical, and Anti-Corrosion Properties of Two-Dimensional ZnO: First-Principles Study. Crystals 2024, 14, 179. https://doi.org/10.3390/cryst14020179
Elwahab FA, Teleb NH, Abdelsalam H, Abd-Elkader OH, Zhang Q. Insights into the Electronic, Optical, and Anti-Corrosion Properties of Two-Dimensional ZnO: First-Principles Study. Crystals. 2024; 14(2):179. https://doi.org/10.3390/cryst14020179
Chicago/Turabian StyleElwahab, Fatma Abd, Nahed H. Teleb, Hazem Abdelsalam, Omar H. Abd-Elkader, and Qinfang Zhang. 2024. "Insights into the Electronic, Optical, and Anti-Corrosion Properties of Two-Dimensional ZnO: First-Principles Study" Crystals 14, no. 2: 179. https://doi.org/10.3390/cryst14020179







